Abstract
In 1991, Frostell and colleagues reported that breathing low concentrations of nitric oxide (NO) decreased pulmonary artery pressure (PAP) in awake lambs with experimental pulmonary arterial hypertension (PAH) [1]. Subsequently, efforts of multiple research groups studying animals and patients led to approval of inhaled NO by the US Food and Drug Administration in 1999 and the European Medicine Evaluation Agency and European Commission in 2001. Inhaled NO is currently indicated for the treatment of term and near-term neonates with hypoxemia and PAH. Since regulatory approval, several studies have suggested that NO inhalation can prevent chronic lung disease in premature infants. In addition, unanticipated systemic effects of inhaled NO may lead to treatments for a variety of disorders including ischemia-reperfusion injury.
This review summarizes the pharmacology and physiological effects of breathing NO. The application of inhaled NO to hypoxemic neonates with PAH is discussed including recent studies exploring the use of inhaled NO to prevent bronchopulmonary dysplasia in premature infants. This review also highlights the application of inhaled NO to treat adults with cardiopulmonary disease, strategies to augment the efficacy of inhaled NO, and potential applications of the systemic effects of the gas.
Inhaled NO: mechanisms of action, methods of administration, and safety issues
Nitric oxide/cGMP signal transduction system
NO is a free radical with a short half-life in biological fluids. NO produced endogenously or provided by exogenous sources reacts with a variety of cellular targets in the lung leading to a plethora of responses including vasorelaxation and bronchodilation, as well as inhibition of mitochondrial respiration, inhibition of platelet and leukocyte activation, and modulation of vascular smooth muscle cell proliferation [reviewed in reference 2]. NO is synthesized from L-arginine and oxygen by a family of three NO synthases (NOSs), all of which are expressed in the lung. Endothelial NOS (eNOS or NOS3) appears to play an important role in maintaining low pulmonary vascular tone [3].
NO stimulates soluble guanylate cyclase (sGC) to synthesize cGMP that, in turn, activates cGMP-dependent protein kinase (PKG) leading to vascular relaxation. The actions of cGMP are limited via its catabolism by phosphodiesterases (PDEs). NO can elicit effects via cGMP-independent mechanisms including interactions with heme-containing molecules (in addition to sGC) and proteins containing reactive thiol groups [reviewed in reference 4]. NO also interacts with superoxide radical (O2−), thereby limiting NO bioavailability and resulting in the formation of the potent oxidant peroxynitrite (ONOO−). In the presence of oxygenated hemoglobin (Hb), NO is rapidly metabolized to nitrate with formation of met-Hb. Met-Hb in erythrocytes is rapidly reduced to ferrous-Hb by met-Hb reductase.
Inhaled NO is a selective pulmonary vasodilator
Drugs that generate NO, such as nitroglycerin and sodium nitroprusside, have long been used to reduce blood pressure and treat angina pectoris. Systemically-administered NO-donor compounds can dilate the pulmonary vasculature, but their efficacy is limited by systemic hypotension. In lung injury, these drugs can impair matching of ventilation with perfusion leading to systemic arterial hypoxemia. Frostell and colleagues reasoned that NO administered via inhalation would relax the pulmonary vasculature but, upon reaching the bloodstream, would be scavenged by Hb thereby preventing systemic vasodilation [1]. These investigators studied awake lambs with pulmonary vasoconstriction induced by intravenous administration of U46619, a thromboxane mimetic, or induced by breathing low oxygen concentrations. Inhalation of NO gas dose-dependently decreased PAP and pulmonary vascular resistance (PVR) in sheep with PAH but not in sheep with normal pulmonary vascular tone. The pulmonary vasodilator effects of breathing NO were readily reversible upon discontinuation of the gas. Breathing NO up to 80 parts per million (ppm) did not alter systemic blood pressure, a finding which has been consistently observed over a wide range of species, including man.
Methods of administration and safety issues
NO is a colorless and odorless gas that readily reacts with oxygen to form the pulmonary irritant NO2. As a result, NO gas must be stored in nitrogen or another inert gas and administered to the patient in a manner designed to minimize the duration of exposure to oxygen. Because the pulmonary vasodilator effects of NO are transient when the gas is discontinued, it must be administered continuously with careful monitoring of NO and NO2 concentrations. Commercially-available equipment permits the safe delivery of NO gas in intubated and spontaneously-breathing patients. Met-Hb levels should be followed, particularly in patients breathing 80 ppm or more NO. Methemoglobinemia typically responds to a reduction of the inhaled concentration or to discontinuation of NO therapy. Abrupt discontinuation of NO inhalation can result in “rebound” pulmonary hypertension leading to a decreased cardiac output and systemic hypotension. The risk of rebound pulmonary hypertension can be minimized by gradually weaning the concentration of inhaled NO gas delivered to the patient. In the presence of severe left ventricular (LV) failure, administration of NO to relieve pulmonary vasoconstriction may augment LV filling and raise the pulmonary capillary wedge pressure (PCWP). Thus, caution should be advised when patients with severe LV failure are treated with inhaled NO.
Perinatal and pediatric uses of inhaled NO
NO is an important regulatory molecule in the perinatal lung
NO plays a significant role in the normal reduction of PVR at birth during the transition from a fetal to a newborn circulatory system [5]. Many forms of newborn lung disease are associated with endothelial cell injury and decreased NO/cGMP signaling. Studies in newborns suggest that premature constriction of the ductus arteriosus, septic mediators, and meconium aspiration cause pulmonary endothelial cell injury. For example, in utero ductus arteriosus ligation in the fetal lamb causes flow-induced endothelial cell dysfunction and reduced NOS3 expression [6], as well as decreased NO-dependent vasorelaxation [7]. Moreover, several congenital cardiac lesions, such as atrial and ventricular septal defects, atrioventricular canals, persistent ductus arteriosus, and transposition of the great vessels without a ventricular septal defect, can produce endothelial cell injury and decrease NO-mediated pulmonary vasorelaxation in the infant lung [8].
Inhaled NO is a selective pulmonary vasodilator in newborn animals
Multiple studies have demonstrated that inhaled NO decreases PVR in newborn animals with PAH. For example, in fetal and newborn lambs with normal pulmonary arteries and acute pulmonary vasoconstriction, inhaled NO rapidly and selectively decreased PVR [reviewed in reference 9]. In newborn lambs with pulmonary vascular disease and PAH induced by premature ligation of the ductus arteriosus, inhaled NO caused dose-dependent vasodilatation and improved systemic oxygenation levels and survival rates [10, 11].
Inhaled NO can attenuate pulmonary disease in newborn animals
In newborn rats, sustained hypoxia or exposure to endothelial toxins causes pulmonary artery muscularization and hypertension that is similar to what is observed in congenital heart disease patients with PAH. In these models, prolonged NO inhalation has been observed to attenuate pulmonary vascular remodeling and RV hypertrophy [12]. Moreover, in newborn rats with monocrotaline-induced endothelial injury, Roberts and colleagues reported that inhaled NO prevented abnormal pulmonary vascular remodeling even in the absence of pulmonary vasoconstriction [13].
Accumulating evidence suggests that inhaled NO can also enhance the development of the injured premature and newborn lung. Lung injury disrupts normal alveolar and pulmonary microvascular development in premature lambs and baboons, as well as newborn mice and rats [14]. In recent studies, prolonged NO inhalation has been reported to increase alveologenesis in chronically-ventilated premature baboons [15] and lambs [16]. Moreover, breathing NO augmented alveolar development in hypoxemic newborn NOS3-deficient mice [17, 18]. Taken together, these findings suggest that inhaled NO may play an important role in improving pulmonary alveolar and vascular maturation in the injured premature and newborn lung.
Inhaled NO increases systemic oxygenation in hypoxemic newborns with persistent pulmonary hypertension of the newborn
Pilot studies revealed that inhalation of NO increases systemic oxygenation in severely hypoxemic newborns with persistent pulmonary hypertension of the newborn (PPHN) [19, 20]. The ability of inhaled NO to increase systemic oxygen levels was confirmed in several multicenter randomized controlled studies of term and near-term babies with hypoxemia and PAH [21–23]. In one study, systemic oxygenation was observed to increase in nearly half of patients with severe PPHN who received inhaled NO [21]. Importantly, the magnitude of the increase in oxygen levels in babies treated with inhaled NO was directly proportional to the degree of hypoxia before NO inhalation, and, in 75% of the infants breathing NO, the increased systemic oxygenation was maintained over time. In another study of newborns with PPHN, the optimal concentration of inhaled NO level was investigated [24]: although the maximum increase in systemic oxygenation was observed in babies breathing 80 ppm NO, as little as 5 to 20 ppm NO increased the arterial oxygen levels above those measured in babies not breathing NO. Several studies reported that, although inhaled NO did not reduce mortality in these patients, it did decrease the need for extracorporeal membrane oxygenation (ECMO), an invasive procedure that requires systemic heparinization and is not available in all intensive care nurseries [21–23].
Inhaled NO in bronchopulmonary dysplasia
Bronchopulmonary dysplasia (BPD) is a chronic lung disease of prematurely born infants that results from the inhibition or disruption of normal pulmonary alveolar and microvascular development, in part, as a result of oxygen- and ventilator-induced lung injury [25]. A number of prospective randomized controlled masked trials have now evaluated the efficacy of inhaled NO in preventing BPD and/or death in premature infants [for a comprehensive review see reference 26]. Although an early study observed that inhaled NO did not decrease the incidence of BPD [27], a subsequent single center trial involving 207 premature infants conducted by Schrieber and colleagues suggested that inhaled NO decreases the incidence of BPD and death in prematurely born infants [28]. These latter observations were supported by data from a subsequent multicenter trial in which inhaled NO was noted to decrease the rate of BPD and death in premature infants with a birth weight > 1,000 g [29]. In a more recent study of 793 newborns who were 34 weeks of gestational age or less, Kinsella and colleagues reported that inhaled NO decreased the incidence of BPD in the subset of 129 infants with a birth weight of at least 1,000 g [30]. Moreover, in a multicenter study of 582 premature infants with a birth weight 1,250 g, in which inhaled NO was commenced 7 to 21 days after birth, Ballard and coworkers reported that NO inhalation improved survival without BPD [31]. Although these results are encouraging when considered together, additional studies need to be performed before the precise role of inhaled NO in preventing BPD can be defined.
Safety of inhaled NO in the newborn
Although studies in newborns and infants suggest that inhaled NO is safe [32, 33], the long-term pulmonary and extrapulmonary effects of inhaled NO are unknown. In particular, the effects of chronic NO inhalation on normal pulmonary cell proliferation and differentiation, as well as on alveolar and microvascular development, are unknown.
NO can increase cGMP levels in platelets and inhibit their function [reviewed in reference 34]. The potential of inhaled NO to alter platelet function [35] is of particular concern for immature infants since abnormal hemostatic activity increases the incidence or evolution of intraventricular hemorrhage (IVH) and associated neurologic injury. In one study, breathing NO was associated with increased mortality and rates of IVH in infants weighing 1000 g or less [29]. Nevertheless, data from several other studies suggest that inhaled NO exposure does not increase the incidence or severity of IVH in premature babies [27, 28, 31, 36, 37]. In fact, in one study of premature infants, inhaled NO was reported to decrease the frequency of IVH and its sequelae [30].
It is uncertain whether or not an improvement in pulmonary disease in some premature babies who have breathed NO is associated with better neurologic development. In a European multicenter trial of nearly 100 premature babies, inhaled NO was not observed to affect the incidence of death or severe disability at one year, when corrected for postnatal age [38]. On the other hand, a more rigorous evaluation of older premature babies treated in another trial suggests that inhaled NO improves neurologic outcomes [39].
Uses of inhaled NO in adults with cardiopulmonary diseases
Inhaled NO and pulmonary vasoreactivity testing in the cardiac catheterization laboratory
In patients with pulmonary arterial hypertension (PAH), demonstration of a positive response to vasodilator agents correlates with a favorable long-term clinical outcome [40]. While a number of vasodilators, including intravenous prostacyclin and calcium channel blockers, have been utilized for diagnostic testing in PAH patients, systemic administration of these agents can produce severe hypotension, increased intrapulmonary right-to-left shunting, and death. In contrast, a number of studies have indicated that inhaled NO can be safely and effectively used to assess the capacity for pulmonary vasodilation in pediatric [41] and adult patients [42] with PAH, without causing systemic hypotension. The ability of inhaled NO to decrease PAP or PVR can be used to predict the subsequent response to therapy with oral vasodilators, such as nifedipine [42], and a better mid-term survival in adult patients with PAH due to congenital heart disease [43].
Treatment of pulmonary hypertension in patients undergoing cardiac surgery
Pulmonary hypertension leading to acute right heart failure can complicate the management of patients during and after cardiac surgery employing cardiopulmonary bypass (CPB). A number of uncontrolled studies have demonstrated that inhalation of NO (20–40 ppm) effectively decreases PAP when coronary artery bypass grafting (CABG) or surgery for valvular heart disease is complicated by perioperative pulmonary hypertension [44].
Pulmonary hypertension in cardiac transplant recipients is a major cause of right heart failure and early death. Inhaled NO has been reported to selectively reduce RV afterload and enhance RV stroke work after cardiac transplantation [45]. A positive pulmonary vasodilator response to NO inhalation has been used as a criterion to select patients for cardiac transplantation [46].
Although there is physiologic data and clinical experience suggesting that NO inhalation may be beneficial in patients during or after cardiac surgery complicated by PAH and RV dysfunction, whether or not inhaled NO can improve clinically-important outcomes remains to be determined by randomized controlled trials.
Treatment of right heart failure after insertion of left ventricular assist device
RV dysfunction occurs in 20–50% of patients after insertion of a LV assist device (LVAD) [47]. The ability of the RV to pump sufficient blood to the LVAD is critically dependent on RV contractility and afterload, both of which may be adversely effected by the primary disease process and/or cardiopulmonary bypass. RV failure after LVAD placement is associated with increased morbidity and mortality and often requires placement of a RV assist device (RVAD) [48]. In a randomized, double-blind trial, Argenziano and coworkers demonstrated that inhaled NO decreased PAP and increased LVAD flow in LVAD recipients with PAH [49]. Many clinicians employ a therapeutic trial of inhaled NO to improve RV function prior to the consideration of RVAD implantation, but a formal clinical trial demonstrating that NO inhalation can obviate the need for RVAD placement remains to be completed.
Treatment of cardiogenic shock due to RV myocardial infarction
RV injury and dysfunction may complicate inferior myocardial infarction (MI) due to right coronary artery occlusion. If coronary revascularization is delayed, cardiogenic shock may develop despite preserved LV function and is associated with a high mortality rate. In a small series of patients with cardiogenic shock due to RVMI, Inglessis and colleagues reported that breathing NO (80 ppm) acutely decreased PAP and improved cardiac index by 24%, likely by unloading the RV [50]. In RVMI patients with a patent foramen ovale, treatment with inhaled NO markedly reduced the right-to-left shunt and improved systemic oxygenation. Further studies are required to learn if NO inhalation will decrease the morbidity and mortality associated with cardiogenic shock due to RVMI.
Treatment of pulmonary ischemia-reperfusion injury
Ischemia-reperfusion (I-R) injury is one of the major causes of early graft failure after lung transplantation. Adhesion and sequestration of leukocytes by activated pulmonary endothelium is believed to be an important contributor to I-R injury. NO possesses anti-inflammatory properties, and inhaled NO has been shown to attenuate pulmonary I-R injury in preclinical studies [51]. While two uncontrolled clinical studies suggested that inhaled NO may be beneficial in patients who develop lung I-R injury [52, 53], a small randomized, placebo-controlled study demonstrated that inhaling NO (20 ppm) commencing 10 minutes after reperfusion did not affect the physiological or clinical outcome of patients after lung transplantation [54]. Additional studies will be required to define the therapeutic role of inhaled NO in patients who have developed I-R injury after lung transplantation.
Acute respiratory distress syndrome in adults
In clinical studies of patients with severe acute respiratory distress syndrome (ARDS), inhaled NO has been shown to produce selective pulmonary vasodilation and improve systemic oxygenation [55]. Although follow-up clinical studies, as well as a number of studies in animal models of acute lung injury, have confirmed a physiological benefit of inhaled NO therapy, subsequent randomized clinical trials reported disappointing outcome results. Inhaled NO therapy did not affect mortality rate, duration of mechanical ventilation, or the number of days alive and off mechanical ventilation in the two single-center pilot trials with small sample sizes (n=40 [56] and 30 [57]) and two larger multi-center randomized trials (n=177 [58] and 286 [59]). Whether or not inhaled NO can improve clinical outcome in a subgroup of severely hypoxemic ARDS patients has not yet been conclusively resolved.
Chronic obstructive pulmonary disease (COPD)
Severe COPD is frequently complicated by pulmonary hypertension and hypoxemia. Systemic hypoxemia in COPD is typically caused by mismatching of ventilation and perfusion rather than the intra- and extra-pulmonary right-to-left shunting seen in ARDS and PPHN. While inhaled NO is a more potent pulmonary vasodilator than oxygen in COPD patients, breathing inhaled NO admixed with air can vasodilate the poorly ventilated lung regions reducing systemic oxygenation [60]. However, when NO gas is breathed in combination with modest oxygen enrichment (e.g. 1 L/min), PaO2 is increased to a greater extent than when breathing supplemental oxygen alone [61]. Along these lines, a randomized controlled trial of COPD patients demonstrated that the combined use of supplemental oxygen and inhaled NO for a period of 3 months via a portable inspiratory pulsing device decreased PAP and PVR and increased cardiac output without decreasing systemic oxygenation, whereas pulmonary hemodynamics did not change in patients on supplemental oxygen alone [62]. Further studies are needed to determine the effect of long-term ambulatory breathing of pulsed NO and oxygen on the quality of life and mortality rate of patients with severe COPD.
Experimental methods to improve upon the efficacy of inhaled NO
In an important fraction of adult and pediatric patients, NO inhalation fails to reduce the PVR and/or improve oxygenation. Moreover, because the pulmonary vasodilator effects of inhaled NO are transient after NO is discontinued, continuous therapy is required. In addition, the utilization of the lowest effective concentration may decrease the risk of met-Hb, augment the matching of ventilation and perfusion, and, minimize any theoretical risk of lung toxicity. Based upon an understanding of the NO/cGMP signal transduction system, several research groups have developed strategies to augment and/or prolong the efficacy of inhaled NO. For example, inhibition of the cGMP-specific PDE, PDE5, with zaprinast prolonged the pulmonary vasodilator effects of NO inhalation in awake adult lambs with U46619-induced PAH [63] and augmented the effects of inhaled NO in newborn lambs with PAH induced by prenatal ligation of the ductus arteriosus [64]. Steinhorn and colleagues reported that administration of superoxide dismutase can augment the efficacy of inhaled NO in newborn lambs with PAH likely by decreasing superoxide-mediated scavenging of NO [65]. In studies of awake adult lambs with U46619-induced PAH, Evgenov and colleagues reported that agents that sensitize sGC to NO can augment and prolong the pulmonary vasodilator effects of inhaled NO [66].
These observations in animal models have been extended to patients with pulmonary hypertension. For example, sildenafil augmented and prolonged the pulmonary vasodilator effects of inhaled NO in patients with pulmonary hypertension due to pulmonary vascular disease [67] or congestive heart failure [68]. Importantly, sildenafil was also found to attenuate the pulmonary vascular rebound associated with discontinuing NO inhalation [69] and may facilitate weaning from NO inhalation in patients with cardiovascular instability.
“Unexpected” extrapulmonary effects of breathing NO
Because breathing NO can decrease PVR without altering systemic blood pressure, it was initially proposed that the actions of inhaled NO were limited to the lungs. It was hypothesized that any NO reaching the bloodstream would be rapidly scavenged by oxy-Hb leading to the production of nitrate and met-Hb [1]. However, as early as 1993, there was a report that NO inhalation could inhibit platelet function [35]. Subsequently, studies describing a wide range of systemic effects of breathing NO were published, as summarized recently by McMahon and Doctor [70] and highlighted in the Table. In this section, the ability of NO inhalation to decrease I-R injury will be reviewed, and circumstances where breathing NO can modulate systemic vascular tone will be discussed. In addition, potential mechanisms by which inhaled NO may exert its systemic effects will be outlined.
Table.
Biological effects of breathing NO with selected physiological impacts and potential therapeutic applications. Representative references are included.
| Biological Effect | Impact | Therapeutic Application | References |
|---|---|---|---|
| pulmonary vasodilation | reduction in pulmonary artery pressure | pulmonary arterial hypertension | [1] |
| decrease in extrapulmonary right to left shunting and improved systemic oxygenation | pulmonary hypertension of the newborn and congenital heart disease | [19, 86, 87] | |
| decrease in right ventricular afterload | RV failure after LVAD placement | [49] | |
| RVMI complicated by cardiogenic shock | [50] | ||
| RV failure after cardiac surgery | [44] | ||
| improved matching of lung ventilation and perfusion | increased systemic oxygenation | acute lung injury/pneumonia | [55, 58, 59] |
| chronic pulmonary disease | [62] | ||
| pulmonary vascular and alveolar development | decreased vascular SMC hyperplasia and increased alveolarization in the injured developing lung | prevent or ameliorate pulmonary hypertension in infants with congenital heart disease and chronic lung disease in premature infants | [12, 15, 16, 18] |
| systemic effects of breathing NO | platelet inhibition | decreased thrombosis after coronary thrombolysis (dogs) | [35, 88] |
| leukocyte inhibition | improved mesenteric blood flow after ischemia and reperfusion (cats) | [71] | |
| decreased infarct size in cardiac ischemia-reperfusion injury | [74, 75] | ||
| oxidization of extra-erythrocytic hemoglobin | decreased hemolysis-induced vasoconstriction and renal dysfunction (dogs) | [78] |
Inhaled NO and ischemia-reperfusion injury
Observations that NO can form stable, biologically-active adducts with protein thiol groups led Fox-Robichaud and colleagues to examine whether or not inhaled NO could be used to deliver NO to peripheral tissues [71]. Studying post-capillary venules of cats subjected to intestinal ischemia and reperfusion, these investigators observed that breathing 80 ppm NO, but not 20 ppm, prevented the reduction of blood flow, the increase in leukocyte activation (rolling, adherence, and emigration), and vascular leakage. The investigators also observed that breathing NO prevented the arteriolar constriction and neutrophil activation induced by the administration of L-NAME, a NOS inhibitor. However, in contrast to NO-donor compounds, inhaled NO did not prevent the increase in mucosal permeability induced by intestinal I-R suggesting that the systemic effects of breathing NO may be limited to the vascular compartment [72].
Based upon these observations, Geury and colleagues evaluated the ability of breathing NO to preserve cardiac function after myocardial I-R injury [73]. Hearts from rats that had breathed air with or without NO (10 ppm) for 4 hours were isolated and perfused. Coronary perfusion was stopped for 30 minutes followed by reperfusion for 40 minutes. The investigators observed that pretreatment with inhaled NO led to improved systolic and diastolic function after I-R. These findings were extended by Hataishi and colleagues, who examined the ability of breathing NO to decrease MI size in mice subjected to 30, 60, and 120 minutes of cardiac ischemia followed by reperfusion [74]. They observed that breathing NO for 20 minutes before and 24 hours after reperfusion decreased MI size and improved systolic and diastolic function. Breathing 40 and 80 ppm NO decreased myocardial I-R injury equally, but 20 ppm was ineffective. Breathing NO decreased cardiac neutrophil accumulation, and leukocyte depletion prevented the beneficial effects of NO on MI size. Studying a porcine model of cardiac I-R injury, Janssens and colleagues recently reported that breathing NO (80 ppm) decreased MI size, as well as microvascular obstruction [75]. Taken together with the observations of Fox-Robichaud et al. [71], these results suggest that when inhaled concentrations of NO required to elicit systemic effects (in vivo) are greater than those required to elicit pulmonary vascular effects. Based upon these considerations, a randomized clinical trial has been initiated to test whether or not breathing NO can decrease MI size in patients who undergo percutaneous coronary revascularization for an acute coronary artery occlusion.
Inhaled NO and systemic vascular tone
Whether or not breathing NO can modulate systemic vascular tone remains controversial. Clinically significant systemic hypotension has not been observed in adults and babies with a wide variety of disorders breathing NO concentrations up to 80 ppm. However, inhalation of 80 ppm NO has been reported to attenuate the ability of L-NAME to increase blood pressure in cats [72]. Cannon and colleagues reported that breathing 80 ppm NO increased forearm blood flow in healthy subjects treated with an intrabrachial artery infusion of L-NMMA (another NOS inhibitor) to block endogenous NO synthesis [76]. In contrast, Hataishi and colleagues observed that breathing NO did not decrease systemic vascular resistance (SVR) in L-NAME-treated mice or in NOS3-deficient mice [77].
Inhaled NO has also been reported to decrease SVR in a canine model of endothelial dysfunction associated with hemolysis [78]: Minneci et al. induced hemolysis by intravenous administration of free water and observed that the presence of Hb in the plasma induced hypertension and renal insufficiency, likely via the scavenging of endothelium-derived NO. Breathing 80 ppm NO attenuated the hemolysis-induced hypertension and renal dysfunction by oxidizing nearly all of the plasma Hb to met-Hb (85–90%) and preventing the scavenging of endogenous NO. The authors proposed that NO inhalation may attenuate the hypertension, vascular disease, and prothrombotic state characteristic of diseases associated with hemolysis, such as sickle cell anemia.
How does inhaled NO exerts its systemic effects?
With the exception of the case of hemolysis, the mechanisms by which NO, once inhaled, is able to induce responses in the periphery remain incompletely understood. One hypothesis is that during NO inhalation, blood cells are exposed to high concentrations of NO in the lung prior to scavenging of the gas by red blood cells, and NO-exposed blood cells are responsible for the systemic effects of inhaled NO (Figure). For example, exposure of neutrophils to NO in the pulmonary circulation may inhibit their activation at the site of intestinal or cardiac I-R injury. However, this hypothesis does not fully account for the dilator effects of breathing NO seen in some vascular beds. Moreover, Fox-Robichaud et al. [71] reported that the ability of blood leukocytes to bind to immobilized platelet monomers ex vivo was not altered when the blood was obtained from cats breathing NO.
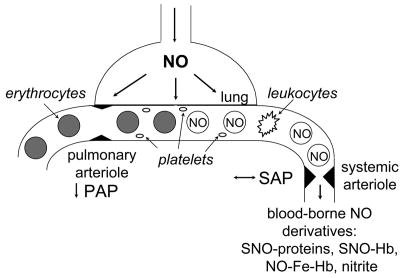
Figure.
Inhaled NO is a selective pulmonary vasodilator with actions on the systemic vasculature. A schematic of an alveolar-capillary unit is presented highlighting the ability of inhaled NO to dilate pulmonary arterioles and reduce pulmonary artery pressure (PAP). Although inhaled NO does not dilate systemic arterioles or alter systemic arterial pressure (SAP) under normal conditions, inhaled NO does have systemic effects which are described in the text and may be mediated by circulating cells exposed to NO in the lungs and blood-borne NO derivatives: SNO-proteins—S-nitroso proteins including SNO-albumin; SNO-Hb—S-nitroso-hemoglobin (nitrosylated on Cys93 of the β chain); NO-Fe-Hb—nytrosyl-hemoglobin; and nitrite.
An alternate hypothesis is that some of the inhaled NO escapes scavenging by Hb and is carried in a stable, bioavailable form to the periphery (Figure). Loscalzo and colleagues proposed that endogenously-synthesized NO reacts with low and high molecular weight thiols in blood, including serum albumin [79, 80]. These S-nitrosothiols can deliver NO to tissues distant from the site of their synthesis. Stamler and colleagues observed that NO reacts with the thiol group on Cys93 of the Hb β chain: the S-nitrosylation of Hb (SNO-Hb) is dependent on the tissue oxygen tension, enabling erythrocytes to load NO on Hb in the high oxygen environment of the lungs and release low molecular weight S-nitrosothiols in the relatively hypoxic periphery [reviewed in reference 70]. NO can also be oxidized in the blood to nitrite, which regenerates NO in the presence of nitrite reductases including deoxy-Hb [reviewed in reference 81]. Erythrocyte SNO-Hb and nitrite have both been implicated as potential mediators of the systemic vasodilation induced by tissue hypoxia. NO may also react with other plasma species including which are capable of regenerating NO including N-nitrosamines, iron-nitrosyls, and nitrated lipids.
Even before it was appreciated that breathing NO could selectively dilate the pulmonary vasculature, several research groups examined the metabolic fate of inhaled NO. In 1975, Oda and colleagues reported NO inhalation led to the formation of nitrosyl-Hb in the blood of mice, rats, and rabbits [82]. In 1987, Yoshida and Kasama reported that breathing 15NO (145 ppm for 123 minutes) led to the formation of nitrosyl-Hb, nitrite, and nitrate [83]. More recently, Cannon et al. found that, in healthy adults breathing 80 ppm NO for 1 hour, blood nitrosyl-Hb concentrations increased markedly, whereas plasma nitrite increased only modestly [76]. Arterial concentrations of both products were greater than venous levels suggesting that both were consumed in peripheral tissues. Cannon and colleagues did not detect an increase in plasma S-nitrosothiols or SNO-Hb after breathing NO for 1 hour. In contrast, Ng and colleagues reported that, in cats, breathing 80 ppm NO increased plasma levels of S-nitrosoalbumin and nitrite, both of which were further increased in the setting of intestinal ischemia and reperfusion [84]. Arterial levels of S-nitrosoalbumin were higher than venous levels whereas the converse was true for nitrite levels suggesting that S-nitrosoalbumin was consumed in the periphery and nitrite was generated.
The potential importance of nitrite as a mediator of inhaled NO’s impact on I-R injury was highlighted in recent studies of Duranski and colleagues [85]: they observed that small increases in blood nitrite levels (such as might be attained during NO inhalation) were sufficient to decrease I-R injury in the liver and heart.
Controversy as to the identity of the circulating NO-derived products whose levels increase during NO inhalation may be attributable to differences in the methods used to detect these products. Nonetheless, there appears to be a consensus developing that bioavailable NO-derivatives contribute to the effects of breathing NO on the periphery. However, many questions remain including: Do NO-derived products accumulate in tissues during NO inhalation? Are cGMP-dependent or cGMP-independent mechanisms responsible for the systemic effects of inhaled NO?
Summary
In the past fifteen years, extensive basic science and clinical research has enabled the safe and rapid translation of inhaled NO’s selective pulmonary vasodilator effects to the treatment of critically-ill newborns and adults. Randomized clinical trials have demonstrated the utility of inhaled NO in term and near-term babies with PAH and hypoxia and have suggested that breathing NO may prevent chronic lung disease (BPD) in premature infants. Future advances in gas delivery technology and strategies to augment the efficacy of inhaled NO may enable application of this therapy to chronic disease such as PAH or COPD. Additional research and, in particular, randomized clinical trials are required to translate preclinical studies indicating that inhaled NO may have beneficial effects in the systemic circulation including treatment of ischemia-reperfusion injury and the vascular sequelae of hemolytic anemia.
Acknowledgments
Research in the authors’ laboratories is supported by grants from the National Heart Lung and Blood Institute. The authors are grateful to the many past and present post-doctoral fellows and research technicians who have contributed to this research.
Footnotes
Disclosures: All of the authors have served on the Scientific Advisory Board of INOTherapeutics LLC, a company that sells NO gas for inhalation. Dr. Bloch’s research program has been supported in part by a sponsored research agreement between INOTherapeutics LLC and the Massachusetts General Hospital. The Massachusetts General Hospital owns patents covering the use of inhaled NO that it has licensed to INOTherapeutics LLC, and Dr. Zapol receives a portion of the royalties.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Frostell C, Fratacci MD, Wain JC, Jones R, Zapol WM. Inhaled nitric oxide. A selective pulmonary vasodilator reversing hypoxic pulmonary vasoconstriction. Circulation. 1991;83:2038–47. doi: 10.1161/01.cir.83.6.2038. [DOI] [PubMed] [Google Scholar]
- 2.Feil R, Lohmann SM, de Jonge H, Walter U, Hofmann F. Cyclic GMP-dependent protein kinases and the cardiovascular system: insights from genetically modified mice. Circ Res. 2003;93:907–16. doi: 10.1161/01.RES.0000100390.68771.CC. [DOI] [PubMed] [Google Scholar]
- 3.Fagan KA, Tyler RC, Sato K, Fouty BW, Morris KG, Jr, Huang PL, et al. Relative contributions of endothelial, inducible, and neuronal NOS to tone in the murine pulmonary circulation. Am J Physiol. 1999;277:L472–8. doi: 10.1152/ajplung.1999.277.3.L472. [DOI] [PubMed] [Google Scholar]
- 4.Hare JM, Stamler JS. NO/redox disequilibrium in the failing heart and cardiovascular system. J Clin Invest. 2005;115:509–17. doi: 10.1172/JCI200524459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abman SH, Chatfield BA, Hall SL, McMurtry IF. Role of endothelium-derived relaxing factor during transition of pulmonary circulation at birth. Am J Physiol. 1990;259:H1921–H7. doi: 10.1152/ajpheart.1990.259.6.H1921. [DOI] [PubMed] [Google Scholar]
- 6.Shaul PW, Yuhanna IS, German Z, Chen Z, Steinhorn RH, Morin FC., 3rd Pulmonary endothelial NO synthase gene expression is decreased in fetal lambs with pulmonary hypertension. Am J Physiol. 1997;272:L1005–12. doi: 10.1152/ajplung.1997.272.5.L1005. [DOI] [PubMed] [Google Scholar]
- 7.Steinhorn RH, Russell JA, Morin FC., III Disruption of cGMP production in pulmonary arteries isolated from fetal lambs with pulmonary hypertension. Am J Physiol. 1995;268:H1483–H9. doi: 10.1152/ajpheart.1995.268.4.H1483. [DOI] [PubMed] [Google Scholar]
- 8.Celermajer DS, Cullen S, Deanfield JE. Impairment of endothelium-dependent pulmonary artery relaxation in children with congenital heart disease and abnormal pulmonary hemodynamics. Circulation. 1993;87:440–6. doi: 10.1161/01.cir.87.2.440. [DOI] [PubMed] [Google Scholar]
- 9.Kinsella JP, Abman SH. Inhaled nitric oxide in the premature infant: animal models and clinical experience. Semin Perinatol. 1997;21:418–25. doi: 10.1016/s0146-0005(97)80007-9. [DOI] [PubMed] [Google Scholar]
- 10.Zayek M, Cleveland D, Morin FC., III Treatment of persistent pulmonary hypertension in the newborn lamb by inhaled nitric oxide. J Pediatr. 1993;122:743–50. doi: 10.1016/s0022-3476(06)80020-x. [DOI] [PubMed] [Google Scholar]
- 11.Zayek M, Wild L, Roberts JD, Jr, Morin FC., III Effect of nitric oxide on the survival rate and incidence of lung injury in newborn lambs with persistent pulmonary hypertension. J Pediatr. 1993;123:947–52. doi: 10.1016/s0022-3476(05)80393-2. [DOI] [PubMed] [Google Scholar]
- 12.Roberts JD, Jr, Roberts CT, Jones RC, Zapol WM, Bloch KD. Continuous nitric oxide inhalation reduces pulmonary arterial structural changes, right ventricular hypertrophy, and growth retardation in the hypoxic newborn rat. Circulation Research. 1995;76:215–22. doi: 10.1161/01.res.76.2.215. [DOI] [PubMed] [Google Scholar]
- 13.Roberts JD, Jr, Chiche JD, Weimann J, Steudel W, Zapol WM, Bloch KD. Nitric oxide inhalation decreases pulmonary artery remodeling in the injured lungs of rat pups. Circ Res. 2000;87:140–5. doi: 10.1161/01.res.87.2.140. [DOI] [PubMed] [Google Scholar]
- 14.Bourbon J, Boucherat O, Chailley-Heu B, Delacourt C. Control mechanisms of lung alveolar development and their disorders in bronchopulmonary dysplasia. Pediatr Res. 2005;57:38R–46R. doi: 10.1203/01.PDR.0000159630.35883.BE. [DOI] [PubMed] [Google Scholar]
- 15.McCurnin DC, Pierce RA, Chang LY, Gibson LL, Osborne-Lawrence S, Yoder BA, et al. Inhaled NO improves early pulmonary function and modifies lung growth and elastin deposition in a baboon model of neonatal chronic lung disease. Am J Physiol Lung Cell Mol Physiol. 2005;288:L450–9. doi: 10.1152/ajplung.00347.2004. [DOI] [PubMed] [Google Scholar]
- 16.Bland RD, Albertine KH, Carlton DP, Macritchie AJ. Inhaled nitric oxide effects on lung structure and function in chronically ventilated preterm lambs. Am J Respir Crit Care Med. 2005;172:899–906. doi: 10.1164/rccm.200503-384OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balasubramaniam V, Tang JR, Maxey A, Plopper CG, Abman SH. Mild hypoxia impairs alveolarization in the endothelial nitric oxide synthase-deficient mouse. Am J Physiol Lung Cell Mol Physiol. 2003;284:L964–71. doi: 10.1152/ajplung.00421.2002. [DOI] [PubMed] [Google Scholar]
- 18.Balasubramaniam V, Maxey AM, Morgan DB, Markham NE, Abman SH. Inhaled NO restores lung structure in eNOS-deficient mice recovering from neonatal hypoxia. Am J Physiol Lung Cell Mol Physiol. 2006;291:L119–27. doi: 10.1152/ajplung.00395.2005. [DOI] [PubMed] [Google Scholar]
- 19.Roberts JD, Jr, Polaner DM, Lang P, Zapol WM. Inhaled nitric oxide in persistent pulmonary hypertension of the newborn. Lancet. 1992;340:818–9. doi: 10.1016/0140-6736(92)92686-a. [DOI] [PubMed] [Google Scholar]
- 20.Kinsella JP, McQueston JA, Rosenberg AA, Abman SH. Hemodynamic effects of exogenous nitric oxide in ovine transitional pulmonary circulation. Am J Physiol. 1992;263:H875–80. doi: 10.1152/ajpheart.1992.263.3.H875. [DOI] [PubMed] [Google Scholar]
- 21.Roberts JD, Jr, Fineman JR, Morin FCr, Shaul PW, Rimar S, Schreiber MD, et al. Inhaled nitric oxide and persistent pulmonary hypertension of the newborn. N Engl J Med. 1997;336:605–10. doi: 10.1056/NEJM199702273360902. [DOI] [PubMed] [Google Scholar]
- 22.Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure. The Neonatal Inhaled Nitric Oxide Study Group. N Engl J Med. 1997;336:597–604. doi: 10.1056/NEJM199702273360901. [DOI] [PubMed] [Google Scholar]
- 23.Clark RH, Kueser TJ, Walker MW, Southgate WM, Huckaby JL, Perez JA, et al. Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. Clinical Inhaled Nitric Oxide Research Group. N Engl J Med. 2000;342:469–74. doi: 10.1056/NEJM200002173420704. [DOI] [PubMed] [Google Scholar]
- 24.Davidson D, Barefield ES, Kattwinkel J, Dudell G, Damask M, Straube R, et al. Inhaled nitric oxide for the early treatment of persistent pulmonary hypertension of the term newborn: A randomized, double-masked, placebo-controlled, dose-response, multicenter study. Pediatrics. 1998;101:325–34. doi: 10.1542/peds.101.3.325. [DOI] [PubMed] [Google Scholar]
- 25.Kinsella JP, Greenough A, Abman SH. Bronchopulmonary dysplasia. Lancet. 2006;367:1421–31. doi: 10.1016/S0140-6736(06)68615-7. [DOI] [PubMed] [Google Scholar]
- 26.Steinhorn R, Porta N. Use of inhaled nitric oxide in the preterm infant. Current Opinion in Pediatrics. 2007;19:137–41. doi: 10.1097/MOP.0b013e328082e2ed. [DOI] [PubMed] [Google Scholar]
- 27.Kinsella JP, Walsh WF, Bose CL, Gerstmann DR, Labella JJ, Sardesai S, et al. Inhaled nitric oxide in premature neonates with severe hypoxaemic respiratory failure: a randomised controlled trial. Lancet. 1999;354:1061–5. doi: 10.1016/s0140-6736(99)03558-8. [DOI] [PubMed] [Google Scholar]
- 28.Schreiber MD, Gin-Mestan K, Marks JD, Huo D, Lee G, Srisuparp P. Inhaled nitric oxide in premature infants with the respiratory distress syndrome. N Engl J Med. 2003;349:2099–107. doi: 10.1056/NEJMoa031154. [DOI] [PubMed] [Google Scholar]
- 29.Van Meurs KP, Wright LL, Ehrenkranz RA, Lemons JA, Ball MB, Poole WK, et al. Inhaled nitric oxide for premature infants with severe respiratory failure. N Engl J Med. 2005;353:13–22. doi: 10.1056/NEJMoa043927. [DOI] [PubMed] [Google Scholar]
- 30.Kinsella JP, Cutter GR, Walsh WF, Gerstmann DR, Bose CL, Hart C, et al. Early inhaled nitric oxide therapy in premature newborns with respiratory failure. N Engl J Med. 2006;355:354–64. doi: 10.1056/NEJMoa060442. [DOI] [PubMed] [Google Scholar]
- 31.Ballard RA, Truog WE, Cnaan A, Martin RJ, Ballard PL, Merrill JD, et al. Inhaled nitric oxide in preterm infants undergoing mechanical ventilation. N Engl J Med. 2006;355:343–53. doi: 10.1056/NEJMoa061088. [DOI] [PubMed] [Google Scholar]
- 32.Gross I. Recent advances in respiratory care of the term neonate. Ann N Y Acad Sci. 2000;900:151–8. doi: 10.1111/j.1749-6632.2000.tb06225.x. [DOI] [PubMed] [Google Scholar]
- 33.Kinsella JP. Inhaled nitric oxide therapy in premature newborns. Curr Opin Pediatr. 2006;18:107–11. doi: 10.1097/01.mop.0000193291.09894.6c. [DOI] [PubMed] [Google Scholar]
- 34.Schwarz UR, Walter U, Eigenthaler M. Taming platelets with cyclic nucleotides. Biochem Pharmacol. 2001;62:1153–61. doi: 10.1016/s0006-2952(01)00760-2. [DOI] [PubMed] [Google Scholar]
- 35.Hogman M, Frostell C, Arnberg H, Hedenstierna G. Bleeding time prolongation and NO inhalation. Lancet. 1993;341:1664–5. doi: 10.1016/0140-6736(93)90802-n. [DOI] [PubMed] [Google Scholar]
- 36.Early compared with delayed inhaled nitric oxide in moderately hypoxaemic neonates with respiratory failure: a randomised controlled trial. The Franco-Belgium Collaborative NO Trial Group. Lancet. 1999;354:1066–71. [PubMed] [Google Scholar]
- 37.Hascoet JM, Fresson J, Claris O, Hamon I, Lombet J, Liska A, et al. The safety and efficacy of nitric oxide therapy in premature infants. J Pediatr. 2005;146:318–23. doi: 10.1016/j.jpeds.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 38.Field D, Elbourne D, Truesdale A, Grieve R, Hardy P, Fenton AC, et al. Neonatal Ventilation With Inhaled Nitric Oxide Versus Ventilatory Support Without Inhaled Nitric Oxide for Preterm Infants With Severe Respiratory Failure: the INNOVO multicentre randomised controlled trial (ISRCTN 17821339) Pediatrics. 2005;115:926–36. doi: 10.1542/peds.2004-1209. [DOI] [PubMed] [Google Scholar]
- 39.Mestan KK, Marks JD, Hecox K, Huo D, Schreiber MD. Neurodevelopmental outcomes of premature infants treated with inhaled nitric oxide. N Engl J Med. 2005;353:23–32. doi: 10.1056/NEJMoa043514. [DOI] [PubMed] [Google Scholar]
- 40.Rich S, Kaufmann E, Levy PS. The effect of high doses of calcium-channel blockers on survival in primary pulmonary hypertension. N Engl J Med. 1992;327:76–81. doi: 10.1056/NEJM199207093270203. [DOI] [PubMed] [Google Scholar]
- 41.Atz AM, Adatia I, Lock JE, Wessel DL. Combined effects of nitric oxide and oxygen during acute pulmonary vasodilator testing. J Am Coll Cardiol. 1999;33:813–9. doi: 10.1016/s0735-1097(98)00668-8. [DOI] [PubMed] [Google Scholar]
- 42.Ricciardi MJ, Knight BP, Martinez FJ, Rubenfire M. Inhaled nitric oxide in primary pulmonary hypertension: a safe and effective agent for predicting response to nifedipine. J Am Coll Cardiol. 1998;32:1068–73. doi: 10.1016/s0735-1097(98)00361-1. [DOI] [PubMed] [Google Scholar]
- 43.Post MC, Janssens S, Van de Werf F, Budts W. Responsiveness to inhaled nitric oxide is a predictor for mid-term survival in adult patients with congenital heart defects and pulmonary arterial hypertension. Eur Heart J. 2004;25:1651–6. doi: 10.1016/j.ehj.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 44.Fullerton DA, Jones SD, Jaggers J, Piedalue F, Grover FL, McIntyre RC., Jr Effective control of pulmonary vascular resistance with inhaled nitric oxide after cardiac operation. J Thorac Cardiovasc Surg. 1996;111:753–62. doi: 10.1016/s0022-5223(96)70335-5. [DOI] [PubMed] [Google Scholar]
- 45.Ardehali A, Hughes K, Sadeghi A, Esmailian F, Marelli D, Moriguchi J, et al. Inhaled nitric oxide for pulmonary hypertension after heart transplantation. Transplantation. 2001;72:638–41. doi: 10.1097/00007890-200108270-00013. [DOI] [PubMed] [Google Scholar]
- 46.Fojon S, Fernandez-Gonzalez C, Sanchez-Andrade J, Lopez-Perez JM, Hermida LF, Rodriguez JA, et al. Inhaled nitric oxide through a noninvasive ventilation device to assess reversibility of pulmonary hypertension in selecting recipients for heart transplant. Transplant Proc. 2005;37:4028–30. doi: 10.1016/j.transproceed.2005.09.151. [DOI] [PubMed] [Google Scholar]
- 47.Frazier OH, Rose EA, Macmanus Q, Burton NA, Lefrak EA, Poirier VL, et al. Multicenter clinical evaluation of the HeartMate 1000 IP left ventricular assist device. Ann Thorac Surg. 1992;53:1080–90. doi: 10.1016/0003-4975(92)90393-i. [DOI] [PubMed] [Google Scholar]
- 48.Dang NC, Topkara VK, Mercando M, Kay J, Kruger KH, Aboodi MS, et al. Right heart failure after left ventricular assist device implantation in patients with chronic congestive heart failure. J Heart Lung Transplant. 2006;25:1–6. doi: 10.1016/j.healun.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 49.Argenziano M, Choudhri AF, Moazami N, Rose EA, Smith CR, Levin HR, et al. Randomized, double-blind trial of inhaled nitric oxide in LVAD recipients with pulmonary hypertension. Ann Thorac Surg. 1998;65:340–5. doi: 10.1016/s0003-4975(97)01307-6. [DOI] [PubMed] [Google Scholar]
- 50.Inglessis I, Shin JT, Lepore JJ, Palacios IF, Zapol WM, Bloch KD, et al. Hemodynamic effects of inhaled nitric oxide in right ventricular myocardial infarction and cardiogenic shock. J Am Coll Cardiol. 2004;44:793–8. doi: 10.1016/j.jacc.2004.05.047. [DOI] [PubMed] [Google Scholar]
- 51.Struber M, Harringer W, Ernst M, Morschheuser T, Hein M, Bund M, et al. Inhaled nitric oxide as a prophylactic treatment against reperfusion injury of the lung. Thorac Cardiovasc Surg. 1999;47:179–82. doi: 10.1055/s-2007-1013137. [DOI] [PubMed] [Google Scholar]
- 52.Date H, Triantafillou AN, Trulock EP, Pohl MS, Cooper JD, Patterson GA. Inhaled nitric oxide reduces human lung allograft dysfunction. J Thorac Cardiovasc Surg. 1996;111:913–9. doi: 10.1016/s0022-5223(96)70364-1. [DOI] [PubMed] [Google Scholar]
- 53.Ardehali A, Laks H, Levine M, Shpiner R, Ross D, Watson LD, et al. A prospective trial of inhaled nitric oxide in clinical lung transplantation. Transplantation. 2001;72:112–5. doi: 10.1097/00007890-200107150-00022. [DOI] [PubMed] [Google Scholar]
- 54.Meade MO, Granton JT, Matte-Martyn A, McRae K, Weaver B, Cripps P, et al. A randomized trial of inhaled nitric oxide to prevent ischemia-reperfusion injury after lung transplantation. Am J Respir Crit Care Med. 2003;167:1483–9. doi: 10.1164/rccm.2203034. [DOI] [PubMed] [Google Scholar]
- 55.Rossaint R, Falke KJ, Lopez F, Slama K, Pison U, Zapol WM. Inhaled nitric oxide for the adult respiratory distress syndrome. N Engl J Med. 1993;328:399–405. doi: 10.1056/NEJM199302113280605. [DOI] [PubMed] [Google Scholar]
- 56.Michael JR, Barton RG, Saffle JR, Mone M, Markewitz BA, Hillier K, et al. Inhaled nitric oxide versus conventional therapy: effect on oxygenation in ARDS. Am J Respir Crit Care Med. 1998;157:1372–80. doi: 10.1164/ajrccm.157.5.96-10089. [DOI] [PubMed] [Google Scholar]
- 57.Troncy E, Collet JP, Shapiro S, Guimond JG, Blair L, Ducruet T, et al. Inhaled nitric oxide in acute respiratory distress syndrome: a pilot randomized controlled study. Am J Respir Crit Care Med. 1998;157:1483–8. doi: 10.1164/ajrccm.157.5.9707090. [DOI] [PubMed] [Google Scholar]
- 58.Dellinger RP, Zimmerman JL, Taylor RW, Straube RC. Placebo and inhaled nitric oxide mortality the same in ARDS clinical trial. Crit Care Med. 1998;26:619. doi: 10.1097/00003246-199803000-00043. [DOI] [PubMed] [Google Scholar]
- 59.Lundin S, Mang H, Smithies M, Stenqvist O, Frostell C. Inhalation of nitric oxide in acute lung injury: results of a European multicentre study. The European Study Group of Inhaled Nitric Oxide. Intensive Care Med. 1999;25:911–9. doi: 10.1007/s001340050982. [DOI] [PubMed] [Google Scholar]
- 60.Barbera JA, Roger N, Roca J, Rovira I, Higenbottam TW, Rodriguez-Roisin R. Worsening of pulmonary gas exchange with nitric oxide inhalation in chronic obstructive pulmonary disease. Lancet. 1996;347:436–40. doi: 10.1016/s0140-6736(96)90011-2. [DOI] [PubMed] [Google Scholar]
- 61.Yoshida M, Taguchi O, Gabazza EC, Kobayashi T, Yamakami T, Kobayashi H, et al. Combined inhalation of nitric oxide and oxygen in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 1997;155:526–9. doi: 10.1164/ajrccm.155.2.9032189. [DOI] [PubMed] [Google Scholar]
- 62.Vonbank K, Ziesche R, Higenbottam TW, Stiebellehner L, Petkov V, Schenk P, et al. Controlled prospective randomised trial on the effects on pulmonary haemodynamics of the ambulatory long term use of nitric oxide and oxygen in patients with severe COPD. Thorax. 2003;58:289–93. doi: 10.1136/thorax.58.4.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ichinose F, Adrie C, Hurford WE, Zapol WM. Prolonged pulmonary vasodilator action of inhaled nitric oxide by Zaprinast in awake lambs. J Appl Physiol. 1995;78:1288–95. doi: 10.1152/jappl.1995.78.4.1288. [DOI] [PubMed] [Google Scholar]
- 64.Thusu KG, Morin FC, 3rd, Russell JA, Steinhorn RH. The cGMP phosphodiesterase inhibitor zaprinast enhances the effect of nitric oxide. Am J Respir Crit Care Med. 1995;152:1605–10. doi: 10.1164/ajrccm.152.5.7582302. [DOI] [PubMed] [Google Scholar]
- 65.Steinhorn RH, Albert G, Swartz DD, Russell JA, Levine CR, Davis JM. Recombinant human superoxide dismutase enhances the effect of inhaled nitric oxide in persistent pulmonary hypertension. Am J Respir Crit Care Med. 2001;164:834–9. doi: 10.1164/ajrccm.164.5.2010104. [DOI] [PubMed] [Google Scholar]
- 66.Evgenov OV, Ichinose F, Evgenov NV, Gnoth MJ, Falkowski GE, Chang Y, et al. Soluble guanylate cyclase activator reverses acute pulmonary hypertension and augments the pulmonary vasodilator response to inhaled nitric oxide in awake lambs. Circulation. 2004;110:2253–9. doi: 10.1161/01.CIR.0000144469.01521.8A. [DOI] [PubMed] [Google Scholar]
- 67.Lepore JJ, Maroo A, Pereira NL, Ginns LC, Dec GW, Zapol WM, et al. Effect of sildenafil on the acute pulmonary vasodilator response to inhaled nitric oxide in adults with primary pulmonary hypertension. Am J Cardiol. 2002;90:677–80. doi: 10.1016/s0002-9149(02)02586-9. [DOI] [PubMed] [Google Scholar]
- 68.Lepore JJ, Maroo A, Bigatello LM, Dec GW, Zapol WM, Bloch KD, et al. Hemodynamic effects of sildenafil in patients with congestive heart failure and pulmonary hypertension: combined administration with inhaled nitric oxide. Chest. 2005;127:1647–53. doi: 10.1378/chest.127.5.1647. [DOI] [PubMed] [Google Scholar]
- 69.Namachivayam P, Theilen U, Butt WW, Cooper SM, Penny DJ, Shekerdemian LS. Sildenafil prevents rebound pulmonary hypertension after withdrawal of nitric oxide in children. Am J Respir Crit Care Med. 2006;174:1042–7. doi: 10.1164/rccm.200605-694OC. [DOI] [PubMed] [Google Scholar]
- 70.McMahon TJ, Doctor A. Extrapulmonary effects of inhaled nitric oxide: role of reversible S-nitrosylation of erythrocytic hemoglobin. Proc Am Thorac Soc. 2006;3:153–60. doi: 10.1513/pats.200507-066BG. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fox-Robichaud A, Payne D, Hasan SU, Ostrovsky L, Fairhead T, Reinhardt P, et al. Inhaled NO as a viable antiadhesive therapy for ischemia/reperfusion injury of distal microvascular beds. J Clin Invest. 1998;101:2497–505. doi: 10.1172/JCI2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kubes P, Payne D, Grisham MB, Jourd-Heuil D, Fox-Robichaud A. Inhaled NO impacts vascular but not extravascular compartments in postischemic peripheral organs. Am J Physiol. 1999;277:H676–82. doi: 10.1152/ajpheart.1999.277.2.H676. [DOI] [PubMed] [Google Scholar]
- 73.Guery B, Neviere R, Viget N, Foucher C, Fialdes P, Wattel F, et al. Inhaled NO preadministration modulates local and remote ischemia-reperfusion organ injury in a rat model. J Appl Physiol. 1999;87:47–53. doi: 10.1152/jappl.1999.87.1.47. [DOI] [PubMed] [Google Scholar]
- 74.Hataishi R, Rodrigues AC, Neilan TG, Morgan JG, Buys E, Shiva S, et al. Inhaled nitric oxide decreases infarction size and improves left ventricular function in a murine model of myocardial ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2006;291:H379–84. doi: 10.1152/ajpheart.01172.2005. [DOI] [PubMed] [Google Scholar]
- 75.Liu X, Huang Y, Santos J, Swinnen M, Pellens M, Gillijns H, et al. Nitric oxide inhalation decreases myocardial injury in a porcine model of cardiac ischemia and reperfusion. Circulation. 2005;112:A375. Abstract. [Google Scholar]
- 76.Cannon RO, 3rd, Schechter AN, Panza JA, Ognibene FP, Pease-Fye ME, Waclawiw MA, et al. Effects of inhaled nitric oxide on regional blood flow are consistent with intravascular nitric oxide delivery. J Clin Invest. 2001;108:279–87. doi: 10.1172/JCI12761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hataishi R, Zapol WM, Bloch KD, Ichinose F. Inhaled nitric oxide does not reduce systemic vascular resistance in mice. Am J Physiol Heart Circ Physiol. 2006;290:H1826–9. doi: 10.1152/ajpheart.00938.2005. [DOI] [PubMed] [Google Scholar]
- 78.Minneci PC, Deans KJ, Zhi H, Yuen PS, Star RA, Banks SM, et al. Hemolysis-associated endothelial dysfunction mediated by accelerated NO inactivation by decompartmentalized oxyhemoglobin. J Clin Invest. 2005;115:3409–17. doi: 10.1172/JCI25040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stamler JS, Jaraki O, Osborne J, Simon DI, Keaney J, Vita J, et al. Nitric oxide circulates in mammalian plasma primarily as an S-nitroso adduct of serum albumin. Proc Natl Acad Sci U S A. 1992;89:7674–7. doi: 10.1073/pnas.89.16.7674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scharfstein JS, Keaney JF, Jr, Slivka A, Welch GN, Vita JA, Stamler JS, et al. In vivo transfer of nitric oxide between a plasma protein-bound reservoir and low molecular weight thiols. J Clin Invest. 1994;94:1432–9. doi: 10.1172/JCI117480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gladwin MT, Raat NJ, Shiva S, Dezfulian C, Hogg N, Kim-Shapiro DB, et al. Nitrite as a vascular endocrine nitric oxide reservoir that contributes to hypoxic signaling, cytoprotection, and vasodilation. Am J Physiol Heart Circ Physiol. 2006;291:H2026–35. doi: 10.1152/ajpheart.00407.2006. [DOI] [PubMed] [Google Scholar]
- 82.Oda H, Kusumoto S, Nakajima T. Nitrosyl-hemoglobin formation in the blood of animals exposed to nitric oxide. Arch Environ Health. 1975;30:453–6. doi: 10.1080/00039896.1975.10666749. [DOI] [PubMed] [Google Scholar]
- 83.Yoshida K, Kasama K. Biotransformation of nitric oxide. Environ Health Perspect. 1987;73:201–5. doi: 10.1289/ehp.8773201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ng ES, Jourd’heuil D, McCord JM, Hernandez D, Yasui M, Knight D, et al. Enhanced S-nitroso-albumin formation from inhaled NO during ischemia/reperfusion. Circ Res. 2004;94:559–65. doi: 10.1161/01.RES.0000117771.63140.D6. [DOI] [PubMed] [Google Scholar]
- 85.Duranski MR, Greer JJ, Dejam A, Jaganmohan S, Hogg N, Langston W, et al. Cytoprotective effects of nitrite during in vivo ischemia-reperfusion of the heart and liver. J Clin Invest. 2005;115:1232–40. doi: 10.1172/JCI22493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kinsella JP, Neish SR, Shaffer E, Abman SH. Low-dose inhalational nitric oxide in persistent pulmonary hypertension of the newborn. Lancet. 1992;340:819–20. doi: 10.1016/0140-6736(92)92687-b. [DOI] [PubMed] [Google Scholar]
- 87.Roberts JD, Jr, Lang P, Bigatello LM, Vlahakes GJ, Zapol WM. Inhaled nitric oxide in congenital heart disease. Circulation. 1993;87:447–53. doi: 10.1161/01.cir.87.2.447. [DOI] [PubMed] [Google Scholar]
- 88.Adrie C, Bloch KD, Moreno PR, Hurford WE, Guerrero JL, Holt R, et al. Inhaled nitric oxide increases coronary artery patency after thrombolysis. Circulation. 1996;94:1919–26. doi: 10.1161/01.cir.94.8.1919. [DOI] [PubMed] [Google Scholar]