Abstract
Developmental exposure to unrelated neurotoxicants can nevertheless converge on common final targets so as to exacerbate damage or functional deficits. We examined the effects of developmental exposure to terbutaline, a β2-adrenergic receptor agonist used to arrest preterm labor, and chlorpyrifos, a widely used organophosphate pesticide, on serotonin (5HT) systems. Treatments were chosen to parallel periods typical of human developmental exposures, terbutaline (10 mg/kg) on postnatal days (PN) 2-5 and chlorpyrifos (5 mg/kg) on PN11-14, with assessments conducted in juvenile and adolescent stages (PN21, PN30 and PN45), comparing each agent alone as well as sequential administration of both. By itself, terbutaline produced persistent 5HT presynaptic hyperactivity as evidenced by increased 5HT turnover in brain regions containing 5HT terminal zones; this effect was similar to that seen in earlier studies with chlorpyrifos administration during the same early postnatal period. Later administration of chlorpyrifos (PN11-14) produced a transient increase in 5HT turnover during the juvenile stage, and the sequential exposure paradigm, terbutaline followed by chlorpyrifos, showed a corresponding increase in effect over either agent alone. In combination with our earlier work on 5HT receptors, these results indicate that terbutaline is a developmental neurotoxicant that targets the 5HT system, findings that lend a mechanistic underpinning to clinical indications of elevated childhood psychiatric disorders in the offspring of women treated with β-agonist tocolytics. Equally importantly, the interaction between terbutaline and chlorpyrifos suggests that tocolytic therapy may alter the subsequent susceptibility to common environmental toxicants.
Keywords: Adolescence, Brain development, Chlorpyrifos, Organophosphate insecticides, Preterm labor, Serotonin turnover, Terbutaline, Tocolysis
Introduction
It is increasingly evident that events in fetal stages contribute to the subsequent emergence of disease states in childhood, adolescence or adulthood [16,21,22,36,41,50,53,63,79,82]. Although a variety of social and economic factors undoubtedly underlie the marked increases noted for obesity and affective or anxiety disorders, it has also been proposed that environmental factors that target the functioning of serotonin (5HT), the neurotransmitter most closely implicated in the control of appetite and mood, play a significant role [16,63,79,82]. Because disparate agents can converge on the programming of 5HT development and function, drugs and chemicals that have different fundamental mechanisms of action in the fetal brain can nonetheless produce similar final outcomes because they ultimately converge on common sets of developmental signals (reviews, [85,86].
In the current study, we focused on two seemingly unrelated agents that ultimately affect the programming of 5HT synaptic development: terbutaline, a β-adrenergic receptor (βAR) agonist, and chlorpyrifos, one of the most widely-used organophosphate pesticides. Terbutaline is used “off-label” as a tocolytic to arrest preterm labor, which occurs in approximately 20% of pregnancies in the U.S. [9,43]; although controlled studies show that it is ineffective for maintenance tocolysis, it is still commonly given for that purpose [42,44,46,62,78]. The passage of terbutaline across the placenta leads to inappropriate hyperstimulation of βARs in the fetal brain, leading to morphological abnormalities and persistent alterations in synaptic development and function [3,28,59,60,66,72]. Clinical studies indicate that maintenance tocolytic therapy with βAR agonists leads to a significantly increased incidence of psychiatric disorders as compared to preterm infants not exposed to these agents, with the differences first emerging in childhood up to the preadolescent stage [54]. Recently, these findings have been extended to include autism [17]. In our earlier work with terbutaline administered to developing rats, we found that treatments simulating the neurodevelopmental period corresponding to its use in preterm labor produced persistent overexpression of 5HT receptors in adulthood [3], a finding characteristic of upregulation associated with deficient synaptic function.
Interestingly, we had similar findings for prenatal or early neonatal chlorpyrifos exposure [1-3,5,69], suggesting a convergence of these two otherwise unrelated agents on a common set of functional deficits related to 5HT systems. Indeed, a connection of organophosphate exposures to autism has been proposed [19,75], just as for terbutaline. Although recent U.S. regulations have discontinued chlorpyrifos use in the home [80,81], its major application in agriculture continues unabated, as does its residential use elsewhere in the world [14]. Indeed, organophosphates account for over 50% of insecticide application worldwide, with chlorpyrifos as the leading agent [14]. Additionally, given the ubiquitous exposure of the human population to this agent [8,12,14], there is an ongoing legacy of adverse neurodevelopmental consequences to be explored, and a recent study points out how exposures below acceptable guidelines nevertheless have an impact on children's cognitive performance [57].
Much more is known about the mechanisms, sequence of events and long-term consequences on 5HT function for chlorpyrifos than for terbutaline. Prenatal or early neonatal chlorpyrifos exposure produces permanent upregulation of 5HT receptors in association with increased presynaptic 5HT activity and persistent behavioral deficits, effects that are consistent with miswiring of 5HT circuits [1-5,56,69,70]. Importantly many of these aspects emerge in juvenile to periadolescent stages after a period of apparent normality, thus implicating a change in the programming of 5HT function [5,56,70]. Accordingly, the current study focuses on whether neonatal terbutaline exposure, which also produces 5HT receptor upregulation in adulthood [3], similarly exhibits 5HT presynaptic hyperactivity in juvenile and/or adolescent stages. We also determined whether sequential exposure to terbutaline followed by chlorpyrifos might exacerbate the abnormalities of 5HT function; given the widespread use of terbutaline in preterm labor and the ubiquitous exposure to organophosphates, this sequence mimics a common scenario involving a subpopulation that might be especially vulnerable to organophosphate-related developmental neurotoxicity [3,48,60]. We used treatments appropriate to the corresponding peak periods of human fetal or neonatal exposures, given the fact that the rat is an altricial species [15,61]. We gave terbutaline on postnatal days (PN) 2-5, analogous to the common use of this drug during the second trimester of human pregnancy [43,44]; given the developmental timetable for development of the rat brain [15,61], the four day treatment regimen corresponds to exposure over several weeks' duration in human fetal development, thus mimicking maintenance tocolysis. Chlorpyrifos was administered on PN11-14, corresponding to the higher human exposures in newborns and young children both in typical domestic settings and in agricultural communities [24,25,31,37,45,52]. Notably, chlorpyrifos by itself produces greater alterations in 5HT systems when given earlier, either in late gestation or just after birth [1-5,56,69,70]. However, for our purposes, we wanted a period where the sensitivity to chlorpyrifos alone would be detectable but waning, so as to be able to identify an interaction with terbutaline indicative of exacerbation of adverse effects. Evaluations were conducted in the juvenile stage (PN21) as well as in early (PN30) and late adolescence (PN45). Presynaptic activity was determined from the 5HT turnover rate as assessed by ratio of the 5HT metabolite, 5-hydroxyindoleacetic acid (5HIAA) to 5HT [71,84]. We examined brain regions with prominent 5HT innervation that are known to be targets for terbutaline and chlorpyrifos [1-3,5,69,70] and that comprise the majority of 5HT projections (cerebral cortex, striatum, hippocampus), as well as regions containing 5HT cell bodies (midbrain, brainstem).
Methods
All experiments using live animals were reviewed and approved by the Duke University Institutional Animal Care and Use Committee, and were carried out in accordance with the declaration of Helsinki and with the Guide for the Care and Use of Laboratory Animals as adopted and promulgated by the National Institutes of Health. Twenty-four timed-pregnant Sprague-Dawley rats were housed in breeding cages, with a 12-h light-dark cycle and free access to food and water. Pups from all litters were randomized on the day after birth and redistributed to the dams with litter sizes of 10 pups to ensure standardized nutrition and maternal care. The animals were then divided into four treatment groups with six litters per group: controls, those receiving terbutaline alone, those receiving chlorpyrifos alone, and those receiving terbutaline followed by chlorpyrifos. To enable comparison across all four groups, all animals were given two batteries of injections; that is, animals receiving terbutaline alone were also given injections of the vehicle used for chlorpyrifos injections on PN11-14 whereas animals receiving chlorpyrifos alone were given injections of the vehicle used for terbutaline injection on PN2-5. The corresponding controls similarly received both sets of vehicle injections. Randomization was repeated at intervals of several days to distribute any differences in maternal caretaking equally among all litters. Weaning was carried out on PN21. For each experiment, each litter contributed no more than one male and one female for each treatment group.
Terbutaline sulfate (10 mg/kg s.c.; Sigma Chemical Co., St. Louis, MO) or equivalent volumes of saline (1 ml/kg) were administered daily on PN2-5. This regimen elicits robust β-adrenergic receptor stimulation in the neonate, simulating the effects seen with its use as a tocolytic, including cardiac activation and enhancement of lung surfactant synthesis [6,28,39,40,49]. For tocolytic therapy in humans, doses typically lie in the range of 0.5 mg/kg/day, but can also be as high as 1-2 mg/kg/day [29,43]. In light of the fact that terbutaline has a much shorter half-life in the rat [77] we used a proportionally higher dose.
On PN11-14, animals were treated with either chlorpyrifos (5 mg/kg s.c.) or vehicle (1 ml/kg) on postnatal days 11-14. Chlorpyrifos was dissolved in dimethylsulfoxide to provide consistent absorption [83]. This dose, which lies within the range of estimated fetal and neonatal exposures from environmental exposure or home use of chlorpyrifos [24,31,52], has been shown previously to produce delayed neurotoxicity in the developing rat brain without eliciting signs of systemic toxicity as evaluated by signs of cholinergic hyperstimulation, weight loss or impaired viability [13,20,64,67,73,74,83]; notably, the neurodevelopmental defects include effects on 5HT systems that emerge in adolescence and persist into adulthood [1-3,5,69,70].
Animals were decapitated on PN21, PN30 and PN45, with 12 animals for each treatment group at each age, evenly divided between males and females. Blunt cuts were made through the cerebellar peduncles, whereupon the cerebellum (including flocculi) was lifted from the underlying tissue; a cut was then made rostral to the thalamus to separate the forebrain from the midbrain+brainstem. The forebrain was further dissected further into cerebral cortex, hippocampus and striatum, and the midbrain was separated from the brainstem, with the exception that on PN21, the striatum was included in the region designated as “cerebral cortex.” Tissues were frozen immediately in liquid nitrogen and maintained at −45°C until assayed. All assays were run such that all the animals for all four groups were evaluated simultaneously to ensure that day-to-day assay variations did not generate spurious treatment effects.
Tissues were thawed and homogenized in ice-cold 0.1 M perchloric acid and sedimented for 20 min at 18,000 × g. The supernatant solution was collected and aliquots were used for analysis of 5HT and 5HIAA by high-performance liquid chromatography with electrochemical detection [71,84]. Concurrently-run standards containing 5HT and 5HIAA (Sigma Chemical Co., St. Louis, MO), were used to calculate the regional content of each neurochemical. Transmitter levels were calculated as ng per g tissue and ng per region, and, because there were no treatment-related differences in brain region weights (see Results), the same effects were obtained with either calculation. Because 5HT turnover is calculated as the ratio of 5HIAA to 5HT, the values are independent of whether region or tissue weight is used as the denominator term.
The studies involved a multivariate design, with factors of treatment, age, brain region and sex. Accordingly, initial evaluations were conducted using a global ANOVA incorporating all factors, using log-transformed values because of heterogeneity of variance among the different regions and ages. Where significant treatment effects were found, these were followed by lower-order ANOVAs and finally, individual values that differed from the corresponding control or from other treatment groups were established post-hoc using Fisher's Protected Least Significant Difference. We did not find significant sex differences in any of the treatment effects (no treatment × sex interactions), so values are shown combined for males and females, although the factor of sex was retained in performing the statistical evaluations. In order to facilitate visual comparison of treatment effects in different regions and at different ages, results are reported as the percentage change from control values but the statistical evaluations were always conducted on the original data. For reference, the corresponding control determinations appear in Table 1.
TABLE 1.
Control Values
| Age | Region | 5HT (ng/g tissue) | 5HT (ng/region) | 5HT turnover ratio |
|---|---|---|---|---|
| PN21 | cerebral cortex | 157 ± 4 | 128 ± 4 | 1.27 ± 0.09 |
| hippocampus | 143 ± 5 | 13.8 ± 0.6 | 1.70 ± 0.09 | |
| midbrain | 464 ± 34 | 106 ± 8 | 1.40 ± 0.09 | |
| brainstem | 394 ± 14 | 47 ± 2 | 1.15 ± 0.06 | |
| PN30 | cerebral cortex | 181 ± 6 | 146 ± 4 | 0.81 ± 0.08 |
| striatum | 258 ± 6 | 24.0 ± 1.0 | 1.11 ± 0.04 | |
| hippocampus | 164 ± 9 | 16.7 ± 1.0 | 1.30 ± 0.14 | |
| midbrain | 499 ± 27 | 126 ± 6 | 0.76 ± 0.05 | |
| brainstem | 394 ± 14 | 49 ± 2 | 0.74 ± 0.03 | |
| PN45 | cerebral cortex | 203 ± 9 | 173 ± 9 | 0.65 ± 0.05 |
Because of the absence of sex differences for these measures, values are shown combined for males and females. For the measurements on PN21, the region designated as “cerebral cortex” includes the striatum.
Results
Multivariate ANOVA incorporating all treatments, regions, ages and both sexes, indicated significant main treatment effects for both 5HT content (p < 0.05) and turnover (p < 0.0001). Main effects were also detected for each treatment individually as compared to control: terbutaline, p < 0.03 for content and p < 0.0001 for turnover; chlorpyrifos, p < 0.03 for turnover; combined treatment, p < 0.04 for content and p < 0.0001 for turnover. In addition, the effects of terbutaline were substantially greater than those of chlorpyrifos (p < 0.0006 for turnover), as were the effects of combined treatment (p < 0.04 for content, p < 0.0001 for turnover). Neither terbutaline nor chlorpyrifos treatment alone or in combination, elicited a significant deficit in body or brain region weights at any age tested (data not shown), in agreement with earlier studies [3,48,60].
Significant treatment effects on 5HT systems were readily detected at weaning (PN21). For 5HT content, there was a small but significant overall reduction associated with all of the treatments (Fig. 1A). However, there was a much more robust change in 5HT turnover, with substantial increases evoked by each of the treatments (Fig. 1B). By itself, neonatal terbutaline treatment elicited a 20-40% elevation, with the largest changes seen in the terminal zones of the cerebral cortex and hippocampus. Although chlorpyrifos exposure also produced significant overall differences, these were less consistent across the various regions and achieved significance only in the brainstem, which contains 5HT cell bodies. By far the largest effect was seen with sequential treatment (terbutaline followed by chlorpyrifos), with increases in turnover ranging from 30-60% and achieving statistical significance in all brain regions; although the combined effect was most notable in the brainstem, the regional differences need to be interpreted with caution, since there was no treatment × region interaction. The effects of terbutaline alone or in combination with chlorpyrifos were significantly greater than those achieved with chlorpyrifos alone (p < 0.04 and p < 0.003, respectively).
Figure 1.
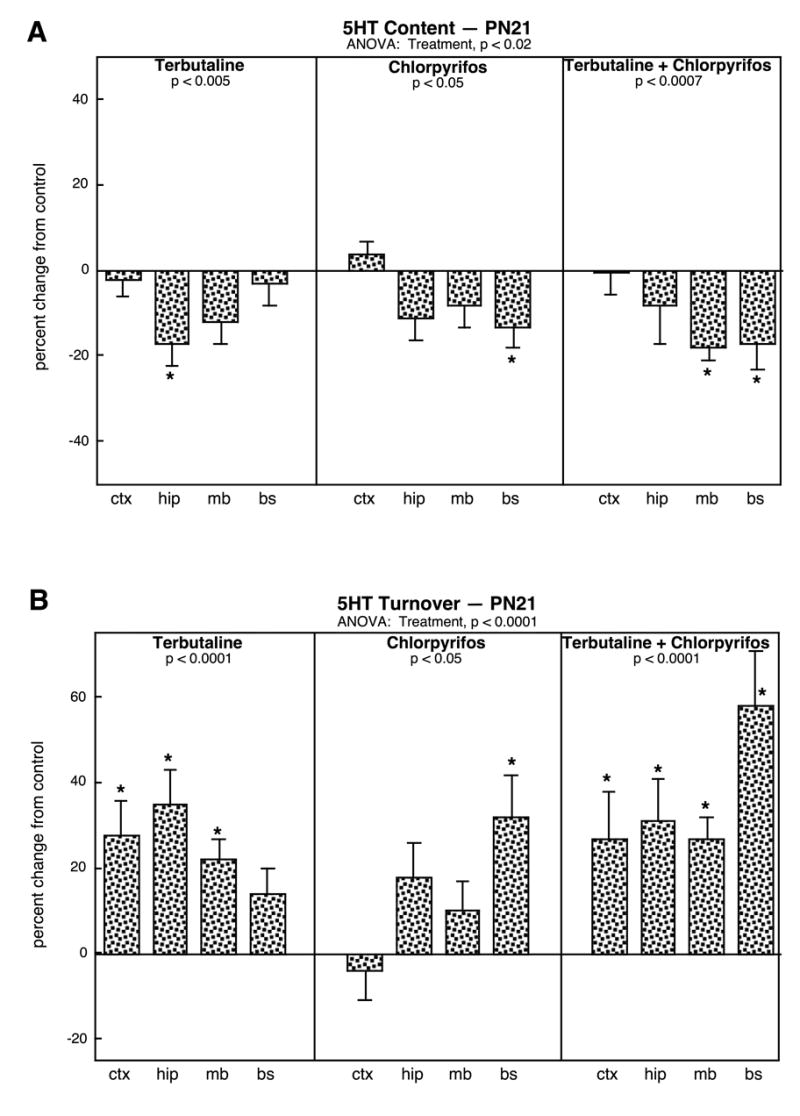
Effects of neonatal treatment with terbutaline (PN2-5), chlorpyrifos (PN11-14), or terbutaline followed by chlorpyrifos on 5HT systems assessed on PN21: (A) 5HT content, (B) 5HT turnover. Data are means and standard errors, presented as the percent change from the control values shown in Table 1. Because of the absence of treatment interactions with sex, values are shown combined for males and females. ANOVA across all regions and treatments appears at the top of each panel and lower-order tests for each treatment compared to control are shown within the panels. Asterisks denote individual regions for which the treatments produce significant differences. For this age, the region designated as cerebral cortex also includes the striatum. Abbreviations: ctx, cerebral cortex; hip, hippocampus; mb, midbrain; bs, brainstem.
By early adolescence (PN30) the minor effects on 5HT content were no longer detectable (Fig. 2A) but robust effects of terbutaline alone or in combination with chlorpyrifos were still notable (Fig. 2B). By itself, terbutaline treatment elicited 30-40% increases in 5HT turnover in regions containing high concentrations of 5HT nerve terminals (cerebral cortex, striatum) and little or no change in those containing 5HT cell bodies (midbrain, brainstem). In contrast, the effects of chlorpyrifos exposure alone receded by PN30, so that there were no longer any detectable differences in 5HT turnover either globally or in any of the individual regions. Sequential exposure to terbutaline followed by chlorpyrifos evoked a pattern almost identical to that seen with terbutaline alone. Again, the significant effects of terbutaline alone or in combination with chlorpyrifos were statistically distinguishable from the nonsignificant changes seen with chlorpyrifos alone (p < 0.05 and p < 0.03, respectively).
Figure 2.
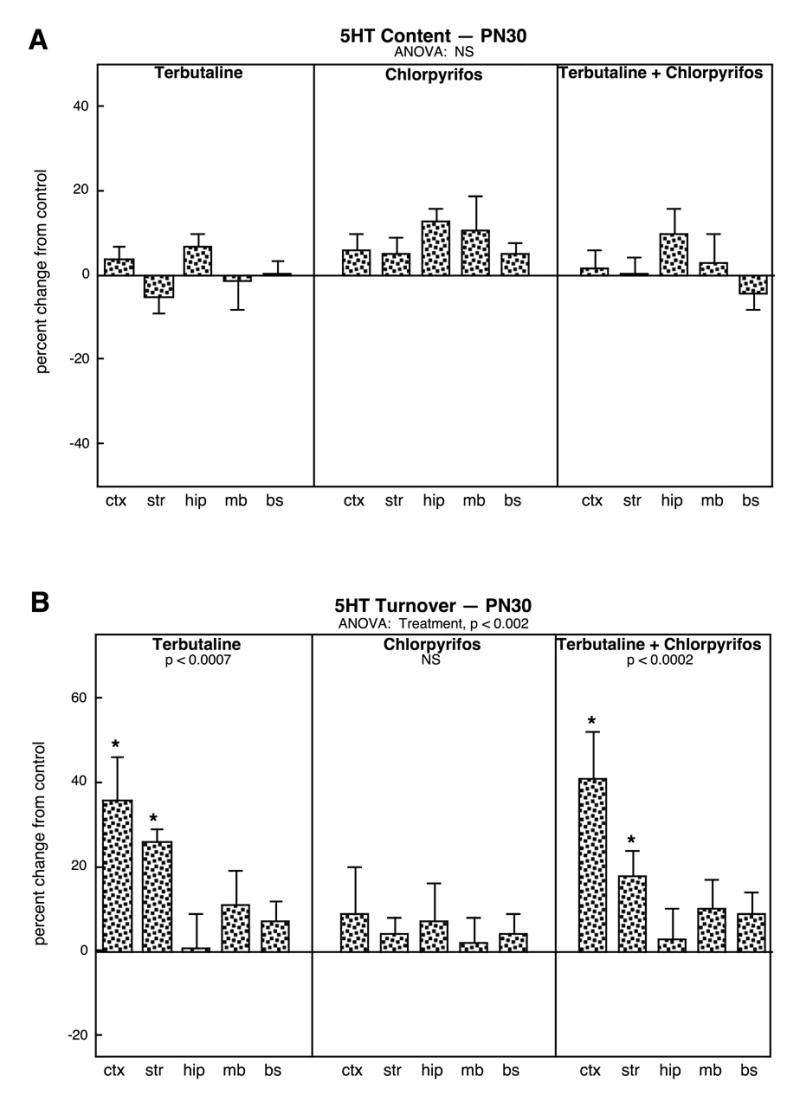
Effects of neonatal treatment with terbutaline (PN2-5), chlorpyrifos (PN11-14), or terbutaline followed by chlorpyrifos on 5HT systems assessed on PN30: (A) 5HT content, (B) 5HT turnover. Data are means and standard errors, presented as the percent change from the control values shown in Table 1. Because of the absence of treatment interactions with sex, values are shown combined for males and females. ANOVA across all regions and treatments appears at the top of each panel and lower-order tests for each treatment compared to control are shown within the panels. Asterisks denote individual regions for which the treatments produce significant differences. Abbreviations: NS, not significant; ctx, cerebral cortex; str, striatum; hip, hippocampus; mb, midbrain; bs, brainstem.
To determine the persistence of effects on 5HT synaptic function into late adolescence, we further examined 5HT content and turnover on PN45 in the cerebral cortex, the region that showed the largest changes on PN30. Again, there were no effects on 5HT content with any of the treatments but 5HT turnover remained significantly elevated with the terbutaline treatment alone or with terbutaline followed by chlorpyrifos (Fig. 3). In addition to their differences from control values, these two effects were significantly distinguishable from the value obtained with chlorpyrifos alone (p < 0.02 and p < 0.05, respectively).
Figure 3.
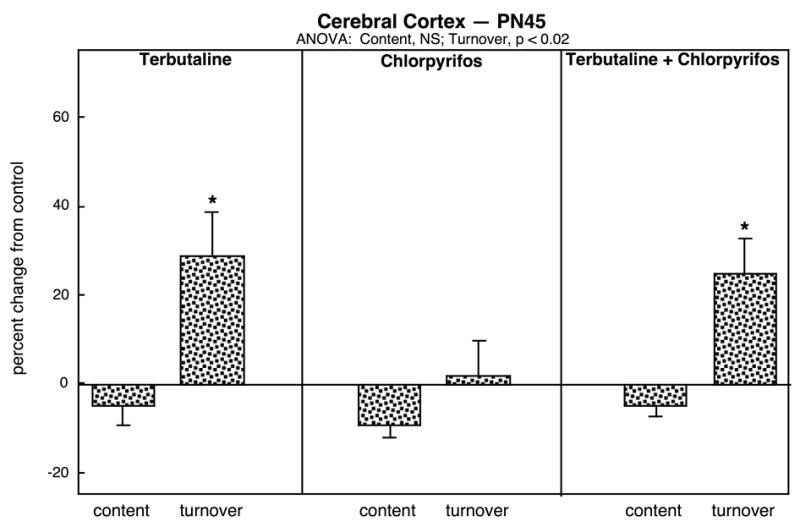
Effects of neonatal treatment with terbutaline (PN2-5), chlorpyrifos (PN11-14), or terbutaline followed by chlorpyrifos on cerebrocortical 5HT content and turnover assessed on PN45. Data are means and standard errors, presented as the percent change from the control values shown in Table 1. Because of the absence of treatment interactions with sex, values are shown combined for males and females. ANOVA across all treatments appears at the top of the panel and asterisks denote significant differences from control values. Abbreviation: NS, not significant.
Discussion
In our earlier studies with neonatal terbutaline treatment, we found overexpression of both the 5HT presynaptic transporter and 5HT receptors in adulthood [3]. Notably, however, the primary effects were seen in the regions containing 5HT cell bodies (midbrain, brainstem) and in one area with 5HT terminals, the hippocampus, whereas the cerebral cortex and striatum were largely spared. This pattern is consistent with damage to developing nerve terminals and consequent reactive sprouting [10,32,38] but the persistence of the upregulation of these static markers of 5HT synaptic integrity suggests that underlying functional abnormalities remain. In the current study, we found initial overall reductions in 5HT content, consistent with nerve terminal damage. This was accompanied by presynaptic 5HT hyperactivity, as monitored by 5HT turnover, throughout juvenile and adolescent stages of development, with the most notable and persistent changes occurring in the cerebral cortex and striatum, the very same regions that appeared to be spared the upregulation of 5HT receptors in our earlier study [3]. The most likely explanation of this pattern is that 5HT synapses are “miswired,” namely that, consequent to the initial damage, sprouting reestablishes innervation but to inappropriate loci within the affected regions, so that presynaptic hyperactivity occurs as compensation for defective synaptic function. Confirmation of this interpretation will require anatomical evaluations; prior work has established that developmental exposures to terbutaline or related β-adrenergic receptor agonists do indeed cause structural alterations consistent with miswiring [33,59] but our findings point to the need to supplement these with specific histological markers to identify the locations and potential misconnections of 5HT projections and their target cells containing 5HT receptors.
Much more is known about the impact of developmental exposure to chlorpyrifos on 5HT systems [1-5,56,69,70]. When this organophosphate is given either late in gestation or in the early neonatal period, with the latter corresponding to the stage at which we administered terbutaline in the present study, 5HT receptors are globally upregulated in adulthood [5], accompanied by elevated 5HT turnover indicative of presynaptic hyperactivity that emerges in juvenile to adolescent stages [2,70]. Despite these increases, 5HT-related behaviors are deficient, consistent with patterns seen in animal models of depression [1]. Thus, there is a striking resemblance between our findings for terbutaline and those for prenatal or early neonatal chlorpyrifos exposure. On a functional plane, then, our results for terbutaline are entirely consonant with the clinical findings of higher incidence of psychiatric disorders [54] and autism [17] in children exposed to β-agonists as a consequence of tocolytic therapy for preterm labor, and provide a mechanistic link between such exposures and adverse neurodevelopmental outcomes.
As seen here and in our earlier work [2,3,5], shifting chlorpyrifos exposure to PN11-14, a later neonatal stage, reduces the impact on 5HT systems. In our previous studies, upregulation of 5HT receptors was much less notable than for earlier chlorpyrifos exposure and 5HT turnover was unaffected in adulthood [2,3,5]. Here, we found a significant but transient elevation in 5HT turnover at the juvenile stage (PN21), consistent with a lessened susceptibility to chlorpyrifos as compared to exposure paradigms in earlier developmental periods; this receded by early adolescence (PN30). Accordingly, it appears that there is a critical period of highest susceptibility of developing 5HT systems to disruption by chlorpyrifos, centered around the immediate perinatal period, the same phase in which the sensitivity to adverse effects of terbutaline is maximal [59]. But why should these two otherwise dissimilar agents converge on the same types of outcomes? Both agents have a lasting effect on the development of cell signaling mechanisms involved in neural cell differentiation, most notably for the adenylyl cyclase/cyclic AMP transduction cascade that integrates multiple neuronal and hormonal inputs [64-66]. Another possibility is that both terbutaline and chlorpyrifos are capable of evoking oxidative stress [7,18,30,35,55,58,68]. Monoamine systems are among the most vulnerable to oxidative damage [11,26,51] and the immature brain is especially susceptible because of its high oxygen demand, unique membrane lipid composition [30], and lower reserves of protective enzymes and antioxidants [34]. The developing nervous system is also deficient in glia, the cells which serve as the primary scavengers of reactive oxidative species [76]. However, when we assessed lipid peroxidation after early neonatal exposure to terbutaline or chlorpyrifos, we found evidence for oxidative damage only with exposure in the second postnatal week [68]; since we assessed only lipid peroxidation, other types of oxidative damage may have gone undetected, so our results do not absolutely rule out oxidative stress as the common mechanism underlying the convergent effects of terbutaline and chlorpyrifos, and clearly, this issue should be pursued further. Indeed, the same genetic factors that influence the sensitivity to organophosphates like chlorpyrifos, also play a role in susceptibility to oxidative damage. Polymorphisms in paraoxonase, the enzyme that degrades chlorpyrifos and related pesticides, govern individual susceptibility to organophosphate toxicity [27] but the actual physiological role of this enzyme is in the detoxification of lipid peroxides [47], so that the same polymorphisms may then determine the neurodevelopmental outcomes after terbutaline use in preterm labor. Again, this is an area for future investigation that may then resolve both underlying mechanisms and the uncovering of a subpopulation with especial vulnerability to perinatal brain damage from a variety of agents. For example, one would expect to find the same relationship of paraoxonase polymorphisms to autism after developmental terbutaline exposure [17] as has been proposed for organophosphates [19,75].
Regardless of the cellular mechanisms that underlie the similar outcomes for 5HT systems after developmental exposure to terbutaline or chlorpyrifos, our results also point to potential interactions with coexposure to the two agents, a realistic exposure scenario given the high incidence of preterm labor and ubiquitous exposure to organophosphates. At the juvenile stage, animals receiving the combined treatment showed greater effects than with either agent alone, suggesting a worsening of the defects in net 5HT synaptic function. Since the PN11-14 chlorpyrifos regimen does cause significant lipid peroxidation indicative of oxidative stress [68], again it is conceivable that the prior treatment with terbutaline depletes antioxidant defenses, thus rendering developing 5HT systems more vulnerable to the subsequent chlorpyrifos exposure. Furthermore, given that the effects of chlorpyrifos on 5HT systems are already waning by the PN11-14 treatment period used in the present study, we would anticipate even greater interactions with terbutaline for earlier periods of chlorpyrifos exposure [1-5,56,69,70]. In any case, given the emergence of psychiatric disorders [54] and autism [17] in the offspring of women given β-agonist tocolytics, these findings raise the possibility that this therapeutic intervention produces a subpopulation with elevated susceptibility to common environmental toxicants. Recent work shows that even nonsymptomatic, routine exposures to organophosphates produce cognitive impairment in children [23,57] and our findings point to the need to identify factors that may increase the likelihood of these adverse outcomes.
In conclusion, in combination with our earlier work [3], the current results indicate that terbutaline treatment during the neurodevelopmental period corresponding to its use in preterm labor, produces abnormalities of 5HT function that emerge in juvenile and adolescent stages and that remain prominent into adulthood [3]. The outcomes distinctly resemble those seen with chlorpyrifos administered in the same early neonatal period [2,3,5,56,69,70], an exposure known to produce depression-like behavioral patterns [1]. In light of the elevated incidence of childhood psychiatric disorders [54] and autism [17] in the offspring of women who received β-agonist tocolytic therapy, the present findings provide a mechanistic underpinning for these relationships. Given that neurodevelopmental variability between brain regions is undoubtedly higher in humans than in rats, further neuroanatomical studies of the 5HT system in this model may help to clarify the findings in human disorders. Finally, the fact that sequential exposure to terbutaline and chlorpyrifos exacerbates the changes in 5HT function in juvenile rats, raises the possibility that drug therapy used in preterm labor contributes to a subpopulation of individuals who are especially vulnerable to common environmental neurotoxicants.
Acknowledgments
Research was supported by NIH ES10356. The authors thank Charlotte Tate for technical assistance. The authors have served as expert witnesses on behalf of governmental entities, corporations and/or individuals, but have no conflicts of interest.
Abbreviations
- 5HIAA
5-hydroxyindoleacetic acid
- 5HT
5-hydroxytryptamine (serotonin)
- ANOVA
analysis of variance
- βAR
β-adrenergic receptor
- PN
postnatal day
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Aldridge JE, Levin ED, Seidler FJ, Slotkin TA. Developmental exposure of rats to chlorpyrifos leads to behavioral alterations in adulthood, involving serotonergic mechanisms and resembling animal models of depression. Environ Health Perspect. 2005;113:527–531. doi: 10.1289/ehp.7867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aldridge JE, Meyer A, Seidler FJ, Slotkin TA. Alterations in central nervous system serotonergic and dopaminergic synaptic activity in adulthood after prenatal or neonatal chlorpyrifos exposure. Environ Health Perspect. 2005;113:1027–1031. doi: 10.1289/ehp.7968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aldridge JE, Meyer A, Seidler FJ, Slotkin TA. Developmental exposure to terbutaline and chlorpyrifos: pharmacotherapy of preterm labor and an environmental neurotoxicant converge on serotonergic systems in neonatal rat brain regions. Toxicol Appl Pharmacol. 2005;203:134–144. doi: 10.1016/j.taap.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Aldridge JE, Seidler FJ, Meyer A, Thillai I, Slotkin TA. Serotonergic systems targeted by developmental exposure to chlorpyrifos: effects during different critical periods. Environ Health Perspect. 2003;111:1736–1743. doi: 10.1289/ehp.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aldridge JE, Seidler FJ, Slotkin TA. Developmental exposure to chlorpyrifos elicits sex-selective alterations of serotonergic synaptic function in adulthood: critical periods and regional selectivity for effects on the serotonin transporter, receptor subtypes, and cell signaling. Environ Health Perspect. 2004;112:148–155. doi: 10.1289/ehp.6713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Auman JT, Seidler FJ, Tate CA, Slotkin TA. β-Adrenoceptor-mediated cell signaling in the neonatal heart and liver: responses to terbutaline. Am J Physiol. 2001;281:R1895–R1901. doi: 10.1152/ajpregu.2001.281.6.R1895. [DOI] [PubMed] [Google Scholar]
- 7.Bagchi D, Bagchi M, Hassoun EA, Stohs SJ. In vitro and in vivo generation of reactive oxygen species, DNA damage and lactate dehydrogenase leakage by selected pesticides. Toxicology. 1995;104:129–140. doi: 10.1016/0300-483x(95)03156-a. [DOI] [PubMed] [Google Scholar]
- 8.Barr DB, Allen R, Olsson AO, Bravo R, Caltabiano LM, Montesano A, Nguyen J, Udunka S, Walden D, Walker RD, Weerasekera G, Whitehead RD, Jr, Schober SE, Needham LL. Concentrations of selective metabolites of organophosphorus pesticides in the United States population. Environ Res. 2005;99:314–326. doi: 10.1016/j.envres.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 9.Berkowitz GS, Papiernik E. Epidemiology of preterm birth. Epidemiol Rev. 1993;15:414–443. doi: 10.1093/oxfordjournals.epirev.a036128. [DOI] [PubMed] [Google Scholar]
- 10.Blue ME, Molliver ME. 6-Hydroxydopamine induces serotonergic axon sprouting in cerebral cortex of newborn rat. Dev Brain Res. 1987;32:255–269. doi: 10.1016/0165-3806(87)90106-4. [DOI] [PubMed] [Google Scholar]
- 11.Blum D, Torch S, Lambeng N, Nissou M, Benabid AL, Sadoul R, Verna JM. Molecular pathways involved in the neurotoxicity of 6-OHDA, dopamine and MPTP: contribution to the apoptotic theory in Parkinson's disease. Prog Neurobiol. 2001;65:135–172. doi: 10.1016/s0301-0082(01)00003-x. [DOI] [PubMed] [Google Scholar]
- 12.Bouvier G, Seta N, Vigouroux-Villard A, Blanchard O, Momas I. Insecticide urinary metabolites in nonoccupationally exposed populations. J Toxicol Environ Health. 2005;8:485–512. doi: 10.1080/10937400591007284. [DOI] [PubMed] [Google Scholar]
- 13.Campbell CG, Seidler FJ, Slotkin TA. Chlorpyrifos interferes with cell development in rat brain regions. Brain Res Bull. 1997;43:179–189. doi: 10.1016/s0361-9230(96)00436-4. [DOI] [PubMed] [Google Scholar]
- 14.Casida JE, Quistad GB. Organophosphate toxicology: safety aspects of nonacetylcholinesterase secondary targets. Chem Res Toxicol. 2004;17:983–998. doi: 10.1021/tx0499259. [DOI] [PubMed] [Google Scholar]
- 15.Clancy B, Finlay BL, Darlington RB, Anand KJS. Extrapolating brain development from experimental species to humans. NeuroToxicology. 2007 doi: 10.1016/j.neuro.2007.01.014. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clark PM. Programming of the hypothalamo-pituitary-adrenal axis and the fetal origins of adult disease hypothesis. Eur J Pediatr. 1998;157:S7–S10. doi: 10.1007/pl00014289. [DOI] [PubMed] [Google Scholar]
- 17.Connors SL, Crowell DE, Eberhart CG, Copeland J, Newschaffer CJ, Spence SJ, Zimmerman AW. β2-Adrenergic receptor activation and genetic polymorphisms in autism: data from dizygotic twins. J Child Neurol. 2005;20:876–884. doi: 10.1177/08830738050200110401. [DOI] [PubMed] [Google Scholar]
- 18.Crumpton TL, Seidler FJ, Slotkin TA. Is oxidative stress involved in the developmental neurotoxicity of chlorpyrifos? Dev Brain Res. 2000;121:189–195. doi: 10.1016/s0165-3806(00)00045-6. [DOI] [PubMed] [Google Scholar]
- 19.D'Amelio M, Ricci I, Sacco R, Liu X, D'Agruma L, Muscarella LA, Guarnieri V, Militerni R, Bravaccio C, Elia M, Schneider C, Melmed R, Trillo S, Pascucci T, Puglisi-Allegra S, Reichelt KL, Macciardi F, Holden JJA, Persico AM. Paraoxonase gene variants are associated with autism in North America, but not in Italy: possible regional specificity in gene-environment interactions. Mol Psychiat. 2005;10:1006–1016. doi: 10.1038/sj.mp.4001714. [DOI] [PubMed] [Google Scholar]
- 20.Dam K, Garcia SJ, Seidler FJ, Slotkin TA. Neonatal chlorpyrifos exposure alters synaptic development and neuronal activity in cholinergic and catecholaminergic pathways. Dev Brain Res. 1999;116:9–20. doi: 10.1016/s0165-3806(99)00067-x. [DOI] [PubMed] [Google Scholar]
- 21.Dodic M, Peers A, Coghlan JP, May CN, Lumbers E, Yu ZY, Wintour EM. Altered cardiovascular haemodynamics and baroreceptor-heart rate reflex in adult sheep after prenatal exposure to dexamethasone. Clin Sci. 1999;97:103–109. [PubMed] [Google Scholar]
- 22.Dodic M, Samuel C, Moritz K, Wintour EM, Morgan J, Grigg L, Wong J. Impaired cardiac functional reserve and left ventricular hypertrophy in adult sheep after prenatal dexamethasone exposure. Circ Res. 2001;89:623–629. doi: 10.1161/hh1901.097086. [DOI] [PubMed] [Google Scholar]
- 23.Eskenazi B, Marks AR, Bradman A, Harley K, Barr DB, Johnson C, Morga N, Jewell NP. Organophosphate pesticide exposure and neurodevelopment in young Mexican-American children. Environ Health Perspect. 2007 doi: 10.1289/ehp.9828. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fenske RA, Black KG, Elkner KP, Lee C, Methner MM, Soto R. Potential exposure and health risks of infants following indoor residential pesticide applications. Am J Pub Health. 1990;80:689–693. doi: 10.2105/ajph.80.6.689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fenske RA, Lu CS, Barr D, Needham L. Children's exposure to chlorpyrifos and parathion in an agricultural community in central Washington State. Environ Health Perspect. 2002;110:549–553. doi: 10.1289/ehp.02110549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Frost DO, Cadet JL. Effects of methamphetamine-induced neurotoxicity on the development of neural circuitry: a hypothesis. Brain Res Rev. 2000;34:103–118. doi: 10.1016/s0165-0173(00)00042-4. [DOI] [PubMed] [Google Scholar]
- 27.Furlong CE, Cole TB, Jarvik GP, Pettan-Brewer C, Geiss GK, Richter RJ, Shih DM, Tward AD, Lusis AJ, Costa LG. Role of paraoxonase (PON1) status in pesticide sensitivity: genetic and temporal determinants. Neurotoxicology. 2005;26:651–659. doi: 10.1016/j.neuro.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 28.Garofolo MC, Seidler FJ, Cousins MM, Tate CA, Qiao D, Slotkin TA. Developmental toxicity of terbutaline: critical periods for sex-selective effects on macromolecules and DNA synthesis in rat brain, heart, and liver. Brain Res Bull. 2003;59:319–329. doi: 10.1016/s0361-9230(02)00925-5. [DOI] [PubMed] [Google Scholar]
- 29.Goldenberg RL. The management of preterm labor. Obstetrics and Gynecology. 2002;100:1020–1037. doi: 10.1016/s0029-7844(02)02212-3. [DOI] [PubMed] [Google Scholar]
- 30.Gupta RC. Brain regional heterogeneity and toxicological mechanisms of organophosphates and carbamates. Toxicol Mech Meth. 2004;14:103–143. doi: 10.1080/15376520490429175. [DOI] [PubMed] [Google Scholar]
- 31.Gurunathan S, Robson M, Freeman N, Buckley B, Roy A, Meyer R, Bukowski J, Lioy PJ. Accumulation of chlorpyrifos on residential surfaces and toys accessible to children. Environ Health Perspect. 1998;106:9–16. doi: 10.1289/ehp.981069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamon M, Nelson DL, Mallat M, Bourgoin S. Are 5-HT receptors involoved in the sprouting of serotonergic terminals following neonatal 5,7-dihydroxytryptamine treatment in the rat? Neurochem Int. 1981;3:69–79. doi: 10.1016/0197-0186(81)90051-6. [DOI] [PubMed] [Google Scholar]
- 33.Izdebska-Straszak G, Gubala E, Jedrzejowska-Szypulka H, Klencki M, Wiczkowski A, Jarzab B. Permanent effects of postnatal administration of β-adrenergic ligands on the volume of sexually dimorphic nucleus of the preoptic area (SDN-POA) in rats. Neuroendocrinol Lett. 2006;27:105–113. [PubMed] [Google Scholar]
- 34.James SJ, Slikker W, Melnyk S, New E, Pogribna M, Jernigan S. Thimerosal neurotoxicity is associated with glutathione depletion: protection with glutathione precursors. Neurotoxicology. 2005;26:1–8. doi: 10.1016/j.neuro.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 35.Jett DA, Navoa RV. In vitro and in vivo effects of chlorpyrifos on glutathione peroxidase and catalase in developing rat brain. Neurotoxicology. 2000;21:141–145. [PubMed] [Google Scholar]
- 36.Khan IY, Lakasing L, Poston L, Nicolaides KH. Fetal programming for adult disease: where next? J Maternal Fetal Neonatal Med. 2003;13:292–299. doi: 10.1080/jmf.13.5.292.299. [DOI] [PubMed] [Google Scholar]
- 37.Koch D, Lu C, Fisker-Andersen J, Jolley L, Fenske RA. Temporal association of children's pesticide exposure and agricultural spraying: report of a longitudinal biological monitoring study. Environ Health Perpsect. 2002;110:829–833. doi: 10.1289/ehp.02110829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kostrzewa RM, Garey RE. Sprouting of noradrenergic terminals in rat cerebellum following neonatal treatment with 6-hydroxydopa. Brain Res. 1977;124:385–391. doi: 10.1016/0006-8993(77)90898-8. [DOI] [PubMed] [Google Scholar]
- 39.Kudlacz EM, Navarro HA, Eylers JP, Dobbins SS, Lappi SE, Slotkin TA. Selective linkage of β-adrenergic receptors to functional responses in developing rat lung and liver: phosphatidic acid phosphatase, ornithine decarboxylase and lung liquid reabsorption. J Dev Physiol. 1989;12:129–134. [PubMed] [Google Scholar]
- 40.Kudlacz EM, Navarro HA, Slotkin TA. Phosphatidic acid phosphatase in neonatal rat lung: effects of prenatal dexamethasone or terbutaline treatment on basal activity and on responsiveness to beta adrenergic stimulation. J Pharmacol Exp Ther. 1989;250:236–240. [PubMed] [Google Scholar]
- 41.Lackland DT, Egan BM, Ferguson PL. Low birth weight as a risk factor for hypertension. J Clin Hypertension. 2003;5:133–136. doi: 10.1111/j.1524-6175.2003.01353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lam F, Bergauer NK, Jacques D, Coleman SK, Stanziano GJ. Clinical and cost-effectiveness of continuous subcutaneous terbutaline versus oral tocolytics for treatment of recurrent preterm labor in twin gestations. J Perinatol. 2001;21:444–450. doi: 10.1038/sj.jp.7210553. [DOI] [PubMed] [Google Scholar]
- 43.Lam F, Elliott J, Jones JS, Katz M, Knuppel RA, Morrison J, Newman R, Phelan J, Willcourt R. Clinical issues surrounding the use of terbutaline sulfate for preterm labor. Obstet Gynecol Survey. 1998;53:S85–S95. doi: 10.1097/00006254-199811002-00001. [DOI] [PubMed] [Google Scholar]
- 44.Lam F, Istwan NB, Jacques D, Coleman SK, Stanziano GJ. Managing perinatal outcomes: the clinical benefit and cost-effectiveness of pharmacologic treatment of recurrent preterm labor. Managed Care. 2003;12:39–46. [PubMed] [Google Scholar]
- 45.Landrigan PJ, Claudio L, Markowitz SB, Berkowitz GS, Brenner BL, Romero H, Wetmur JG, Matte TD, Gore AC, Godbold JH, Wolff MS. Pesticides and inner-city children: exposures, risks, and prevention. Environ Health Perspect. 1999;107 3:431–437. doi: 10.1289/ehp.99107s3431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lewis R, Mercer BM, Salama M, Walsh MA, Sibai BM. Oral terbutaline after parenteral tocolysis: a randomized, double-blind, placebo-controlled trial. Am J Obstet Gynecol. 1996;175:834–837. doi: 10.1016/s0002-9378(96)80008-1. [DOI] [PubMed] [Google Scholar]
- 47.Mackness B, Durrington PN, Mackness MI. The paraoxonase gene family and coronary heart disease. Curr Opin Lipidol. 2002;13 doi: 10.1097/00041433-200208000-00002. [DOI] [PubMed] [Google Scholar]
- 48.Meyer A, Seidler FJ, Aldridge JE, Slotkin TA. Developmental exposure to terbutaline alters cell signaling in mature rat brain regions and augments the effects of subsequent neonatal exposure to the organophosphorus insecticide, chlorpyrifos. Toxicol Appl Pharmacol. 2005;203:154–166. doi: 10.1016/j.taap.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 49.Navarro HA, Kudlacz EM, Slotkin TA. Control of adenylate cyclase activity in developing rat heart and liver: effects of prenatal exposure to terbutaline or dexamethasone. Biol Neonate. 1991;60:127–136. doi: 10.1159/000243398. [DOI] [PubMed] [Google Scholar]
- 50.Nyirenda MJ, Seckl JR. Intrauterine events and the programming of adulthood disease: the role of fetal glucocorticoid exposure. Int J Mol Med. 1998;2:607–614. doi: 10.3892/ijmm.2.5.607. [DOI] [PubMed] [Google Scholar]
- 51.Olanow CW, Arendash GW. Metals and free radicals in neurodegeneration. Curr Opin Neurol. 1994;7:548–558. doi: 10.1097/00019052-199412000-00013. [DOI] [PubMed] [Google Scholar]
- 52.Ostrea EM, Morales V, Ngoumgna E, Prescilla R, Tan E, Hernandez E, Ramirez GB, Cifra HL, Manlapaz ML. Prevalence of fetal exposure to environmental toxins as determined by meconium analysis. Neurotoxicology. 2002;23:329–339. doi: 10.1016/s0161-813x(02)00077-3. [DOI] [PubMed] [Google Scholar]
- 53.Phillips DIW. Endocrine programming and fetal origins of adult disease. Trends Endocrinol Metab. 2002;13:363. doi: 10.1016/s1043-2760(02)00696-3. [DOI] [PubMed] [Google Scholar]
- 54.Pitzer M, Schmidt MH, Esser G, Laucht M. Child development after maternal tocolysis with β-sympathomimetic drugs. Child Psychiat Hum Dev. 2001;31:165–182. doi: 10.1023/a:1026419720410. [DOI] [PubMed] [Google Scholar]
- 55.Qiao D, Seidler FJ, Slotkin TA. Oxidative mechanisms contributing to the developmental neurotoxicity of nicotine and chlorpyrifos. Toxicol Appl Pharmacol. 2005;206:17–26. doi: 10.1016/j.taap.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 56.Raines KW, Seidler FJ, Slotkin TA. Alterations in serotonin transporter expression in brain regions of rats exposed neonatally to chlorpyrifos. Dev Brain Res. 2001;130:65–72. doi: 10.1016/s0165-3806(01)00211-5. [DOI] [PubMed] [Google Scholar]
- 57.Rauh VA, Garfinkel R, Perera R, Andrews H, Hoepner L, Barr D, Whitehead D, Tang D, Whyatt RM. Impact of prenatal chlorpyrifos exposure on neurodevelopment in the first 3 years of life among inner-city children. Pediatrics. 2006;118:1845–1859. doi: 10.1542/peds.2006-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Remondino A, Kwon SH, Communal C, Pimentel DR, Sawyer DB, Singh K, Colucci WS. β-Adrenergic receptor-stimulated apoptosis in cardiac myocytes is mediated by reactive oxygen species/c-Jun NH2-terminal kinase-dependent activation of the mitochondrial pathway. Circ Res. 2003;92:136–138. doi: 10.1161/01.res.0000054624.03539.b4. [DOI] [PubMed] [Google Scholar]
- 59.Rhodes MC, Seidler FJ, Abdel-Rahman A, Tate CA, Nyska A, Rincavage HL, Slotkin TA. Terbutaline is a developmental neurotoxicant: effects on neuroproteins and morphology in cerebellum, hippocampus and somatosensory cortex. J Pharmacol Exp Ther. 2004;308:529–537. doi: 10.1124/jpet.103.060095. [DOI] [PubMed] [Google Scholar]
- 60.Rhodes MC, Seidler FJ, Qiao D, Tate CA, Cousins MM, Slotkin TA. Does pharmacotherapy for preterm labor sensitize the developing brain to environmental neurotoxicants? Cellular and synaptic effects of sequential exposure to terbutaline and chlorpyrifos in neonatal rats. Toxicol Appl Pharmacol. 2004;195:203–217. doi: 10.1016/j.taap.2003.11.008. [DOI] [PubMed] [Google Scholar]
- 61.Rodier PM. Structural-functional relationships in experimentally induced brain damage. Prog Brain Res. 1988;73:335–348. doi: 10.1016/S0079-6123(08)60514-2. [DOI] [PubMed] [Google Scholar]
- 62.Rust OA, Bofill JA, Arriola RM, Andrew ME, Morrison JC. The clinical efficacy of oral tocolytic therapy. Am J Obstet Gynecol. 1996;175:838–842. doi: 10.1016/s0002-9378(96)80009-3. [DOI] [PubMed] [Google Scholar]
- 63.Slikker W, Schwetz BA. Childhood obesity: the possible role of maternal smoking and impact on public health. J Child Health. 2003;1:29–40. [Google Scholar]
- 64.Slotkin TA. Developmental cholinotoxicants: nicotine and chlorpyrifos. Environ Health Perspect. 1999;107 1:71–80. doi: 10.1289/ehp.99107s171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Slotkin TA. Developmental neurotoxicity of organophosphates: a case study of chlorpyrifos. In: Gupta RC, editor. Toxicity of Organophosphate and Carbamate Pesticides. Elsevier Academic Press; San Diego: 2005. pp. 293–314. [Google Scholar]
- 66.Slotkin TA, Auman JT, Seidler FJ. Ontogenesis of β-adrenoceptor signaling: implications for perinatal physiology and for fetal effects of tocolytic drugs. J Pharmacol Exp Ther. 2003;306:1–7. doi: 10.1124/jpet.102.048421. [DOI] [PubMed] [Google Scholar]
- 67.Slotkin TA, Cousins MM, Tate CA, Seidler FJ. Persistent cholinergic presynaptic deficits after neonatal chlorpyrifos exposure. Brain Res. 2001;902:229–243. doi: 10.1016/s0006-8993(01)02387-3. [DOI] [PubMed] [Google Scholar]
- 68.Slotkin TA, Oliver CA, Seidler FJ. Critical periods for the role of oxidative stress in the developmental neurotoxicity of chlorpyrifos and terbutaline, alone or in combination. Dev Brain Res. 2005;157:172–180. doi: 10.1016/j.devbrainres.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 69.Slotkin TA, Seidler FJ. The alterations in CNS serotonergic mechanisms caused by neonatal chlorpyrifos exposure are permanent. Dev Brain Res. 2005;158:115–119. doi: 10.1016/j.devbrainres.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 70.Slotkin TA, Seidler FJ. Prenatal chlorpyrifos exposure elicits presynaptic serotonergic and dopaminergic hyperactivity at adolescence: critical periods for regional and sex-selective effects. Reprod Toxicol. 2007 doi: 10.1016/j.reprotox.2006.07.010. in press. [DOI] [PubMed] [Google Scholar]
- 71.Slotkin TA, Seidler FJ, Ali SF. Cellular determinants of reduced adaptability of the aging brain: neurotransmitter utilization and cell signaling responses after MDMA lesions. Brain Res. 2000;879:163–173. doi: 10.1016/s0006-8993(00)02767-0. [DOI] [PubMed] [Google Scholar]
- 72.Slotkin TA, Tate CA, Cousins MM, Seidler FJ. β-Adrenoceptor signaling in the developing brain: sensitization or desensitization in response to terbutaline. Dev Brain Res. 2001;131:113–125. doi: 10.1016/s0165-3806(01)00282-6. [DOI] [PubMed] [Google Scholar]
- 73.Slotkin TA, Tate CA, Cousins MM, Seidler FJ. Functional alterations in CNS catecholamine systems in adolescence and adulthood after neonatal chlorpyrifos exposure. Dev Brain Res. 2002;133:163–173. doi: 10.1016/s0165-3806(02)00284-5. [DOI] [PubMed] [Google Scholar]
- 74.Song X, Seidler FJ, Saleh JL, Zhang J, Padilla S, Slotkin TA. Cellular mechanisms for developmental toxicity of chlorpyrifos: targeting the adenylyl cyclase signaling cascade. Toxicol Appl Pharmacol. 1997;145:158–174. doi: 10.1006/taap.1997.8171. [DOI] [PubMed] [Google Scholar]
- 75.Szpir M. New thinking on neurodevelopment. Environ Health Perspect. 2006;114:A101–A107. doi: 10.1289/ehp.114-a100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tanaka J, Toku K, Zhang B, Isihara K, Sakanaka M, Maeda N. Astrocytes prevent neuronal death induced by reactive oxygen and nitrogen species. Glia. 1999;28:85–96. doi: 10.1002/(sici)1098-1136(199911)28:2<85::aid-glia1>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 77.Tegner K, Nilsson HT, Persson CGA, Persson K, Ryrfeldt Å. Elimination pathways of terbutaline. Eur J Resp Dis. 1984;65 134:93–100. [PubMed] [Google Scholar]
- 78.Thornton JG. Maintenance tocolysis. Br J Obstet Gynæacol. 2005;1:118–121. doi: 10.1111/j.1471-0528.2005.00599.x. [DOI] [PubMed] [Google Scholar]
- 79.Toschke AM, Koletzko B, Slikker W, Hermann M, von Kries R. Childhood obesity is associated with maternal smoking in pregnancy. Eur J Pediatr. 2002;161:445–448. doi: 10.1007/s00431-002-0983-z. [DOI] [PubMed] [Google Scholar]
- 80.U.S. Environmental Protection Agency. Administrator's Announcement. 2000 Available: http://www.epa.gov/pesticides/announcement6800.htm. [accessed update 13 October 2004.
- 81.U.S. Environmental Protection Agency. Chlorpyrifos: End-Use Products Cancellation Order. 2002 Available: http://www.epa.gov/fedrgstr/EPA-PEST/2002/January/Day-25/p1764.htm. [accessed 6 December 2004.
- 82.von Kries R, Toschke AM, Koletzko B, Slikker W., Jr Maternal smoking during pregnancy and childhood obesity. Am J Epidemiol. 2002;156:954–961. doi: 10.1093/aje/kwf128. [DOI] [PubMed] [Google Scholar]
- 83.Whitney KD, Seidler FJ, Slotkin TA. Developmental neurotoxicity of chlorpyrifos: cellular mechanisms. Toxicol Appl Pharmacol. 1995;134:53–62. doi: 10.1006/taap.1995.1168. [DOI] [PubMed] [Google Scholar]
- 84.Xu Z, Seidler FJ, Ali SF, Slikker W, Slotkin TA. Fetal and adolescent nicotine administration: effects on CNS serotonergic systems. Brain Res. 2001;914:166–178. doi: 10.1016/s0006-8993(01)02797-4. [DOI] [PubMed] [Google Scholar]
- 85.Yanai J. Neurobehavioral Teratology. Elsevier; Amsterdam: 1984. [Google Scholar]
- 86.Yanai J, Vatury O, Slotkin TA. Cell signaling as a target and underlying mechanism for neurobehavioral teratogenesis. Ann N Y Acad Sci. 2002;965:473–478. doi: 10.1111/j.1749-6632.2002.tb04188.x. [DOI] [PubMed] [Google Scholar]


