Abstract
The aryl hydrocarbon receptor (AhR) is expressed in estrogen receptor (ER)-positive ZR-75 breast cancer cells. Treatment with 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) induces CYP1A1 protein and mRNA levels and also activates inhibitory AhR-Erα crosstalk associated with hormone-induced reporter gene expression. In ZR-75 cells grown under hypoxia, induction of these AhR-mediated responses by TCDD was significantly inhibited. This was not accompanied by decreased nuclear AhR levels or decreased interaction of the AhR complex with the CYP1A1 gene promoter as determined in a chromatin immunoprecipitation assay. Hypoxia-induced loss of Ah-responsiveness was not associated with induction of hypoxia-inducible factor-1α or other factors that sequester the AhR nuclear translocation (Arnt) protein, and overexpression of Arnt under hypoxia did not restore Ah-responsiveness. The p65 subunit of NFκB which inhibits AhR-mediated transactivation was not induced by hypoxia and was primarily cytosolic in ZR-75 cells grown under hypoxic and normoxic conditions. In ZR-75 cells maintained under hypoxic conditions for 24 hr, BRCA1 (an enhancer of AhR-mediated transactivation in breast cancer cells) was significantly decreased and this contributed to loss of Ah-responsiveness. In cells grown under hypoxia for 6 hr, BRCA1 was not decreased, but induction of CYP1A1 by TCDD was significantly decreased. Cotreatment of ZR-75 cells with TCDD plus the protein synthesis inhibitor cycloheximide for 6 hr enhanced CYP1A1 expression in cells grown under hypoxia and normoxia. These results suggest that hypoxia rapidly induces protein(s) that inhibit Ah-responsiveness and these may be similar to constitutively expressed inhibitors of Ah-responsiveness (under normoxia) that are also inhibited by cycloheximide.
Keywords: hypoxia, Ah receptor, BRCA1, CYP1A1 inhibition
INTRODUCTION
The aryl hydrocarbon receptor (AhR) is a ligand-activated transcription factor that is a member of the basic helix-loop-helix family of nuclear transcription factors (Swanson et al., 1993; Wilson et al., 1998). The AhR is primarily cytosolic and addition of ligand results in formation of a nuclear AhR complex containing both the AhR and the AhR nuclear translocator (Arnt) protein (Reyes et al., 1992). The AhR complex interacts with dioxin responsive elements (DREs) in Ah-responsive gene promoters and this activates recruitment of other nuclear coregulatory proteins and the pre-initiation complex resulting in induction of gene expression (Reyes, Reisz-Porszasz, and Hankinson, 1992; Swanson and Bradfield, 1993; Whitlock, Jr., 1999; Wilson and Safe, 1998). The AhR was initially identified as the intracellular receptor for 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and structurally related halogenated aromatic pollutants (Poland et al., 1982; Poland et al., 1976). However, the AhR binds structurally diverse synthetic compounds, combustion by-products, endogenous biochemicals, and chemoprotective phytochemicals (Denison et al., 1998; Denison et al., 2003).
TCDD induces a broad spectrum of AhR-mediated toxic and biochemical responses in laboratory animal models, and induction of many of the toxic responses are species-, sex- and age-dependent. For example, TCDD causes a severe chloracne in human, monkeys, rabbits and some hairless strains of mice but not in most other species. The reasons for these differences in TCDD-induced toxicities are not yet fully understood. TCDD and related compounds also inhibit 17β-estradiol (E2)-induced responses in rat mammary tumors, the rodent uterus, and human breast and endometrial cancer cells (Safe et al., 2003). Research in this laboratory has focused on the mechanisms of inhibitory AhR-estrogen receptor (ER) crosstalk and the development of selective AhR modulators (SAhRMs) for treatment of breast cancer (McDougal et al., 2001; Safe et al., 2001). The mechanisms of AhR-mediated inhibition of E2-induced responses are complex and due to several pathways which may be gene specific (Safe and Wormke, 2003). For example, inhibition of some E2-responsive genes involves direct interactions of the AhR complex with inhibitory dioxin response elements (iDREs) in the cathepsin D, heat shock protein 27, pS2 and c-fos gene promoters (Duan et al., 1999; Gillesby et al., 1997; Krishnan et al., 1995; Porter et al., 2001), whereas inhibition of other genes is iDRE-independent (Safe and Wormke, 2003). Moreover, the AhR ligand-dependent degradation of ERα may be due to the recently reported E3 ubiquitin ligase activity of the AhR (Ohtake et al., 2007).
Previous studies in this laboratory reported that TCDD induced proteasome-dependent degradation of ERα in breast cancer cells, and this AhR-dependent response may contribute to the observed antiestrogenic effects (Wormke et al., 2003). Hypoxia also induces proteasome-dependent degradation of ERα resulting in decreased E2-responsiveness (Stoner et al., 2002); however, we also observed that inhibitory AhR-ERα crosstalk was significantly decreased in breast cancer cells grown under hypoxic conditions (Stoner, 2002), and this is consistent with other reports showing that hypoxia decreases Ah-responsiveness (Chan et al., 1999; Gradin et al., 1996; Kim et al., 2000; Pollenz et al., 1999; Prasch et al., 2004). However, the mechanisms of hypoxia-dependent loss of Ah-responsiveness are unclear. In this study, we show that hypoxia decreases AhR-mediated transactivation in ZR-75 breast cancer cells, and this was not due to decreased expression of AhR or Arnt or their interactions with the CYP1A1 promoter. In addition, decreased expression of induced CYP1A1 under hypoxia was not due to activation of proteasomes, induction of HIF-1α, limiting levels of Arnt, or inhibition by the p65 subunit of NFκB. ZR-75 cells maintained under hypoxia for 24 hr decreased BRCA1 protein, an AhR enhancer, and this could contribute to loss of Ah-responsiveness. BRCA1 expression is not decreased in ZR-75 cells grown under hypoxia for only 6 hr, whereas loss of Ah-responsiveness is observed at this time point. However, cotreatment of breast cancer cells with TCDD and cycloheximide under normoxic or hypoxic conditions for 6 hr enhanced Ah-responsiveness, suggesting that decreased AhR-dependent transactivation after 6 hr under hypoxia may be due to rapid induction of inhibitory factors.
MATERIALS AND METHODS
Cells, Chemicals, Biochemicals and Plasmids
ZR-75 cells were purchased from American Type Culture Collection (ATCC, Manassas, VA) and were cultured in RPMI 1620 medium with phenol red (Sigma Chemical Co., St. Louis, MO) supplemented with 10% fetal bovine serum (JRH Biosciences, Lenexa, KS; or Atlanta Biologicals, Inc., Norcross, GA), 1.5 g/l sodium bicarbonate, 2.38 g/l HEPES, 4.5 g/l dextrose, and 0.11 g/l sodium pyruvate. Cells cultured under normoxic conditions were maintained in 37°C incubators under humidified 5% carbon dioxide:95% air. For hypoxia experiments, cells were cultured in a modular incubator flushed with a gas mixture containing 94% nitrogen, 5% carbon dioxide, and 1% oxygen. Dimethyl sulfoxide (DMSO), E2, CoCl2, cycloheximide, and phosphate buffered saline (PBS) were purchased from Sigma. MG132 was purchased from Calbiochem (EMD Biosciences, Inc., CA). TCDD was prepared in this laboratory and was shown to be >99% pure by gas chromatographic analysis. Reporter lysis buffer and Luciferase Assay Reagent were purchased from Promega Corp. (Madison, WI) and/or Boehringer Mannheim (Indianapolis, IN). β-Galactosidase activity was measured using Tropix Galacto-Light Plus Assay System (Tropix, Bedford, MA, USA). Instant Imager and Lumicount micro-well plate reader were purchased from Packard Instrument Co. (Downers Grove, IL). β-Actin antibody was obtained from Sigma and all other antibodies were from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Human ERα expression plasmid was originally provided by Dr. Ming-Jer Tsai (Baylor College of Medicine, Houston, TX) and was recloned into pcDNA3 in this laboratory. The pVEGF1 construct contains the -2018 to +50 VEGF promoter insert as previously described (Stoner et al., 2004) and was kindly provided by Drs. Gerhard Seimeister and Gunter Finkenzeller (Institute of Molecular Medicine, Tumor Biology Center, Freiburg, Germany). Dioxin response element (DRE)-luciferase (DRE-luc) reporter construct was constructed in this laboratory and contained three tandem consensus DREs. The expression vector pBM5/NEO-M1-1 containing the 2.6-kb human ARNT cDNA was a gift from Dr. O. Hankinson (University of California at Los Angeles).
Transient Transfection Assays
Cells were seeded in DME/F12 medium supplemented with 2.5% charcoal-stripped serum overnight in 12-well plates. Transfection was carried out using GeneJuice (Novagen, EMD Biosciences, Inc., CA) according to the manufacturer’s protocol. Cells were then treated for 24 hr under normoxic conditions in the presence or absence of 500 μM CoCl2 and harvested in 100 μL of cell lysis buffer (Promega Corp.). Luciferase activities in the various treatment groups were performed on 20 μL of cell extract using the luciferase assay system (Promega Corp.) in a luminometer (Packard Instrument Co., Meriden, CT), and results were normalized to β-galactosidase enzyme activity which was carried out on 20 μL of cell extract.
Northern Blot Analysis
Cells were seeded in DME/F12 medium supplemented with 2.5% charcoal-stripped serum overnight in 6-well plates. Cells were then treated with DMSO (D) or 10 nM TCDD for 6 hr in the presence or absence of 500 μM CoCl2 with or without cycloheximide (pretreatment for 45 min), and RNA was extracted using RNAzol B (Tel-Test) following the manufacturer’s protocol; 15-20 μg of RNA were separated on a 1.2% agarose/1 M formaldehyde gel, and transferred to a nylon membrane for 48 hr. RNA was crosslinked by exposing the membrane to UV light for 10 min, and the membrane was baked at 80°C for 2 hr. The membrane was then prehybridized for 18 hr at 60°C using ULTRAhyb-Hybridization Buffer (Ambion, Austin, TX) and hybridized in the same buffer for 24 hr with the [γ32P]-labeled CYP1A1 cDNA probe. The membrane was then washed in 2X SSC (0.3 M sodium chloride, 0.03 M sodium citrate, pH 7) and 0.5% sodium dodecyl sulfate (SDS) for 1 hr, and then washed in 2X SSC for 6 - 8 hr. β-Tubulin mRNA were used as an internal control.
Preparation and Analysis of Nuclear and Cytosolic Proteins
ZR-75 cells were seeded into 100 mm diameter plates in DME/F12 medium supplemented with 2.5% charcoal-stripped serum. Cells were treated with DMSO (D) or 10 nM TCDD (T) and grown in 21% O2 with or without 500 μM CoCl2 for varying times. Nuclear and cytosolic extracts were obtained using the NE-PER nuclear and cytoplasmic extraction kit (Pierce) according to the manufacturer’s instructions. Protein samples were boiled in 1X sample buffer [50 mM Tris-HCl, 2% SDS, 0.1% bromphenol blue, 175 mM β-mercaptoethenol) for 5 min, separated on 7.5 - 10% SDS-PAGE gel for 3 hr at 150 V, and Western blot analysis was performed.
Preparation of Whole Cell Extract and Western Blot Analysis
ZR-75 cells were seeded into six-well plates in DME/F12 medium supplemented with 2.5% charcoal-stripped serum. Cells were exposed to normoxia (21% O2), physiological hypoxia (1% O2), or chemically induced hypoxia (500 μM CoCl2) in the presence of DMSO or 10 nM TCDD for varying times. Cells were harvested with ice-cold lysis buffer (50 mM HEPES [pH 7.5], 500 mM NaCl, 10% [vol/vol] glycerol, 1% Triton X-100, 1.5 mM MgCl2, 1 mM EGTA) and supplemented with protease inhibitor cocktail (Sigma). Equal amounts of protein from each treatment group were boiled in 1X sample buffer for 5 min and separated on 7.5 - 10% gel, and then transferred to polyvinylidene difluoride membrane (BioRad) overnight at 30V. Membranes were blocked in Blotto [5% milk, Tris-buffered saline (10 mM Tris-HCl, pH 8.0, 150 mM NaCl), and 0.05% Tween 20] for 30 min and probed with primary antibodies for 2 - 4 hr. Membranes were washed for 30 min in 1X TBS-Tween, probed with peroxidase-conjugated secondary antibody for 1 - 2 hr, and then washed in 1X TBS-Tween for 30 min. Ten ml of HRP-substrate (Dupont- NEN, Boston, MA) was added and incubated for 1 min and visualized by autoradiography. Protein band intensities were scanned on a JX-330 scanner (SHARP Corp., Mahwah, NJ) using Adobe Photoshop 3.0 (Adobe Systems Inc., Palo Alto, CA).
Small Inhibitory RNA
Validated, non-targeting small inhibitory RNA (siRNA) (Silencer® Negative Control siRNA) (iscramble) was purchased from Ambion (Austin, TX) and siRNA for HIF-1α was purchase from Dharmacon Research (Lafayette, CO). Cells were cultured in six-well plates in DME/F12 medium supplemented with 2.5% fetal bovine serum. After 16–20 hr, siRNA duplexes were transfected using LipofectAMINE Plus Reagent (Invitrogen Life Technologies, Carlsbad, CA). siRNA duplex (0.75 μg) was transfected in each well to give a final concentration of 50 nM. Cells were harvested 48 hr after transfection by manual scraping and analyzed by Western blot.
Real-Time PCR
Cells were seeded in DME/F12 medium supplemented with 2.5% charcoal-stripped serum overnight. Cells were treated with DMSO or 10 nM TCDD for 1, 3, 6 and 12 hr, with or without 500 μM CoCl2. RNA was extracted using Qiagen RNeasy minikit (Qiagen, Valencia, CA, USA) following the manufacturer’s protocol and was reverse transcribed for cDNA synthesis using Superscript II reverse transcriptase (Invitrogen, Carlsbad, CA) according to the manufacturer’s protocol. The cDNA reaction mixture was then used to carry out PCR using SYBR Green PCR Master Mix from PE Applied Biosystems (Warrington, UK) on an ABI Prism 7700 Sequence Detection System (PE Applied Biosystems. The relative quantitation of samples was carried out using comparative CT method. TATA binding protein (TBP) was used for normalization. Primers used to perform PCR were purchased from Integrated DNA technologies (Coralville, IA) and are as follows:
AHRR (Fwd): 5′- GAC GGA TGT AAT GCA CCA GAA -3′
AHRR (Rev): 5′- AAA CTG CAT CGT CAT GAG TGG -3′
TBP (Fwd): 5′- TGC ACA GGA GCC AAG AGT GAA - 3′
TBP (Rev): 5′- CAC ATC ACA GCT CCC CAC CA - 3′
Chromatin Immunoprecipitation (ChIP) Assay
ZR-75 cells (1×107) were treated with DMSO (D) or 10 nM TCDD for 2 and 6 hr in the absence or presence of 500 μM CoCl2. Cells were then fixed with 1.5% formaldehyde for 5 min, and the cross-linking reaction was stopped by addition of 125 mM glycine for 5 min. After washing twice with phosphate buffered saline, cells were scraped and pelleted. Collected cells were hypotonically lysed (5 mM PIPES, pH 8.0, 85 mM KCl, 0.5% CA-630, plus protease inhibitors), and nuclei were collected by centrifugation, then dissolved in sonication buffer (1% SDS, 10 mM EDTA, 50 mM Tris-HCl, pH8.0) and sonicated to desired chromatin length (500 bp ~ 1 kb). The chromatin was precleared by addition of protein A-conjugated beads (PIERCE), and then incubated at 4°C for 1 hr with gentle agitation. The beads were pelleted, and the precleared chromatin supernatants were immunoprecipitated with antibodies (1 ~ 2 μg per ChIP) specific to IgG, Sp1, ERα, Pol II, TRAP220, AhR, and Arnt (all from Santa Cruz Biotechnology) at 4°C overnight. The protein-antibody complexes were collected by addition of 5 μL of protein A-conjugated beads at room temperature for 1 hr. The beads were extensively washed by low salt wash buffer (0.1% SDS, 1% Triton X-100, 150 mM NaCl, 2 mM EDTA, 20 mM Tris-HCl, pH 8.0), high salt buffer (500 mM NaCl instead), LiCl buffer (1% CA-630, 1% sodium deoxycholate, 250 mM or 500 mM LiCl, 1 mM EDTA, 100 mM Tris-HCl, pH 8.0), and TE buffer (0.1% Tween 20, 0.1% SDS, 2 mM EDTA, 50 mM Tris-HCl, pH 8.0). The protein-DNA crosslinks were eluted (1% SDS, 50 mM NaHCO3, 1.5 μg/ml of salmon sperm DNA) and reversed (5 μL of 5 N NaCl, 2 μL 10 μg/μL RNase for 100 μL eluent) at 65°C for 5 - 6 hr. DNA was purified by Qiaquick Spin Columns (Qiagen) followed by PCR amplification. The CYP1A1 primers were: 5′ - CAC CCT TCG ACA GTT CCT CTC - 3′ (forward), and 5′ - GCT AGT GCT TTG ATT GGC AGA G - 3′ (reverse), which amplified a 381-bp region of human CYP1A1 enhancer containing DREs, and the primers for the CYP1A1 insert enhancer region were 5′ - CAC CCT TCG ACA GTT CCT CTC - 3′ (forward) and 5′ - GCT AGT GCT TTG ATT GGC AGA G - 3′ (reverse); primers for the TATA region of the CYP1A1 promoter were 5′-CTC CAA TCC CAG AGA GAC CA - 3′ (forward) and 5′ - GTG AAG GCA CTG CAA CCT - 3′ (reverse). The positive control primers were: 5′ - TAC TAG CGG TTT TAC GGG CG - 3′ (forward), and 5′ - TCG AAC AGG AGG AGC AGA GAG CGA - 3′ (reverse), which amplify a 167-bp region of the human glyceraldehyde-3- phosphate dehydrogenase (GAPDH) gene. The negative control primers were: 5′ - ATG GTT GCC ACT GGG GAT CT - 3′ (forward), and 5′ - TGC CAA AGC CTA GGG GAA GA - 3′ (reverse), which amplified a 174-bp region of genomic DNA between the GAPDH gene and the CNAP1 gene. PCR products were resolved on a 2% agarose gel in the presence of 1:10 000 SYBR gold (Molecular Probes).
Statistical Analysis
Experiments were repeated two or more times, and data are expressed as mean ± SE for at least three replicates for each treatment group. Statistical differences between treatment groups were determined using Super ANOVA and Scheffe’s test. Treatments were considered significantly different from controls if p < 0.05.
RESULTS
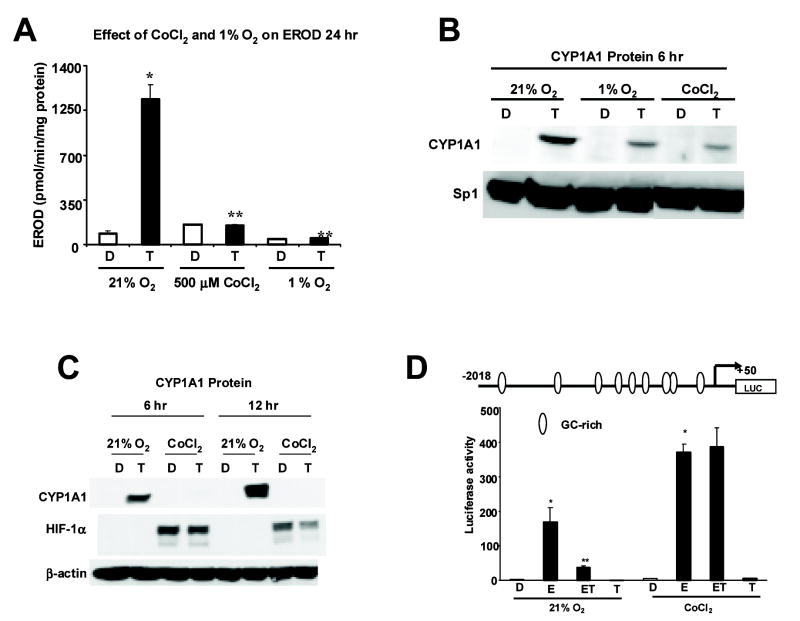
Figure 1A shows that TCDD induces CYP1A1-dependent EROD activity in ZR-75 cells, and this is one of the most sensitive markers of Ah-responsiveness in cancer cell lines and non-tumor tissue. However, induction of EROD activity by TCDD was significantly inhibited when cells were grown under conditions of hypoxia such as 1% O2 or 500 μM cobaltous chloride (CoCl2) (Fig. 1A). CYP1A1 protein was induced by TCDD in MCF-7 cells after treatment for 6 hr, and this induction response was also inhibited when cells were cultured under hypoxic conditions (Fig. 1B). In terms of affecting Ah-responsiveness, both 1% oxygen and cobaltous chloride gave similar responses and the latter reagent was used to simulate hypoxia in subsequent experiments. A direct comparison of the effects of TCDD treatment for 6 or 12 hr shows that CYP1A1 protein was induced under normoxia at both time points, whereas induction of CYP1A1 protein was inhibited in cells grown under hypoxia, and this was accompanied by induction of HIF-1α (Fig. 1C). The inhibitory effects of CoCl2 on induction of CYP1A1 by TCDD showed some variability at the 6 hr time point (Figs. 1B and 1C). TCDD inhibits E2-induced gene expression and reporter gene activity in breast cancer cells transfected with constructs containing E2-responsive promoter inserts (Duan, Porter, Samudio, Vyhlidal, Kladde, and Safe, 1999; Gillesby, Santostefano, Porter, Wu, Safe, and Zacharewski, 1997; Krishnan, Porter, Santostefano, Wang, and Safe, 1995; Porter, Wang, Duan, Qin, Castro-Rivera, and Safe, 2001; Safe and Wormke, 2003). Figure 1D shows that TCDD inhibited induction of luciferase activity by E2 in ZR-75 cells transfected with the E2-responsive pVEGF1 construct containing the -2018 to +50 insert from the VEGF promoter (Stoner, Saville, Wormke, Dean, Burghardt, and Safe, 2002). TCDD alone had minimal effects on activity. This construct is also induced by hypoxia and contains a hypoxia-responsive element at -900. In ZR-75 cells grown under hypoxia, there was a significant increase in basal and E2-inducible luciferase activity. However, E2-induced luciferase activity was not decreased in ZR-75 cells cotreated with E2 plus TCDD, demonstrating the loss of Ah-responsiveness under hypoxic conditions. These results complement data illustrated in Figures 1A - 1C which also show that the effects of TCDD are blocked in ZR-75 cells growth under hypoxia.
Figure 1.
Effects of hypoxia on Ah-responsiveness. [A] Effect of CoCl2 and 1% O2 on EROD activity in ZR-75 cells. ZR-75 cells were treated with DMSO (D) or 10 Nm TCDD (T) and grown in 21% O2 with or without 500 μM CoCl2 or 1% O2 for 24 hr.EROD activity was determined as described in Materials and Methods. TCDD significantly induced EROD activity (*p < 0.05) under normoxic conditions and the induced response was significantly inhibited (**p < 0.05) by 500 μM CoCl2, and 1% O2 conditions. [B and C] Western blot analysis. The effects of 21% O2, 500 μM CoCl2 or 1% O2 on CYP1A1 protein levels in ZR75 cells. Cells were grown in 21% O2 with or without 500 μM CoCl2 or 1% O2 and treated with DMSO (D) or 10 nM TCDD for 6 hr [A and B] or 12 hr [B], and whole cell lysates were analyzed by Western blot analysis as described in the Materials and Methods. Levels of β-actin protein serve as a loading control. Blots illustrated in [B] and [C] were comparable in duplicate studies. [D] VEGF promoter regulation. ZR-75 cells grown under normoxia or 500 μM CoCl2 were transfected with pVEGF1, treated with DMSO (D), 10 nM E2 (E), 10 nM TCDD (T) or their combination (ET), and luciferase activity (normalized to β-gal) was determined as described in the Materials and Methods. Significant (p < 0.05) induction by E2 (*) and inhibition after cotreatment with E2 plus TCDD (**) are indicated.
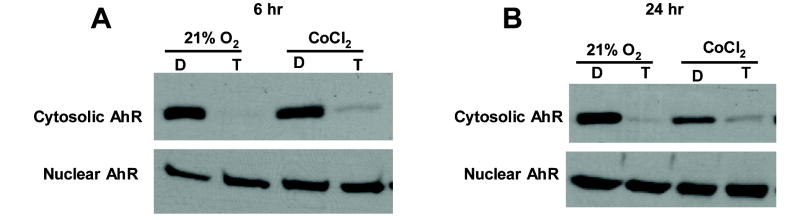
Results in Figure 2A indicate that under conditions of normoxia, the AhR was constitutively expressed in the cytosolic and nuclear fraction in ZR-75 cells as previously reported in MCF-7 cells (Wang et al., 1995). However, after treatment with TCDD for 6 hr, the AhR was primarily located in the nucleus, and similar results were observed under hypoxia for 6 hr (Fig. 2A). In cells treated with DMSO or TCDD for 24 hr, similar results were obtained under normoxia or hypoxia, and levels of the nuclear AhR were comparable in the different treatment groups (Fig. 2B).
Figure 2.
Western blot analysis of cytosolic and nuclear AhR in ZR-75 cells. ZR-75 cells were treated with DMSO (D) or 10 nM TCDD (T) and grown in 21% O2 with or without 500 μM CoCl2 for 6 [A] or 24 hr [B]. Cytosolic and nuclear proteins from ZR-75 cells were obtained and separated by SDS-PAGE (7.5%) as described in Materials and Methods. The immunoblots for each experiment were similar in duplicate experiments and representative blots are illustrated in [A] and [B].
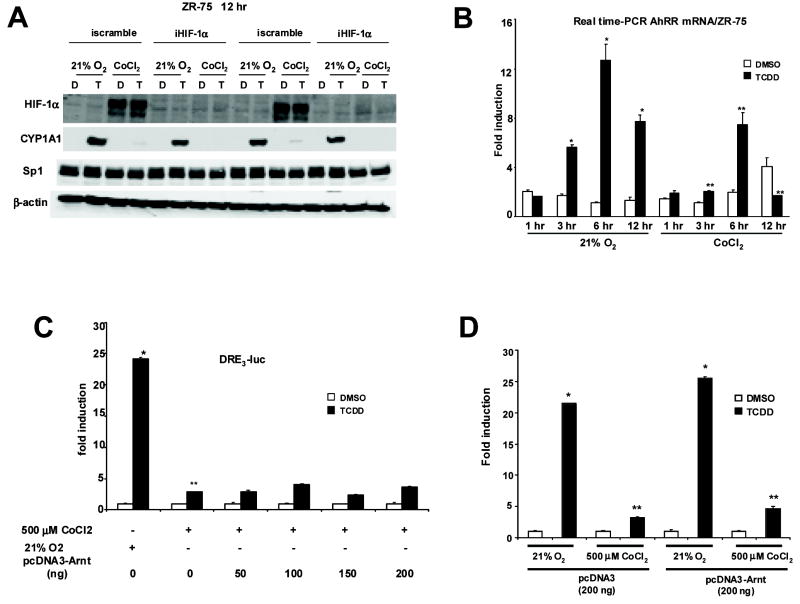
Previous studies have also reported that hypoxia decreases Ah-responsiveness (Chan, Yao, Gu, and Bradfield, 1999; Gradin, McGuire, Wenger, Kvietikova, fhitelaw, Toftgard, Tora, Gassmann, and Poellinger, 1996; Kim and Sheen, 2000; Pollenz, Davarinos, and Shearer, 1999; Prasch, Andreasen, Peterson, and Heideman, 2004), and it has been suggested (Kim and Sheen, 2000) that this may be due to overexpression of HIF-1α which, like the AhR, competitively binds Arnt. Overexpression of HIF1α under hypoxia may competitively decrease availability of Arnt and thereby decrease AhR complex formation. The role of HIF-1α in mediating decreased Ah-responsiveness under hypoxia was addressed using an RNA interference approach (Fig. 3A). In ZR-75 cells grown under normoxia or 500 μM CoCl2 and transfected with a nonspecific small inhibitory RNA (siRNA) iscramble, the results show decreased CYP1A1 and increased HIF-1α protein expression under hypoxia as previously observed in Figure 1. In ZR-75 cells transfected with siRNA for HIF-1α (iHIF-1α), the levels of hypoxia-induced HIF-1α were decreased; however, this did not restore Ah-responsiveness and induction of CYP1A1. Thus, competition for Arnt by HIF-1α was not responsible for hypoxia-dependent loss of Ah-responsiveness. AhR repressor (AhRR) is an Ah-responsive protein that inhibits Ah-responsiveness by forming a transcriptionally-inactive heterodimeric complex with Arnt (Karchner et al., 2002; Mimura et al., 1999). Therefore, we also examined the induction of AhRR mRNA expression by TCDD under normoxia and hypoxia, and the results show that TCDD induced a time-dependent increase in AhRR mRNA levels under normoxia, and this was significantly decreased at all time points under hypoxia (Fig. 3B). Thus, hypoxia also decreased induction of AhRR and, therefore, it is unlikely that AhRR plays a role in hypoxia-induced loss of Ah-responsiveness. These data suggest that under hypoxia, HIF-1α and AhRR are unlikely to decrease Ah-responsiveness by competing with the AhR for binding to Arnt; however, it is possible that other hypoxia-inducible proteins may compete for binding to Arnt. In order to investigate this possibility, the effects of Arnt overexpression on TCDD-induced transactivation was investigated in cells transfected with DRE-luc and increasing amounts of Arnt expression plasmid (Fig. 3C). A comparison of the effects of pcDNA3 vs. pcDNA3-Arnt on Ah-responsiveness under normoxia or 500 μM cobaltous chloride is illustrated in Figure 3D. pcDNA3-Arnt slightly enhanced Ah-responsiveness; however, the ratios of induced luciferase activity (normoxia/hypoxia) were similar with pcDNA3 or pcDNA3-Arnt. The results show that overexpression of Arnt did not affect hypoxia-induced loss of Ah-responsiveness, indicating that Arnt was not limiting in these cells, and it is unlikely that hypoxia induces an Arnt binding protein that results in decreased AhR signaling.
Figure 3.
Role of HIF-1α, AhRR and Arnt on hypoxia-induced loss of Ah-responsiveness. [A] HIF-1α protein expression and HIF-1α protein knockdown in ZR-75 cells by RNA interference. Cells were transfected with iscramble or iHIF-1α for 48 hr in 21% O2 and treated with DMSO (D) or TCDD (T) with or without 500 μM CoCl2 for 12 hr. Whole cell lysates were obtained and analyzed by Western blot as described in the Materials and Methods. Duplicate aliquots of each treatment group are shown and comparable results were observed in a separate experiment. [B] Effects of CoCl2 on AHRR mRNA levels in ZR-75 cells. ZR-75 cells were treated with DMSO (D) or 10 nM TCDD (T) for 1 hr, 3 hr, 6 hr and 12 hr, with or without 500 μM CoCl2, and AHRR mRNA levels were determined by Real-time PCR. Significant (p < 0.05) induction by TCDD under normoxia (*) and inhibition of this response in the presence of CoCl2 (**) are indicated. [C] Effects of increasing amounts of Arnt on pDRE3 in ZR-75 cells. ZR-75 cells were transfected with pDRE3 and increasing amounts of Arnt expression plasmid (pcDNA3-Arnt) for 6hr, treated with DMSO (D) or TCDD (T) with or without 500 μM CoCl2 for 20 hr, and luciferase activity was determined as described in the Materials and Methods. Significant (p < 0.05) induction by TCDD (*) under normoxia or inhibition of this response in presence of CoCl2 (**) are indicated. [D] Comparative effects of pcDNA3 and pcDNA3-Arnt on Ah-responsiveness. Cells were grown and assayed essentially as described above in [C]; however, equal amounts (200 ng) of pcDNA3 and pcDNA3-Arnt were also cotransfected. TCDD significantly (p < 0.05) induced luciferase activity under normoxia (*), and decreased inducibility (**) was observed in cells grown with 500 μM cobaltous chloride added to the medium. Results are expressed as means ± SE for three separate determinations for each treatment group. Ratios of induced activity (normoxia/CoCl2) were not significantly different in cells transfected with pcDNA3 (empty vector) or pcDNA3-Arnt.
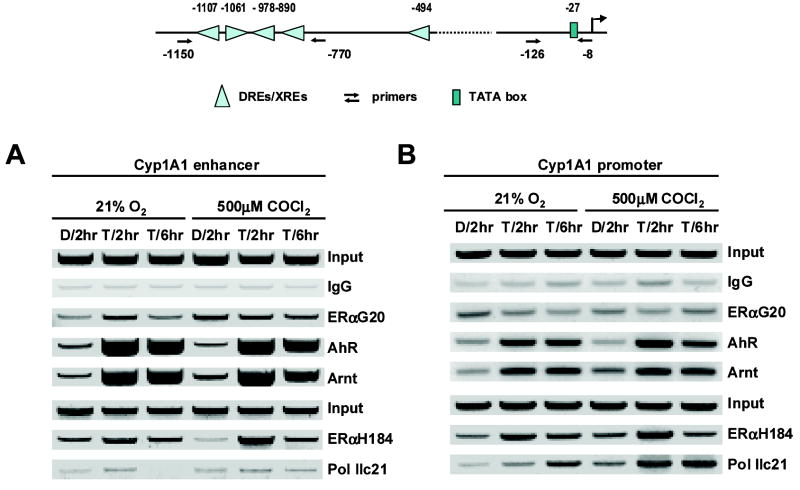
We further investigated hypoxia-induced modulation of AhR/Arnt binding to the enhancer region of the CYP1A1 gene promoter using a chromatin immunoprecipitation (ChIP) assay. The results illustrated in Figure 4A show that under normoxia, treatment with TCDD resulted in recruitment of both AhR and Arnt to the distal region of the CYP1A1 promoter which contains 4 DRE motifs. Pol II was not significantly associated with this complex as previously reported (Hestermann et al., 2003). However, in cells treated with 500 μM CoCl2 for 2 or 6 hr, we also observed comparable recruitment of both AhR and Arnt to the CYP1A1 promoter, suggesting that hypoxia does not directly affect association of this complex with the promoter. We also investigated the effects of hypoxia on interaction of Pol II with the proximal region of the CYP1A1 promoter. Treatment with TCDD resulted in recruitment of Pol II to the CYP1A1 promoter in ZR-75 cells grown under normoxia and hypoxia. Moreover, TCDD also enhanced AhR and Arnt binding to this region of the promoter in cells grown under normoxia and hypoxia. These data show that TCDD-induced interactions of AhR, Arnt and Pol II with distal and proximal regions of the CYP1A1 are comparable under normoxia and hypoxia, suggesting that decreased Ah-responsiveness in ZR-75 cells maintained under hypoxic conditions is not due to decreased association of the AhR complex with promoter DNA or decreased interactions of Pol II with the CYP1A1 promoter. A duplicate of this ChIP assay gave similar results.
Figure 4.
CHIP assay of protein interactions with the CYP1A1 gene promoter. Interactions of proteins with the distal CYP1A1 enhancer [A] and proximal promoter [B] regions were determined in ZR-75 cells grown under normoxia or hypoxia (CoCl2) and treated with DMSO or TCDD or 2 or 6 hr. Antibodies used in the CHIP assay and details of the assay conditions are outlined in the Materials and Methods. A diagram of the CYP1A1 enhancer and proximal promoter regions and the PCR start sites are included in the upper section of the Figure. As a control for this experiment, we showed that TFIIB bound to the GAPDH promoter (positive control) but did not interact with the exon 1 of the CNAP gene (negative control). A similar band distribution was observed in a duplicate experiment examining protein interactions with the CYP1A1 enhancer and promoter regions.
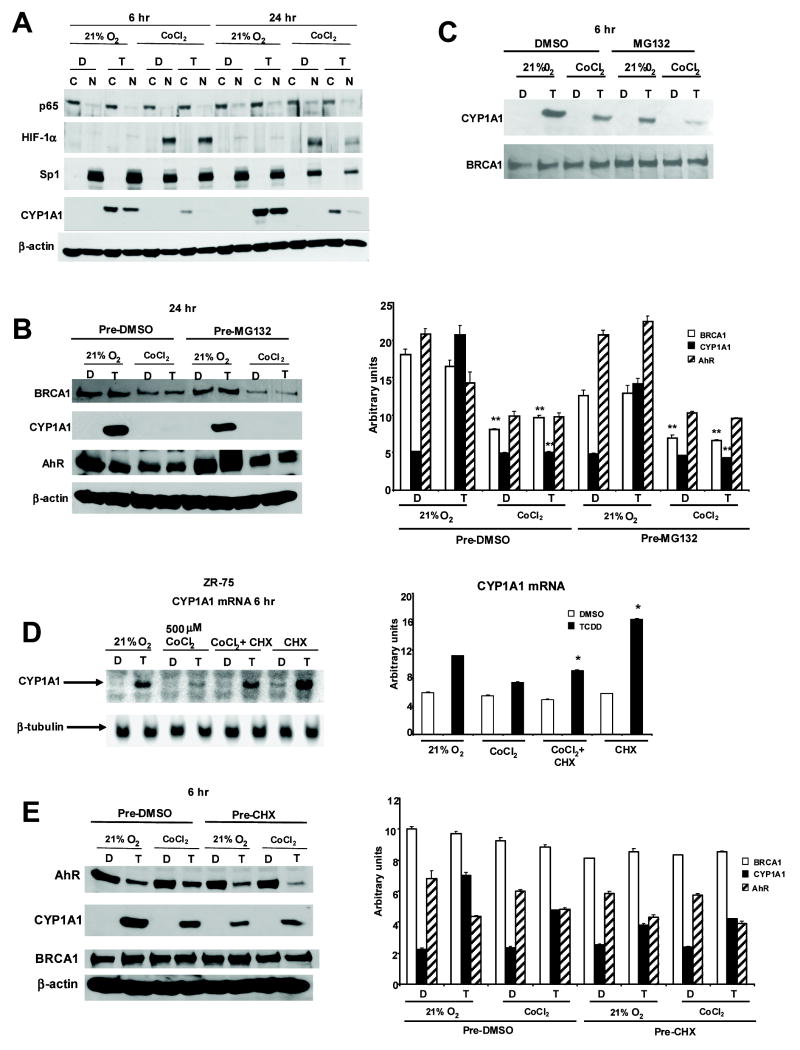
Stress responsive NFκB signaling also inhibits AhR-mediated transactivation (Ke et al., 2001; Tian et al., 1999) and in some cells, hypoxic conditions increase p65 and NFκB expression (Jeong et al., 2005). Therefore, we investigated interactions between hypoxia, NFκB and Ah-responsiveness in ZR-75 cells treated with DMSO or TCDD for 6 and 24 hr under normoxia or hypoxia (Fig. 5A). The results observed after 6 or 24 hr were comparable; hypoxia decreased Ah-responsiveness, increased HIF-1α expression, whereas p65 is primarily cytosolic under normoxia and hypoxia and is not induced by hypoxia in ZR-75 cells. Thus, it is unlikely that p65 plays a role in hypoxia-dependent decreased Ah-responsiveness.
Figure 5.
Role of p65, BRCA1, MG132 and cycloheximide on hypoxia-induced loss of Ah-responsiveness. [A] Western blot analysis of cytosolic and nuclear proteins in ZR-75 cells. ZR-75 cells were treated with DMSO (D) or 10 nM TCDD (T) and grown in 21% O2 with or without 500 μM CoCl2 for 6 or 24 hr. Cytosolic and nuclear protein from ZR-75 cells were obtained and separated by SDS-PAGE (7.5%) as described in Materials and Methods. Western blot analysis after treatment for 24 [B] or 6 hr [C]. ZR-75 cells were pretreated with 10 μM MG132 for 30 min before treating with DMSO (D) or 10 nM TCDD (T) with or without 500μM CoCl2 for 24 [B] or 6 hr [C]. Whole cell lysates were obtained and analyzed by Western blot as described in the Materials and Methods. A significant (p < 0.05) decrease of BRCA1 or induced CYP1A1 protein in cells treated with CoCl2 vs. 21% oxygen is indicated (**) in Figure 5B. [D] Northern blot analysis of CYP1A1 mRNA from ZR-75 cells. Cells were treated with DMSO (D) (lanes 1, 3, 5, 7) or 10 nM TCDD (lanes 2, 4, 6, 8) for 6 hr in the presence or absence of 500μM CoCl2 with or without cycloheximide (CHX) (pretreatment for 45 min). Cell extracts were obtained, and total RNA was isolated and subjected to Northern blot analysis. A significant (p < 0.05) increase in CYP1A1 mRNA levels in cells treated with cycloheximide is indicated. [E] Western blot analysis. ZR-75 cells were pretreated with 25 μM cycloheximide (CHX) for 45 min before treating with DMSO (D) or 10 nM TCDD (T) with or without 500 μM CoCl2 for 6 hr. Whole cell lysates were obtained and analyzed by Western blots as described in the Materials and Methods. means ± SE for three replicate experiments for each treatment group and were normalized to β-actin. Results in Figure 5D are means ± SE for three replicate experiments for each treatment group and were normalized to β-tubulin mRNA. Figures 5A and 5C are representative blots of experiments that were repeated at least two times.
A recent study showed that BRCA1 interacts with Arnt and, in breast cancer cells transfected with a DRE-luc construct, overexpression of BRCA1 (transfected) enhanced Ah-responsiveness; moreover, knockdown of constitutively expressed BRCA1 breast cancer cells decreased induction of luciferase activity by TCDD (Kang et al., 2006). Moreover, since hypoxia decreases BRCA1 expression (Bindra et al., 2005), we further investigated the effects of normoxia and hypoxia on CYP1A1 and BRCA1 protein expression in ZR-75 cells (Figs. 5B and 5C). A comparison of the effects of normoxia and hypoxia in ZR-75 cells treated with DMSO or 10 nM TCDD for 24 (Fig. 5C) showed that in CoCl2-treated cells, there was a significant decrease in TCDD-induced CYP1A1 and BRCA1 in cells treated with DMSO or TCDD (Fig. 5B). This suggests that at the 24 hr time point, the CoCl2-induced loss of BRCA1 contributes to decreased Ah-responsiveness as reported by Kang and coworkers (2006). AhR levels were also decreased in ZR-75 cells grown under hypoxia and MG132 did not affect AhR expression. In contrast, decreased induction of CYP1A1 by TCDD in ZR-75 cells grown under hypoxia for 6 hr was not accompanied by decreased BRCA1 expression (Fig. 5C). The proteasome inhibitor MG132 had minimal effects on Ah-responsiveness in cells maintained under normoxia and hypoxia. Thus, hypoxia-induced loss of Ah-responsiveness after 6 hr was not associated with decreased BRCA1 expression and hypoxia did not directly decrease CYP1A1 through activation of proteasomes.
It has been reported that the protein synthesis inhibitor cycloheximide enhances Ah-responsiveness in breast and other cancer cell lines suggesting endogenous unknown proteins expressed in these cells inhibit induction of CYP1A1 by TCDD (Arellano et al., 1993; Joiakim et al., 2004; Lusska et al., 1992; Ma et al., 2000). Therefore, the induction of analogous or similar inhibitory proteins by hypoxia was investigated in ZR-75 cells treated with DMSO or TCDD and cycloheximide (Fig. 5D). The results show that induction of CYP1A1 mRNA by TCDD (normoxia) was inhibited in cells treated with CoCl2 (hypoxia). Cycloheximide significantly enhanced the fold-induction of CYP1A1 mRNA in ZR-75 cells grown under normoxia as previously reported in other cell lines (Arellano, Wang, and Safe, 1993; Joiakim, Mathieu, Elliott, and Reiners, Jr., 2004; Lusska, Wu, and Whitlock, Jr., 1992; Ma, Renzelli, Baldwin, and Antonini, 2000). In ZR-75 cells treated with CoCl2, the induction of CYP1A1 by TCDD was decreased; however, the fold induction response was significantly increased after treatment with CoCl2 plus cycloheximide (Fig. 5D). The effects of cycloheximide on AhR, BRCA1 and CYP1A1 expression in ZR-75 cells treated with TCDD or DMSO for 6 hr are illustrated in Figure 5E. BRCA1 levels are similar in all treatment groups, whereas TCDD-induced CYP1A1 protein levels were decreased in cells grown under normoxia or hypoxia. This is consistent with the effects of cycloheximide as a protein synthesis inhibitor. However, a comparison of the DMSO vs. cycloheximide treatments indicates that AhR levels were not increased in any treatment group, and this cannot explain cycloheximide-enhanced Ah-responsiveness in normoxic or hypoxic ZR-75 cells (Fig. 5D).
These results show that cycloheximide enhances induction of CYP1A1 by TCDD in ZR-75 cells grown under normoxia or hypoxia, suggesting that hypoxia enhances induction of inhibitory factor(s) that may be similar or different from constitutively expressed proteins that also inhibit Ah-responsiveness. However, in ZR-75 cells, the effects of cycloheximide on induction of CYP1A1 by TCDD are independent of changes in AhR expression.
DISCUSSION
The AhR binds TCDD and structurally-related chlorinated aromatic compounds with high affinity and endogenous biochemicals and diverse synthetic drugs and other aromatic chemicals with lower affinity. In addition, chemoprotective natural products including flavonoids, indole-3-carbinol and related heteroaromatics, green tea components, and other polyhydroxy aromatic antioxidants also bind the AhR (Denison, Seidel, Rogers, Ziccardi, Winter, and Heath-Pagliuso, 1998; Denison and Nagy, 2003) and exhibit both AhR agonist and antagonist activities. Ligand-dependent activation of the AhR inhibits growth of ER-positive breast cancer cells (Safe and Wormke, 2003), and growth inhibitory effects of AhR agonists have also been observed in pancreatic, prostate and ovarian cancer (Koliopanus et al., 2002; Morrow et al., 2004; Rowlands et al., 1993). These observations have led to development of selective AhR modulators (SAhRMs) as a potential new class of drugs for treatment of these cancers (McDougal, Wormke, Calvin, and Safe, 2001; Safe, McDougal, Gupta, and Ramamoorthy, 2001; Safe and Wormke, 2003).
Studies in this laboratory have investigated the mechanisms of inhibitory AhR-ERα crosstalk in breast cancer cells and also the effects of hypoxia on this response. Hypoxia decreased estrogen-responsiveness in breast cancer cells (Stoner, Saville, Wormke, Dean, Burghardt, and Safe, 2002), and also decreases the magnitude of the antiestrogenic activity of AhR agonists in breast cancer cells (Fig. 1D). Since hypoxic conditions could limit the effectiveness of SAhRMs, we further investigated the mechanisms of hypoxia-induced modulation of Ah-responsiveness in breast cancer cells. Previous studies have also reported the effects of hypoxia on AhR function and their results are both complementary and different (Chan, Yao, Gu, and Bradfield, 1999; Gradin, McGuire, Wenger, Kvietikova, fhitelaw, Toftgard, Tora, Gassmann, and Poellinger, 1996; Kim and Sheen, 2000; Pollenz, Davarinos, and Shearer, 1999; Prasch, Andreasen, Peterson, and Heideman, 2004). Some data suggest that overexpression of HIF-1α under hypoxic conditions may decrease Ah-responsiveness through competition with the AhR for binding Arnt (Kim and Sheen, 2000). There is also evidence that overexpression of HIF-1α can decrease AhR:Arnt binding to DNA in a gel mobility shift assay (Chan, Yao, Gu, and Bradfield, 1999). In this study, we also show that hypoxia clearly decreases induction of CYP1A1, EROD activity, and AhRR by TCDD and blocks inhibitory AhR-ERα/Sp1 crosstalk in cells transfected with pVEGF1 (Figs. 1 and 3). Hypoxia also decreased induction of reporter gene activity by TCDD in ZR-75 cells transfected with pDRE3 (Fig. 3C) and hypoxia-induced inhibition of Ah-responsiveness was observed in cells within 6 hr of growth under hypoxic conditions. Previous studies showed that hypoxia activated proteasome-dependent degradation of ERα (Stoner, Saville, Wormke, Dean, Burghardt, and Safe, 2002); however, the proteasome inhibitor MG132 did not reverse the hypoxia-dependent decrease in the induction of CYP1A1 protein by TCDD or the decrease in BRCA1 expression (Fig. 5B). Although hypoxia may decrease cytosolic AhR in ZR-75 cells (Fig. 2), minimal effects on nuclear AhR levels were observed. These data demonstrate that hypoxia inhibits Ah-responsiveness, but this is not due to decreased nuclear AhR (liganded) accumulation or decreased Arnt (data not shown). Moreover, in ChIP assays, the interactions of AhR and Arnt with the enhancer region of the CYP1A1 promoter are similar under hypoxia or normoxia (Fig. 4).
It has previously been suggested that HIF-1α or other proteins that competitively displace the AhR from binding Arnt may that decrease Ah-responsiveness in cells grown under hypoxia (Chan, Yao, Gu, and Bradfield, 1999; Gradin, McGuire, Wenger, Kvietikova, fhitelaw, Toftgard, Tora, Gassmann, and Poellinger, 1996; Kim and Sheen, 2000; Pollenz, Davarinos, and Shearer, 1999; Prasch, Andreasen, Peterson, and Heideman, 2004). However, we show that overexpression of Arnt in transfection assays (Fig. 3C) or knockdown of HIF-1α by RNA interference (Fig. 3A) did not reverse the effects of hypoxia on Ah-responsiveness. It has also been reported that in some cell types, that hypoxia induced p65 and NFκB (Jeong, Hong, Park, Shin, An, and Kim, 2005) and p65 can inhibit Ah-responsiveness and induction of CYP1A1 by TCDD (Ke, Rabson, Germino, Gallo, and Tian, 2001; Tian, Ke, Denison, Rabson, and Gallo, 1999). However, results in Figure 5A demonstrate that hypoxia does not affect p65 expression in ZR-75 cells; moreover, this protein is expressed primarily in the cytosol under all the treatment conditions. Thus, our results would exclude a role for induced HIF-1α, limiting levels of Arnt or increased expression of nuclear p65 in mediating hypoxia-induced loss of Ah-responsiveness in ZR-75 cells.
It has recently been reported that hypoxia decreases BRCA1 expression, and this protein coactivates or enhances Ah-responsiveness in transfection (overexpression) experiments. Moreover, knockdown of constitutively-expressed BRCA1 decreases Ah-responsiveness in human breast cancer cells (Kang, Kim, Kim, Barouki, Cho, Khanna, Rosen, and Bae, 2006). Results in Figure 5B show that hypoxia (24 hr) decreases BRCA1 and this is also accompanied by decreased induction of CYP1A1 by TCDD, suggesting that at this later time point, loss of Ah-responsiveness may be due, in part, to loss of BRCA1 expression.
Breast cancer cells constitutively express protein(s) that decrease induction of CYP1A1 by TCDD and inhibition of protein synthesis by cycloheximide increases the induction of CYP1A1 mRNA levels (Arellano, Wang, and Safe, 1993; Joiakim, Mathieu, Elliott, and Reiners, Jr., 2004; Lusska, Wu, and Whitlock, Jr., 1992; Ma, Renzelli, Baldwin, and Antonini, 2000). Evidence for expression of these inhibitory factors is also observed in ZR-75 cells where cycloheximide treatment increases induction of CYP1A1 mRNA levels by TCDD (Fig. 5D). Moreover, the loss of Ah-responsiveness in ZR-75 cells grown under hypoxia is also significantly reversed in hypoxic cells treated with cycloheximide (Fig. 5C). These results show for the first time that cycloheximide treatment enhances induction of CYP1A1 mRNA by TCDD in breast cancer cells grown under both normoxia and hypoxia for 6 hr. This suggests that hypoxia may also induce unknown inhibitory proteins that decrease Ah-responsiveness in breast cancer cells, and these may be similar to constitutively expressed proteins in breast and other cancer cell lines that inhibit Ah-responsiveness. A previous report suggested that the effects of cycloheximide on superinduction of CYP1A1 by TCDD in mouse Hepa1c1c7 cell may be due to increased accumulation of the nuclear AhR (Ma, Renzelli, Baldwin, and Antonini, 2000). However, in ZR-75 cells, cycloheximide did not affect AhR levels after treatment for 6 hr (Fig. 5E), suggesting that in this cell line enhanced Ah-responsiveness induced by cycloheximide is independent of changes in AhR expression and nuclear uptake.
In summary, results of this study show that hypoxia rapidly decreases Ah-responsiveness in ZR-75 cells and this inhibitory response is not associated with induction of HIF-1α, p65 or limiting levels Arnt. Moreover, TCDD-induced interactions with the CYP1A1 enhancer region are similar under normoxia and hypoxia, suggesting that hypoxia-induced loss of Ah responsiveness is due to other factors. Hypoxia conditions could deplete critical coregulatory proteins required for mediating AhR-dependent activity, and there is evidence for cofactor competition resulting from simultaneous activation of more than one ligand-activated receptor (Meyer et al., 1989). BRCA1 has previously been characterized as a coregulator of AhR-mediated responses (Kang, Kim, Kim, Barouki, Cho, Khanna, Rosen, and Bae, 2006) and therefore, hypoxia-induced downregulation of BRCA1 after 24 hr (Fig. 5B) contributes to the loss of Ah-responsiveness in ZR-75 cells. However, results of the cycloheximide experiments (Fig. 5D and 5E) suggest that hypoxia may rapidly (≤ 6 hr) induce inhibitory factors that are also expressed under normoxic conditions in breast cancer cell (Arellano, Wang, and Safe, 1993; Joiakim, Mathieu, Elliott, and Reiners, Jr., 2004; Lusska, Wu, and Whitlock, Jr., 1992; Ma, Renzelli, Baldwin, and Antonini, 2000). Lee and coworkers (Lee et al., 2006) used a microarray approach to identify genes that may be involved in crosstalk between hypoxia and AhR-mediated transactivation in Hep3B cells. They identified 176 Ah-responsive genes that were influenced by hypoxia indicating the complexity of this interaction. Current studies are investigating the identity and function of specific proteins which may contribute to the rapid hypoxia-induced inhibition of Ah-responsiveness.
Acknowledgments
The financial assistance of the National Institutes of Health (ES09106 and CA76636), the Department of the Defense (DAMD17-03-1-0341), and the Texas Agricultural Experiment Station is gratefully acknowledged.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arellano LO, Wang X, Safe S. Effects of cycloheximide on the induction of CYP1A1 gene expression by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in three human breast cancer cell lines. Carcinogenesis. 1993;14:219–222. doi: 10.1093/carcin/14.2.219. [DOI] [PubMed] [Google Scholar]
- Bindra RS, Gibson SL, Meng A, Westermark U, Jasin M, Pierce AJ, Bristow RG, Classon MK, Glazer PM. Hypoxia-induced down-regulation of BRCA1 expression by E2Fs. Cancer Res. 2005;65:11597–11604. doi: 10.1158/0008-5472.CAN-05-2119. [DOI] [PubMed] [Google Scholar]
- Chan WK, Yao G, Gu Y-Z, Bradfield CA. Cross-talk between the aryl hydrocarbon receptor and hypoxia inducible factor signaling pathways. J Biol Chem. 1999;274:12115–12123. doi: 10.1074/jbc.274.17.12115. [DOI] [PubMed] [Google Scholar]
- Denison MS, Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annu Rev Pharmacol Toxicol. 2003;43:309–334. doi: 10.1146/annurev.pharmtox.43.100901.135828. [DOI] [PubMed] [Google Scholar]
- Denison MS, Seidel SD, Rogers WJ, Ziccardi M, Winter GM, Heath-Pagliuso S. Natural and synthetic ligands for the Ah receptor. In: Puga A, Kendall RJ, editors. Molecular Biology Approaches to Toxicology. Taylor and Francis; London: 1998. pp. 3–33. [Google Scholar]
- Duan R, Porter W, Samudio I, Vyhlidal C, Kladde M, Safe S. Transcriptional activation of c-fos protooncogene by 17β-estradiol: mechanism of aryl hydrocarbon receptor-mediated inhibition. Mol Endocrinol. 1999;13:1511–1521. doi: 10.1210/mend.13.9.0338. [DOI] [PubMed] [Google Scholar]
- Gillesby B, Santostefano M, Porter W, Wu ZF, Safe S, Zacharewski T. Identification of a motif within the 5′-regulatory region on pS2 which is responsible for Ap1 binding and TCDD-mediated suppression. Biochemistry. 1997;36:6080–6089. doi: 10.1021/bi962131b. [DOI] [PubMed] [Google Scholar]
- Gradin K, McGuire J, Wenger RH, Kvietikova I, fhitelaw ML, Toftgard R, Tora L, Gassmann M, Poellinger L. Functional interference between hypoxia and dioxin signal transduction pathways: competition for recruitment of the Arnt transcription factor. Mol Cell Biol. 1996;16:5221–5231. doi: 10.1128/mcb.16.10.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hestermann EV, Brown M. Agonist and chemopreventative ligands induce differential transcriptional cofactor recruitment by aryl hydrocarbon receptor. Mol Cell Biol. 2003;23:7920–7925. doi: 10.1128/MCB.23.21.7920-7925.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong HJ, Hong SH, Park RK, Shin T, An NH, Kim HM. Hypoxia-induced IL-6 production is associated with activation of MAP kinase, HIF-1, and NF-κB on HEI-OC1 cells. Hear Res. 2005;207:59–67. doi: 10.1016/j.heares.2005.04.003. [DOI] [PubMed] [Google Scholar]
- Joiakim A, Mathieu PA, Elliott AA, Reiners JJ., Jr Superinduction of CYP1A1 in MCF10A cultures by cycloheximide, anisomycin, and puromycin: a process independent of effects on protein translation and unrelated to suppression of aryl hydrocarbon receptor proteolysis by the proteasome. Mol Pharmacol. 2004;66:936–947. doi: 10.1124/mol.66.4.. [DOI] [PubMed] [Google Scholar]
- Kang HJ, Kim HJ, Kim SK, Barouki R, Cho CH, Khanna KK, Rosen EM, Bae I. BRCA1 modulates xenobiotic stress-inducible gene expression by interacting with ARNT in human breast cancer cells. J Biol Chem. 2006;281:14654–14662. doi: 10.1074/jbc.M601613200. [DOI] [PubMed] [Google Scholar]
- Karchner SI, Franks DG, Powell WH, Hahn ME. Regulatory interactions among three members of the vertebrate aryl hydrocarbon receptor family: AHR repressor, AHR1, and AHR2. J Biol Chem. 2002;277:6949–6959. doi: 10.1074/jbc.M110779200. [DOI] [PubMed] [Google Scholar]
- Ke S, Rabson AB, Germino JF, Gallo MA, Tian Y. Mechanism of suppression of cytochrome P-450 1A1 expression by tumor necrosis factor-α and lipopolysaccharide. J Biol Chem. 2001;276:39638–39644. doi: 10.1074/jbc.M106286200. [DOI] [PubMed] [Google Scholar]
- Kim JE, Sheen YY. Inhibition of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD)-stimulated Cyp1a1 promoter activity by hypoxic agents. Biochem Pharmacol. 2000;59:1549–1556. doi: 10.1016/s0006-2952(00)00283-5. [DOI] [PubMed] [Google Scholar]
- Koliopanus A, Kleeff J, Xiao Y, Safe S, Zimmerman A, Buchler MW, Friess H. Increased aryl hydrocarbon receptor expression offers a potential therapeutic target in pancreatic cancer. Oncogene. 2002;21:6059–6070. doi: 10.1038/sj.onc.1205633. [DOI] [PubMed] [Google Scholar]
- Krishnan V, Porter W, Santostefano M, Wang X, Safe S. Molecular mechanism of inhibition of estrogen-induced cathepsin D gene expression by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) in MCF-7 cells. Mol Cell Biol. 1995;15:6710–6719. doi: 10.1128/mcb.15.12.6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K, Burgoon LD, Lamb L, Dere E, Zacharewski TR, Hogenesch JB, LaPres JJ. Identification and characterization of genes susceptible to transcriptional cross-talk between the hypoxia and dioxin signaling cascades. Chem Res Toxicol. 2006;19:1284–1293. doi: 10.1021/tx060068d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lusska A, Wu L, Whitlock JP., Jr Superinduction of CYP1A1 transcription by cycloheximide: role of the DNA binding site for the liganded Ah receptor. J Biol Chem. 1992;267:15146–15151. [PubMed] [Google Scholar]
- Ma Q, Renzelli AJ, Baldwin KT, Antonini JM. Superinduction of CYP1A1 gene expression. Regulation of 2,3,7, 8-tetrachlorodibenzo-p-dioxin-induced degradation of Ah receptor by cycloheximide. J Biol Chem. 2000;275:12676–12683. doi: 10.1074/jbc.275.17.12676. [DOI] [PubMed] [Google Scholar]
- McDougal A, Wormke M, Calvin J, Safe S. Tamoxifen-induced antitumorigenic/antiestrogenic action synergized by a selective Ah receptor modulator. Cancer Res. 2001;61:3901–3907. [PubMed] [Google Scholar]
- Meyer ME, Gronemeyer H, Turcotte B, Bocquel MT, Tasset, Chambon P. Steroid hormone receptors compete for factors that mediate their enhancer function. Cell. 1989;57:433–442. doi: 10.1016/0092-8674(89)90918-5. [DOI] [PubMed] [Google Scholar]
- Mimura J, Ema M, Sogawa K, Fujii-Kuriyama Y. Identification of a novel mechanism of regulation of Ah (dioxin) receptor function. Genes Dev. 1999;13:20–25. doi: 10.1101/gad.13.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrow D, Qin C, Smith R, III, Safe S. Aryl hydrocarbon receptor-mediated inhibition of LNCaP prostate cancer cell growth and hormone-induced transactivation. J Steroid Biochem Mol Biol. 2004;88:27–36. doi: 10.1016/j.jsbmb.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Ohtake F, Baba A, Takada I, Okada M, Iwasaki K, Miki H, Takahashi S, Kouzmenko A, Nohara K, Chiba T, Fujii-Kuriyama Y, Kato S. Dioxin receptor is a ligand-dependent E3 ubiquitin ligase. Nature. 2007;446:562–566. doi: 10.1038/nature05683. [DOI] [PubMed] [Google Scholar]
- Poland A, Glover E, Kende AS. Stereospecific, high affinity binding of 2,3,7,8- tetrachlorodibenzo-p-dioxin by hepatic cytosol: evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J Biol Chem. 1976;251:4936–4946. [PubMed] [Google Scholar]
- Poland A, Knutson JC. 2,3,7,8-Tetrachlorodibenzo-p-dioxin and related halogenated aromatic hydrocarbons. Examinations of the mechanism of toxicity. Annu Rev Pharmacol Toxicol. 1982;22:517–554. doi: 10.1146/annurev.pa.22.040182.002505. [DOI] [PubMed] [Google Scholar]
- Pollenz RS, Davarinos NA, Shearer TP. Analysis of aryl hydrocarbon receptor-mediated signaling during physiological hypoxia reveals lack of competition for the aryl hydrocarbon nuclear translocator transcription factor. Mol Pharmacol. 1999;56:1127–1137. doi: 10.1124/mol.56.6.1127. [DOI] [PubMed] [Google Scholar]
- Porter W, Wang F, Duan R, Qin C, Castro-Rivera E, Safe S. Transcriptional activation of heat shock protein 27 gene expression by 17β-estradiol and modulation by antiestrogens and aryl hydrocarbon receptor agonists: estrogenic activity of ICI 164,384. J Mol Endocrinol. 2001;26:31–42. doi: 10.1677/jme.0.0260031. [DOI] [PubMed] [Google Scholar]
- Prasch AL, Andreasen EA, Peterson RE, Heideman W. Interactions between 2,3,7,8-tetrachlorodibenzo-p-dioxin TCDD and hypoxia signaling pathways in zebrafish: hypoxia decreases responses to TCDD in zebrafish embryos. Toxicol Sci. 2004;78:68–77. doi: 10.1093/toxsci/kfh053. [DOI] [PubMed] [Google Scholar]
- Reyes H, Reisz-Porszasz S, Hankinson O. Identification of the Ah receptor nuclear translocator protein (Arnt) as a component of the DNA binding form of the Ah receptor. Science. 1992;256:1193–1195. doi: 10.1126/science.256.5060.1193. [DOI] [PubMed] [Google Scholar]
- Rowlands C, Krishnan V, Wang X, Santostefano M, Safe S, Miller WR, Langdon S. Characterization of the aryl hydrocarbon (Ah) receptor and Ah-responsiveness in human ovarian carcinoma cell lines. Cancer Res. 1993;53:1802–1807. [PubMed] [Google Scholar]
- Safe S, McDougal A, Gupta MS, Ramamoorthy K. Selective Ah receptor modulators (SAhRMs): progress towards development of a new class of inhibitors of breast cancer growth. J Women’s Cancer. 2001;3:37–45. [Google Scholar]
- Safe S, Wormke M. Inhibitory aryl hydrocarbon-estrogen receptor α crosstalk and mechanisms of action. Chem Res Toxicol. 2003;16:807–816. doi: 10.1021/tx034036r. [DOI] [PubMed] [Google Scholar]
- Stoner M. Differential Regulation of Vascular Endothelial Growth Factor (VEGF) Gene Expression by 17β-Estradiol in Human Breast and Endometrial Cancer Cell Lines. Texas A&M University; College Station, TX: 2002. [Google Scholar]
- Stoner M, Saville B, Wormke M, Dean D, Burghardt R, Safe S. Hypoxia induces proteasome-dependent degradation of estrogen receptor a in ZR-75 breast cancer cells. Mol Endocrinol. 2002;16:2231–2242. doi: 10.1210/me.2001-0347. [DOI] [PubMed] [Google Scholar]
- Stoner M, Wormke M, Saville B, Samudio I, Qin C, Abdelrahim M, Safe S. Estrogen regulation of vascular endothelial growth factor gene expression in ZR-75 breast cancer cells through interaction of estrogen receptor α and Sp proteins. Oncogene. 2004;23:1052–1063. doi: 10.1038/sj.onc.1207201. [DOI] [PubMed] [Google Scholar]
- Swanson HI, Bradfield CA. The Ah-receptor: genetics, structure and function. Pharmacogenetics. 1993;3:213–223. doi: 10.1097/00008571-199310000-00001. [DOI] [PubMed] [Google Scholar]
- Tian Y, Ke S, Denison MS, Rabson AB, Gallo MA. Ah receptor and NF-κB interactions, a potential mechanism for dioxin toxicity. J Biol Chem. 1999;274:510–515. doi: 10.1074/jbc.274.1.510. [DOI] [PubMed] [Google Scholar]
- Wang X, Thomsen JS, Santostefano M, Rosengren R, Safe S, Perdew GH. Comparative properties of the nuclear Ah receptor complex from several human cell lines. Eur J Pharmacol. 1995;293:191–205. doi: 10.1016/s0922-4106(05)80044-6. [DOI] [PubMed] [Google Scholar]
- Whitlock JP., Jr Induction of cytochrome P4501A1. Annu Rev Pharmacol Toxicol. 1999;39:103–125. doi: 10.1146/annurev.pharmtox.39.1.103. [DOI] [PubMed] [Google Scholar]
- Wilson CL, Safe S. Mechanisms of ligand-induced aryl hydrocarbon receptor-mediated biochemical and toxic responses. Toxicologic Pathol. 1998;26:657–671. doi: 10.1177/019262339802600510. [DOI] [PubMed] [Google Scholar]
- Wormke M, Stoner M, Saville B, Walker K, Abdelrahim M, Burghardt R, Safe S. The aryl hydrocarbon receptor mediates degradation of the estrogen receptor α through activation of proteasomes. Mol Cell Biol. 2003;23:1843–1855. doi: 10.1128/MCB.23.6.1843-1855.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]