Abstract
The RNA:pseudouridine (Ψ)-synthase family is one of the most complex families of RNA modification enzymes. Ten genes encoding putative RNA:Ψ-synthases have been identified in S. cerevisiae. Most of the encoded enzymes have been characterized experimentally. Only the putative RNA:Ψ-synthase Pus2p (encoded by the YGL063w ORF) had no identified substrate. Here, we analyzed Ψ residues in cytoplasmic and mitochondrial tRNAs extracted from S. cerevisiae strains, carrying disruptions in the PUS1 and/or PUS2 ORFs. Our results demonstrate that Pus2p is a mitochondrial-specific tRNA:Ψ-synthase acting at positions 27 and 28 in tRNAs. The importance of the Asp56 residue in the conserved ARTD motif of the Pus2p catalytic site is demonstrated in vivo. Interestingly, in spite of the absence of a characteristic N-terminal targeting signal, our data strongly suggest an efficient and rapid targeting of Pus2p in yeast mitochondria. In contradiction with the commonly held idea that a unique nuclear gene encodes the enzyme required for both cytoplasmic and mitochondrial tRNA modifications, here we show the existence of an enzyme specifically dedicated to mitochondrial tRNA modification (Pus2p), the corresponding modification in cytoplasmic tRNAs being catalyzed by another protein (Pus1p).
Keywords: S. cerevisiae, pseudouridine, RNA:pseudouridine-synthase, tRNAs, mitochondria
INTRODUCTION
In all organisms, pseudouridine (Ψ) and 2′-O-methylated residues are the most frequent modifications in RNAs (see The RNA Modification Database, URL http://medstat.med.utah.edu/RNAmods/). The Ψ residues are formed by base isomerization of the transcriptionally incorporated uridine residues (Kammen et al. 1988; Huang et al. 1997). This reaction is catalyzed by RNA:Ψ-synthases, which do not require cofactors or energy for catalysis. RNA:Ψ-synthases represent a broad group of proteins that are divided into five distinct families (TruA, TruB, RluA, RsuA, and TruD) on the basis of amino acid sequence homology (see Koonin 1996; Conrad et al. 1998; Kaya and Ofengand 2003). Based on computer analysis of bacterial and yeast genomes, nine genes encoding putative RNA:Ψ-synthases have been identified in Saccharomyces cerevisiae (Koonin 1996). The RNA:Ψ-synthase activity of the products of eight of them has been identified. Only the substrate specificity of the PUS2 gene product has not yet been characterized. RNA:Ψ-synthases Pus3p, Pus4p, and Pus6p are tRNA-specific enzymes acting on both cytoplasmic and mitochondrial tRNAs (Becker et al. 1997; Lecointe et al. 1998; Ansmant et al. 2001). Pus5p is responsible for the modification of the mitochondrial 21S rRNA (Ansmant et al. 2000), and Cbf5p is associated with the H/ACA snoRNA guides and catalyzes Ψ formation in cytoplasmic rRNAs and U2 snRNA (Lafontaine et al. 1998; Kiss et al. 2004; Ma et al. 2005). Pus7p was not identified using sequence homology but by genome-wide screening of GST-tagged yeast ORFs. It was initially characterized as a U2 snRNA-specific Ψ-synthase (Ma et al. 2003), but it also catalyzes tRNA modification at positions 13 and 35 (Behm-Ansmant et al. 2003). We found that two distinct enzymes, Rib2p/Pus8p and Pus9p, are required for Ψ32 formation in tRNAs, depending on their cytoplasmic or mitochondrial localization, and cytoplasmic Rib2p/Pus8p contains both a tRNA:Ψ32-synthase domain and a DRAP-deaminase domain (Behm-Ansmant et al. 2004). Pus1p is an RNA:Ψ-synthase with a multisite specificity (tRNA positions 1, 26, 27, 28, 34, 36, 65, and 67) (Motorin et al. 1998; Behm-Ansmant et al. 2006). In addition, it also modifies U2 snRNA at position 44 (Massenet et al. 1999). In contrast to yeast cytoplasmic tRNAs, their mitochondrial counterparts are significantly less modified; only uridines at positions 27, 28, 31, 32, 38, 39, 55, and 72 may be converted to Ψs. Based on the assumption that a unique enzyme is generally used to modify both cytoplasmic and mitochondrial tRNAs, Pus1p was also expected to be responsible for the formation of residues Ψ27 and Ψ28 commonly present in mitochondrial tRNAs. However, its activity in yeast mitochondria was never tested experimentally. The only remaining RNA:Ψ-synthase without identified substrates (Pus2p) shows strong sequence homology with Pus1p, especially in motifs II and IIa (Fig. 1), hinting at a similar catalytic activity for these two enzymes. Furthermore, in spite of the absence of a characteristic motif for mitochondrial localization in Pus2p, its preferential mitochondrial localization is clearly predicted by the NNPSL software (Reinhardt and Hubbard 1998). Thus, we hypothesized that Pus2p may be the mitochondrial counterpart of Pus1p. Our interest in the discovery of the yeast RNA:Ψ-synthase responsible for modification of mitochondrial tRNAs at positions 27 and 28 was reinforced by the discovery of a direct link between Ψ27 formation in human mitochondrial tRNA and a rare mitochondrial disorder called mitochondrial myopathy and sideroblastic anemia (MLASA) (Patton et al. 2005).
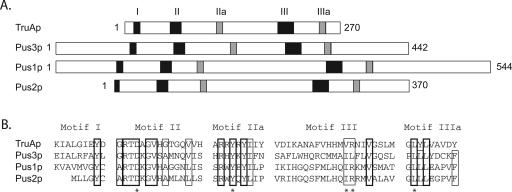
FIGURE 1.
Alignment of the amino acid sequences of the Escherichia coli TruA and S. cerevisiae Pus1p, Pus2p, and Pus3p tRNA:Ψ-synthases of the TruA family. (A) Proteins are schematically represented as bars, whose lengths are proportional to lengths of the polypeptide chains. Motifs I, II, IIa, III, and IIIa of RNA:Ψ-synthases (Del Campo et al. 2004) are represented by black or gray rectangles. (B) The alignments of the amino acid sequences of the conserved blocks I to IIIa are shown. Identical and semiconserved residues are boxed. The thickness of the line is an indication of the level of sequence conservation. The conserved residues (D, Y, I, R, L) in the tRNA:Ψ-synthases active sites are indicated by asterisks.
To test this hypothesis, we analyzed the effects of individual or simultaneous deletions of PUS1 and PUS2 ORFs on the in vivo tRNA pseudouridylation pattern. The loss of PUS1 did not affect pseudouridylation at positions 27 and 28 in mitochondrial tRNAs, while the deletion of PUS2 abolished Ψ27 and Ψ28 formation in these species. Since the enzyme responsible for Ψ72 formation in the mitochondrial tRNAMeti(CAU) has not yet been identified, we also tested the possible activity of Pus2p at position 72 of this tRNA. The data presented demonstrate that Pus2p is a yeast mitochondrial RNA:Ψ-synthase acting at positions 27 and 28, but not at position 72.
RESULTS AND DISCUSSION
Mitochondrial tRNA modifications are affected by PUS2 ORF disruption
To investigate whether Ψ formation at positions 27 and 28 in mitochondrial tRNAs is catalyzed by Pus1p or Pus2p, we tested the effect of individual or simultaneous PUS1 and PUS2 ORF deletions on mitochondrial tRNA pseudouridylation. The haploid S. cerevisiae strain RS453, whose PUS1 ORF was disrupted by insertion of the HIS3 gene (pus1Δ∷HIS3, denoted Δpus1) (Simos et al. 1996), was used to test for the effect of the absence of Pus1p expression. To analyze the consequences of the absence of Pus2p expression, we used the haploid S. cerevisiae strain BY4742, whose PUS2 ORF is disrupted by a Kan r gene replacement (pus2Δ∷Kan r, denoted Δpus2; EUROSCARF). The effects of the simultaneous PUS1 and PUS2 ORF disruptions were tested in the haploid Δpus1/Δpus2 strain, which we isolated by mating of the pus2Δ∷Kan r and the pus1Δ∷HIS3 strains and dissecting the resulting diploids.
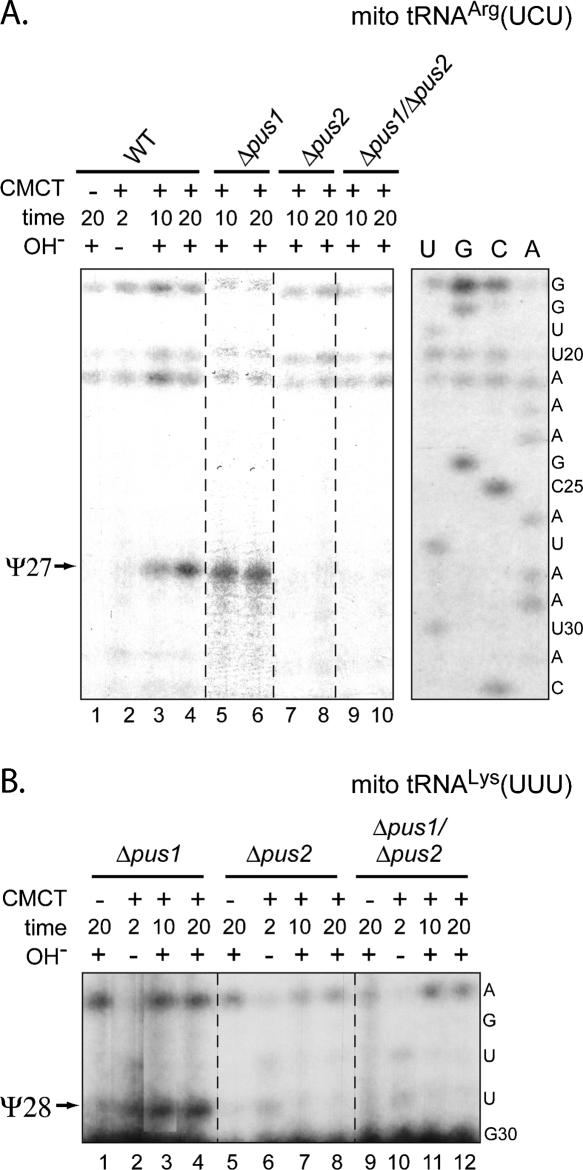
Six out of the 17 sequenced mitochondrial tRNAs in S. cerevisiae bear a Ψ residue at position 27. However, four of them are not suitable for analysis by the CMCT/RT approach because of the presence of a m2G26 or/and m1G37 residue(s), which block(s) primer extension analysis. To avoid this difficulty when testing the Pus1p and Pus2p activity at position 27 we used tRNAArg(UCU), which does not contain these modifications at positions 26 and 37. Pseudouridine at position 28 is found in only one mitochondrial tRNA, the tRNALys(UUU), and fortunately, Ψ28 formation in this tRNA could also be analyzed by the CMCT/RT approach. Disruption of the PUS1 ORF affected neither Ψ27 formation in the mitochondrial tRNAArg(UCU) (Fig. 2A, lanes 5,6) nor Ψ28 formation in mitochondrial tRNALys(UUU) (Fig. 2B, lanes 3,4). In contrast, after deletion of PUS2 ORF, both of these pseudouridylations were abolished (Fig. 2A,B, lanes 7, 8 in both panels). Accordingly, similar results were obtained with the Δpus1/Δpus2 strain carrying both deletions (Fig. 2A,B, panels A and B, lanes 9,10 and 11,12, respectively). Transformation of this strain with a plasmid expressing the WT protein restored Ψ27 formation in tRNAArg(UCU), while no modification was detected in the strain expressing a variant protein with a D56A substitution in Motif II (Fig. 3, lanes 7–10).
FIGURE 2.
Disruption of the PUS2 gene leads to the absence of Ψ27 residues in the mitochondrial tRNAArg(UCU) (A) and Ψ28 in the mitochondrial tRNALys(UUU) (B). Total RNAs extracted from the WT, Δpus1, Δpus2, and Δpus1/Δpus2 strain were modified by CMCT, for 2, 10, and 20 min with (+) or without (−) subsequent alkaline treatment (OH−). A control experiment was performed in the absence of CMCT treatment. Lanes U, G, C, and A correspond to the sequencing ladders obtained with the same oligonucleotide. Primer extension analyses were performed on the mitochondrial tRNAArg(UCU) (A) and tRNALys(UUU) (B) using specific oligonucleotides primers. The reverse transcription stops, corresponding to residue Ψ27 in tRNAArg(UCU) and Ψ28 in tRNALys(UUU), are indicated by arrows.
FIGURE 3.
The aspartic acid residue D56 in the Pus2p active site is essential for the RNA:Ψ-synthase activity. The yeast S. cerevisiae Δpus1/Δpus2 strain was transformed with recombinant p416GalS plasmids, expressing the WT (D56) or mutated (D56A) PUS2 ORF. Total RNAs were extracted from the WT, the untransformed Δpus1/Δpus2, and the two transformed Δpus1/Δpus2 strains. The mitochondrial tRNAArg(UCU) was analyzed by the CMCT/RT approach (same legend as in Fig. 2). The reverse transcription stop, corresponding to residue Ψ27 is indicated by an arrow.
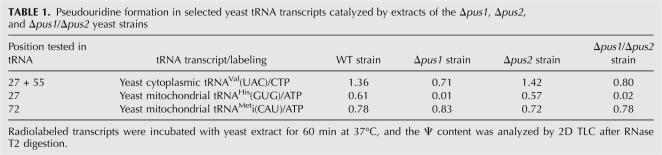
Since Ψ72 in tRNAMeti(CAU) is located too close to the tRNA 3′-extremity to be analyzed by the CMCT/RT approach, the possible implication of Pus2p in Ψ72 formation was only tested in vitro by the nearest-neighbor approach. To this end, the tRNA:Ψ72-synthase activity of cell-free extracts prepared from different strains was tested on a transcript of the mitochondrial tRNAMeti(CAU) labeled by [α-32P]ATP incorporation. As evidenced by thin layer chromatography (TLC) analysis of the labeled RNAse T2 digestion products, none of the gene disruptions affected the activity of the extracts (Table 1, see also Behm-Ansmant et al. 2006). Thus, Pus1p and Pus2p are probably not involved in Ψ formation at position 72.
TABLE 1.
Pseudouridine formation in selected yeast tRNA transcripts catalyzed by extracts of the Δpus1, Δpus2, and Δpus1/Δpus2 yeast strains
Other pseudouridines in cytoplasmic yeast tRNA normally formed by Pus1p (positions 1, 34, 36, 65, 67) are absent in the mitochondrial compartment; thus, positions 27 and 28 are the only possible targets for Pus2p in mitochondria.
Pus2p is unable to insure cytoplasmic tRNA modification
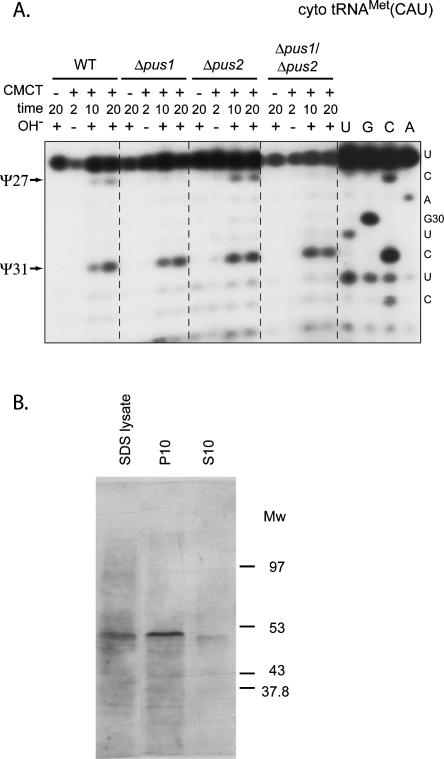
Since Pus1p and Pus2p have redundant activity toward Ψ27 and Ψ28, we tested if Pus2p could complement the absence of active Pus1p for cytoplasmic tRNA modification. Previous studies have already demonstrated the absence of tRNA:Ψ27-synthase activity in extracts from the Δpus1 strain (Motorin et al. 1998). However, this apparent absence of in vitro Pus2p activity could result from protein inactivation or from inappropriate reaction conditions. We therefore verified the absence of in vivo Pus2p activity on cytoplasmic tRNAs by CMCT/RT analysis of the cytoplasmic tRNAMet(CAU) extracted from the Δpus1 strain. As shown in Figure 4, lanes 7, 8,15, and 16, no Ψ residue was detected at position 27 in this tRNA, whereas it was detected in tRNAMet(CAU) extracted from the Δpus2 strain (Fig. 4A, lanes 11,12). Formation of Ψ31, which was used as a control, remained unaffected. Thus, Pus2p is not able to complement the absence of Pus1p activity in vivo.
FIGURE 4.
Formation of residue Ψ27 in the cytoplasmic tRNAMet(CAU) is not affected by disruption of the PUS2 ORF. (A) Total RNAs extracted from the WT, Δpus1, Δpus2, and Δpus1/Δpus2 strains were subjected to CMCT modification (same legend as in Fig. 2). The presence of residues Ψ27 and Ψ31 in tRNAMet(CAU) was tested by primer extension using the oligonucleotide described in Materials and Methods. A sequence ladder was made with the same oligonucleotide. Arrows indicate the reverse transcription stops, corresponding to residues Ψ27 and Ψ31. (B) Western blot analysis of HA-tagged Pus2p in yeast S. cerevisiae strain 26-G-7 (Open BioSystems). Yeast cells lysed by SDS (SDS lysate), insoluble fraction (P10), and S10 extract (S10) were loaded onto 10% PAAG, and separated proteins were transferred to nitrocellulose membrane. Staining was done using monoclonal anti-HA antibodies (12CA5, Roche) and ECL Plus kit (GE Healthcare). Molecular weights of marker proteins are indicated on the left of the panel.
How to explain the inability of Pus2p to modify cytoplasmic tRNAs? Either Pus2p cannot modify cytoplasmic tRNAs due to the absence of the required recognition determinants in these tRNAs or, immediately after protein synthesis, Pus2p is rapidly and predominantly targeted to mitochondria and thus its potential substrates in the cytoplasm escape modification. The first hypothesis seems unlikely since in contrast to their vertebrates counterparts, yeast cytoplasmic and mitochondrial tRNAs have rather similar 2D structures (Wesolowski and Fukuhara 1979; Leehey et al. 1995).
To validate the second hypothesis, we compared the in vitro tRNA:Ψ27-synthase activities of extracts from the WT, Δpus1, Δpus2, and Δpus1/Δpus2 cells on a mitochondrial tRNA known to be modified at position 27 (tRNAHis[GUG]) and on a cytoplasmic tRNA that has Ψ residues at both positions 27 and 55 (tRNAVal[UAC]). The analysis was performed using the nearest neighbor approach. To this end, the mitochondrial tRNAHis(GUG) and the cytoplasmic tRNAVal(UAC) were labeled by [α-32P]ATP and [α-32P]CTP incorporation, respectively. The results obtained are given in Table 1. Formation of Ψ residues were detected at positions 27 and 55 in the cytoplasmic tRNAVal(UAC) and at position 27 in the mitochondrial tRNAHis(GUG), after incubation with the WT and the Δpus2 cell extracts. In contrast, residue Ψ27 was not formed in any of these two tRNAs after incubation in both the Δpus1 and the Δpus1/Δpus2 cell extracts. The following interpretation can be proposed: (1) Pus1p in the extract has an intrinsic capability of modifying mitochondrial as well as cytoplasmic tRNAs, and (2) the absence of tRNA:Ψ27-synthase activity on both the cytoplasmic and the mitochondrial tRNA transcripts in the Δpus1 extract is due to the absence of Pus2p in the cellular extract.
The tight association of Pus2p with the insoluble fraction of disrupted yeast cells, which probably contain the mitochondria, can explain its almost complete loss during preparation of cell-free extract and the observed absence of Pus2p activity in these extracts. Indeed, by expression of an HA-tagged Pus2p in the S. cerevisiae strain 26-G-7 (Open BioSystems), we showed by Western Blot analysis that the S10 cell-free soluble extract is devoid of HA-tagged Pus2p (Fig. 4B).
Taken together, our results show that Pus1p is able to modify both the cytoplasmic and mitochondrial tRNAs in vitro. However, it has a strict cytoplasmic/nuclear localization and therefore only modifies cytoplasmic tRNAs in vivo. In contrast, Pus2p is very rapidly targeted to mitochondria and is not present in a yeast soluble cell fraction. This rapid transport to mitochondria likely explains its absence of activity on cytoplasmic tRNAs.
Nonconventional mitochondrial localization signal in Pus2p
Taken together, our data suggested that Pus2p is efficiently targeted to mitochondria after its synthesis. This was rather surprising since no clear mitochondrial signal peptide was found at the N-terminus of Pus2p. Moreover, among the S. cerevisiae RNA:Ψ-synthases acting in mitochondria (Pus3p, Pus4p, Pus5p, and Pus9p), only Ydl036p (Pus9p) has a characteristic N-terminal targeting signal (Mason 1998; Behm-Ansmant et al. 2004). All other mitochondrial yeast tRNA:Ψ-synthases, although efficiently transported to this compartment, have no characteristic N-terminal signals (Becker et al. 1997; Lecointe et al. 1998; Ansmant et al. 2000, 2001). Nevertheless, based on their amino acid sequences, all of them are predicted to have a predominant mitochondrial localization by the PSORTII software.
On the other hand, a new mechanism was recently found to allow the efficient targeting of proteins without characteristic N-terminal signals to mitochondria. It is based on the presence of a sequence element in the 3′-UTR of mRNAs that allows mRNA concentration and translation in close vicinity of mitochondria (for review, see Jensen and Johnson 2001; Tekotte and Davis 2002). We can exclude the utilization of this mechanism for rapid transport of Pus2p to mitochondria. Indeed, the PUS2 ORF that we expressed in the Δpus2 cells was followed by a foreign 260-base pair (bp) DNA sequence (the CYC1-terminator of p416GalS plasmid), instead of the authentic 3′-UTR of the PUS2 gene. Despite this, the expressed Pus2p efficiently complemented the mitochondrial tRNA:Ψ27-synthase activity in Δpus2 strain. This indicates that Pus2p probably uses another mechanism for efficient transport into mitochondria.
Two distinct RNA:Ψ-synthases act at positions 27 and 28 in tRNAs
Based on several previous studies, up to now it has been accepted that the modification of a given position in tRNAs is ensured by the product(s) of a unique gene in both the cytoplasmic and mitochondrial compartments (Martin and Hopper 1994; Rose et al. 1995; Becker et al. 1997; Lecointe et al. 1998; Ansmant et al. 2001). Either a unique protein is expressed from the gene and it has a dual cytoplasmic and mitochondrial localization (Becker et al. 1997; Lecointe et al. 1998; Ansmant et al. 2001), or two distinct enzymes differing by the presence of an N-terminal mitochondrial targeting signal in one of them are produced from the unique gene by the alternative utilization of AUG initiation codons (Martin and Hopper 1994; Rose et al. 1995). The only reported exception is the Rib2p/Pus8p and Pus9p pair of tRNA:Ψ-synthases: Rib2p/Pus8p is a cytoplasmic tRNA:Ψ32-synthase, while Pus9p can act both in the cytoplasm and mitochondrial compartments with a predominant activity in mitochondria (Behm-Ansmant et al. 2004). Here we show that another pair of RNA:Ψ-synthases is involved in the modification of tRNA positions 27 and 28. Nevertheless, the situation is quite different from the Rib2p/Pus8p-Pus9p pair, since the Pus1p activity is strictly cytoplasmic, while Pus2p activity is strictly mitochondrial.
One possible explanation for the presence of a specific mitochondrial enzyme in addition to Pus1p may be a preferential localization of Pus1p in the nucleus, where it modifies the tRNAIle(UAU) precursor (Simos et al. 1996; Motorin et al. 1998) and U2 snRNA (Massenet et al. 1999). In addition, Pus1p participates in tRNA export from the nucleus in the cytoplasm (Simos et al. 1996; Grosshans et al. 2001). These various functions of Pus1p may be incompatible with its additional mitochondrial localization and may explain the necessity of a dedicated mitochondrial enzyme with a similar substrate specificity (Pus2p).
Although conserved in yeast, the PUS1 and PUS2 genes may be deleted without marked growth phenotype
Another question is to know whether the existence of two redundant tRNA:Ψ-synthases with different sites of action is evolutionarily conserved. A search in genomic databanks of genes that may encode Pus1p and Pus2p homologs revealed the occurrence of genes encoding these two proteins only in yeasts that belong to the Saccharomycetoïdaea and the Schizosaccharomycetoïdaea families. They have a strong similarity with both Pus1p and Pus2p. However, only one gene that may encode a Pus1p/Pus2p homolog was found in vertebrates and other eukaryotes (Chen and Patton 1999, 2000). In relation to this observation, it must be pointed out that in yeasts, modification at position 44 in U2 snRNA is catalyzed by Pus1p (Massenet et al. 1999), whereas a scaRNA guided system is used in vertebrates (Ma et al. 2005). The emergence of the scaRNA guided modification system for UsnRNAs probably had a strong influence on the activity of members of the RNA:Ψ-synthase families. The striking similarity between Pus1p and Pus2p suggests that the presence of two distinct enzymes may have arisen from gene duplication, followed by acquisition of the appropriate subcellular targeting signals.
Despite our observation of nonoverlapping functions of Pus1p and Pus2p, individual and even simultaneous disruptions of their genes had no marked effect on cell viability and growth, even in growth conditions allowing detection of mitochondrial respiratory defects (medium with glycerol as the only carbon source, data not shown). The absence of marked growth phenotype is a rather general property of strains carrying disruption in a gene encoding an RNA modification enzyme. When growth phenotypes were detected, they were almost always due to the lack of an additional protein function distinct from the RNA modification activity.
MATERIALS AND METHODS
Yeast strains
The haploid S. cerevisiae BY4742 strain (denoted WT in this article) and the haploid strain carrying a disruption of the PUS2 gene (pus2Δ∷Kan r, denoted Δpus2), were obtained from the EUROSCARF collection (Germany). The haploid Δpus1 yeast strain was a generous gift of G. Simos (University of Larissa, Larissa, Greece). It was obtained by interruption of the PUS1 gene by the HIS3 auxotrophic marker (pus1Δ∷HIS3) in the genomic DNA of the RS453 S. cerevisiae strain (Simos et al. 1996). Yeast S. cerevisiae strain 26-G-7 expressing 3× HA-tagged Pus2p (insertion position 312) was obtained from Open BioSystems. A haploid strain with simultaneous deletions of the PUS1 and PUS2 genes was generated by mating of the pus2Δ∷Kan r and the pus1Δ∷HIS3 strains. After sporulation of the obtained diploid mutants, asci were separated by microdissection.
Complementation of the disrupted strains
To generate a S. cerevisiae plasmid expressing Pus2p, the coding region of the PUS2 ORF together with the upstream 300-bp region were amplified from the genomic DNA of the S. cerevisiae BY4742 strain. The oligonucleotides used generated a NheI and an EcoRI restriction site at the 5′- and 3′-extremities, respectively. The amplified DNA fragment was inserted at the SmaI site of plasmid pUC18. The resulting construct (pUC18-PUS2) was cleaved by the NheI and EcoRI restriction endonucleases, and the fragment encoding Pus2p was inserted between the XbaI and EcoRI sites of plasmid p416GalS (Mumberg et al. 1994). A point mutation in the putative RNA:Ψ-synthase active site of Pus2p was introduced by PCR-mediated site-directed mutagenesis (D56A, GAC->GCC). All the recombinant genes produced were entirely sequenced. Yeast transformation was performed with the standard lithium acetate procedure (Gietz et al. 1992; Adams et al. 1997).
CMCT/RT mapping of Ψ residues in RNA
Preparation of total RNA from yeast strains and CMCT-RT mapping of Ψ residues were performed as described previously (Bakin and Ofengand 1993; Massenet et al. 1999; Ansmant et al. 2000). The presence of residue Ψ27 in mitochondrial tRNAArg(UCU), Ψ28 in mitochondrial tRNALys(UUU), and Ψ27 in cytoplasmic tRNAMet(CAU) was analyzed using oligonucleotides complementary to residues 46–62, residues 43–72, and residues 40–57, respectively.
Analysis of Ψ residue formation by the nearest neighbor approach
The coding sequences of the mitochondrial tRNAHis(GUG) and tRNAMeti(CAU) were PCR amplified from the genomic DNA of the BY4742 S. cerevisiae strain and inserted downstream from a T7 RNA polymerase promoter at the SmaI site of plasmid pUC18. The pTFM-Val plasmid used for in vitro transcription of cytoplasmic tRNAVal(UAC) was kindly provided by C. Florentz (IBMC, Strasbourg, France). Cell-free S10 extracts preparation and tests of their in vitro RNA:Ψ-synthase activities were performed as previously described (Auxilien et al. 1996; Behm-Ansmant et al. 2003).
Sequence analysis
The NNPSL software (Reinhardt and Hubbard 1998, http://predict.sanger.ac.uk/nnpsl/, Web site) was used for prediction of the Pus2p subcellular localization. This program takes into account the amino acid composition of the whole protein, its predicted secondary structure, and various characteristic features, such as the presence of a mitochondrial targeting sequence or of a potential signal peptidase cleavage site.
ACKNOWLEDGMENTS
We thank H. Grosjean (LEBS, Gif-sur-Yvette, France) for helpful discussions, R. Giegé and C. Florentz (CNRS, Strasbourg, France) for giving us the plasmid containing the yeast tRNAVal(UAC) gene, A. Lescure (CNRS, Strasbourg, France) for help in yeast dissection, and G. Simos (University of Larissa, Larissa, Greece) for the gift of the pus1Δ∷HIS3 yeast strain and helpful discussions. This work was supported by laboratory funds from the “Ministère de l'Éducation Nationale, de l'Enseignement Supérieur et de la Recherche” (MENESR) and the French “Centre National de la Recherche Scientifique”. I.B.-A. was a predoctoral fellow supported by a fellowship from the MENESR.
Footnotes
Abbreviations: 2D, bidimensional; CMCT, 1-cyclohexyl-3-[2-morpholinoethyl] carbodiimide metho-p-toluenesulfonate; NMP, nucleotide monophosphate; Ψ, pseudouridine; ORF, open reading frame; PCR, polymerase chain reaction; RT, reverse transcriptase; TLC, thin layer chromatography; U, uridine; WT, wild-type.
Article published online ahead of print. Article and publication date are at http://www.rnajournal.org/cgi/doi/10.1261/rna.605607.
REFERENCES
- Adams, A., Gottschling, D.E., Kaiser, C.A., Stearns, T. Methods in yeast genetics. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1997. [Google Scholar]
- Ansmant, I., Massenet, S., Grosjean, H., Motorin, Y., Branlant, C. Identification of the Saccharomyces cerevisiae RNA:pseudouridine synthase responsible for formation of Ψ2819 in 21S mitochondrial ribosomal RNA. Nucleic Acids Res. 2000;28:1941–1946. doi: 10.1093/nar/28.9.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ansmant, I., Motorin, Y., Massenet, S., Grosjean, H., Branlant, C. Identification and characterization of the tRNA:Ψ31-synthase (Pus6p) of Saccharomyces cerevisiae . J. Biol. Chem. 2001;276:34934–34940. doi: 10.1074/jbc.M103131200. [DOI] [PubMed] [Google Scholar]
- Auxilien, S., Crain, P.F., Trewyn, R.W., Grosjean, H. Mechanism, specificity and general properties of the yeast enzyme catalysing the formation of inosine 34 in the anticodon of transfer RNA. J. Mol. Biol. 1996;262:437–458. doi: 10.1006/jmbi.1996.0527. [DOI] [PubMed] [Google Scholar]
- Bakin, A., Ofengand, J. Four newly located pseudouridylate residues in Escherichia coli 23S ribosomal RNA are all at the peptidyltransferase center—Analysis by the application of a new sequencing technique. Biochemistry. 1993;32:9754–9762. doi: 10.1021/bi00088a030. [DOI] [PubMed] [Google Scholar]
- Becker, H.F., Motorin, Y., Planta, R.J., Grosjean, H. The yeast gene YNL292w encodes a pseudouridine synthase (Pus4) catalysing the formation of Ψ55 in both mitochondrial and cytoplasmic tRNAs. Nucl. Acids Res. 1997;25:4493–4499. doi: 10.1093/nar/25.22.4493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behm-Ansmant, I., Urban, A., Ma, X., Yu, Y.T., Motorin, Y., Branlant, C. The Saccharomyces cerevisiae U2 snRNA:pseudouridine-synthase Pus7p is a novel multisite-multisubstrate RNA:Ψ-synthase also acting on tRNAs. RNA. 2003;9:1371–1382. doi: 10.1261/rna.5520403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behm-Ansmant, I., Grosjean, H., Massenet, S., Motorin, Y., Branlant, C. Pseudouridylation at position 32 of mitochondrial and cytoplasmic tRNAs requires two distinct enzymes in Saccharomyces cerevisiae . J. Biol. Chem. 2004;279:52998–53006. doi: 10.1074/jbc.M409581200. [DOI] [PubMed] [Google Scholar]
- Behm-Ansmant, I., Massenet, S., Immel, F., Patton, J.R., Motorin, Y., Branlant, C. A previously unidentified activity of yeast and mouse RNA:pseudouridine synthases 1 (Pus1p) on tRNAs. RNA. 2006;12:1583–1593. doi: 10.1261/rna.100806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J., Patton, J.R. Cloning and characterization of a mammalian pseudouridine synthase. RNA. 1999;5:409–419. doi: 10.1017/s1355838299981591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J., Patton, J.R. Pseudouridine synthase 3 from mouse modifies the anticodon loop of tRNA. Biochemistry. 2000;39:12723–12730. doi: 10.1021/bi001109m. [DOI] [PubMed] [Google Scholar]
- Conrad, J., Sun, D., Englund, N., Ofengand, J. The rluC gene of Escherichia coli codes for a pseudouridine synthase that is solely responsible for synthesis of pseudouridine at positions 955, 2504, and 2580 in 23 S ribosomal RNA. J. Biol. Chem. 1998;273:18562–18566. doi: 10.1074/jbc.273.29.18562. [DOI] [PubMed] [Google Scholar]
- Del Campo, M., Ofengand, J., Malhotra, A. Crystal structure of the catalytic domain of RluD, the only rRNA pseudouridine synthase required for normal growth of Escherichia coli . RNA. 2004;10:231–239. doi: 10.1261/rna.5187404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gietz, D., St. Jean, A., Woods, R.A., Schiestl, R.H. Improved method for high efficiency transformation of intact yeast cells. Nucleic Acids Res. 1992;20:1425. doi: 10.1093/nar/20.6.1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosshans, H., Lecointe, F., Grosjean, H., Hurt, E., Simos, G. Pus1p-dependent tRNA pseudouridinylation becomes essential when tRNA biogenesis is compromised in yeast. J. Biol. Chem. 2001;276:46333–46339. doi: 10.1074/jbc.M107141200. [DOI] [PubMed] [Google Scholar]
- Huang, L., Pookanjanatavip, M., Santi, D.V. Catalytic mechanism of RNA pseudouridine synthase. FASEB J. 1997;11:A1320. [Google Scholar]
- Jensen, R.E., Johnson, A.E. Opening the door to mitochondrial protein import. Nat. Struct. Biol. 2001;8:1008–1010. doi: 10.1038/nsb1201-1008. [DOI] [PubMed] [Google Scholar]
- Kammen, H.O., Marvel, C.C., Hardy, L., Penhoet, E.E. Purification, structure, and properties of Escherichia coli tRNA pseudouridine synthase I. J. Biol. Chem. 1988;263:2255–2263. [PubMed] [Google Scholar]
- Kaya, Y., Ofengand, J. A novel unanticipated type of pseudouridine synthase with homologs in bacteria, archaea, and eukarya. RNA. 2003;9:711–721. doi: 10.1261/rna.5230603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiss, A.M., Jady, B.E., Bertrand, E., Kiss, T. Human box H/ACA pseudouridylation guide RNA machinery. Mol. Cell. Biol. 2004;24:5797–5807. doi: 10.1128/MCB.24.13.5797-5807.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin, E.V. Pseudouridine synthases: four families of enzymes containing a putative uridine-binding motif also conserved in dUTPases and dCTP deaminases. Nucleic Acids Res. 1996;24:2411–2415. doi: 10.1093/nar/24.12.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lafontaine, D.L.J., Bousquet-Antonelli, C., Henry, Y., Caizergues-Ferrer, M., Tollervey, D. The box H+ACA snoRNAs carry Cbf5p, the putative rRNA pseudouridine synthase. Genes & Dev. 1998;12:527–537. doi: 10.1101/gad.12.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecointe, F., Simos, G., Sauer, A., Hurt, E.C., Motorin, Y., Grosjean, H. Characterization of yeast protein Deg1 as Pseudouridine synthase (Pus 3) catalysing the formation of Ψ38 and Ψ39 in tRNA anticodon loop. J. Biol. Chem. 1998;273:1316–1323. doi: 10.1074/jbc.273.3.1316. [DOI] [PubMed] [Google Scholar]
- Leehey, M.A., Squassoni, C.A., Friederich, M.W., Mills, J.B., Hagerman, P.J. A noncanonical tertiary conformation of a human mitochondrial transfer RNA. Biochemistry. 1995;34:16235–16239. doi: 10.1021/bi00050a001. [DOI] [PubMed] [Google Scholar]
- Ma, X., Zhao, X., Yu, Y.T. Pseudouridylation (Ψ) of U2 snRNA in S. cerevisiae is catalyzed by an RNA-independent mechanism. EMBO J. 2003;22:1889–1897. doi: 10.1093/emboj/cdg191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma, X., Yang, C., Alexandrov, A., Grayhack, E.J., Behm-Ansmant, I., Yu, Y.T. Pseudouridylation of yeast U2 snRNA is catalyzed by either an RNA-guided or RNA-independent mechanism. EMBO J. 2005;24:2403–2413. doi: 10.1038/sj.emboj.7600718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin, N.C., Hopper, A.K. How single genes provide tRNA processing enzymes to mitochondria, nuclei, and the cytosol. Biochimie. 1994;76:1161–1167. doi: 10.1016/0300-9084(94)90045-0. [DOI] [PubMed] [Google Scholar]
- Mason, T.L. Functional aspects of the three modified nucleotides in yeast mitochondrial large-subunit rRNA. In: Grosjean H., Benne R., editors. The modification and editing of RNA. ASM Press; Washington, DC: 1998. pp. 273–280. [Google Scholar]
- Massenet, S., Motorin, Y., Lafontaine, D.L.J., Hurt, E.C., Grosjean, H., Branlant, C. Pseudouridine mapping in the Saccharomyces cerevisiae spliceosomal UsnRNAs reveals that pseudouridine synthase Pus1p exhibits a dual substrate specificity for U2 snRNA and tRNA. Mol. Cell. Biol. 1999;19:2142–2154. doi: 10.1128/mcb.19.3.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motorin, Y., Keith, G., Simon, C., Foiret, D., Simos, G., Hurt, E., Grosjean, H. The yeast tRNA:pseudouridine synthase Pus1p displays a multisite substrate specificity. RNA. 1998;4:856–869. doi: 10.1017/s1355838298980396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mumberg, D., Muller, R., Funk, M. Regulatable promoters of Saccharomyces cerevisiae: Comparison of transcriptional activity and their use for heterologous expression. Nucl. Acids Res. 1994;22:5767–5768. doi: 10.1093/nar/22.25.5767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton, J.R., Bykhovskaya, Y., Mengesha, E., Bertolotto, C., Fischel-Ghodsian, N. Mitochondrial myopathy and sideroblastic anemia (MLASA): Missense mutation in the pseudouridine synthase 1 (PUS1) gene is associated with the loss of tRNA pseudouridylation. J. Biol. Chem. 2005;280:19823–19828. doi: 10.1074/jbc.M500216200. [DOI] [PubMed] [Google Scholar]
- Reinhardt, A., Hubbard, T. Using neural networks for prediction of the subcellular location of proteins. Nucleic Acids Res. 1998;26:2230–2236. doi: 10.1093/nar/26.9.2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose, A.M., Belford, H.G., Shen, W.C., Greer, C.L., Hopper, A.K., Martin, N.C. Location of N 2,N 2-dimethylguanosine-specific tRNA methyltransferase. Biochimie. 1995;77:45–53. doi: 10.1016/0300-9084(96)88103-x. [DOI] [PubMed] [Google Scholar]
- Simos, G., Tekotte, H., Grosjean, H., Segref, A., Sharma, K., Tollervey, D., Hurt, E.C. Nuclear pore proteins are involved in the biogenesis of functional tRNA. EMBO J. 1996;15:2270–2284. [PMC free article] [PubMed] [Google Scholar]
- Tekotte, H., Davis, I. Intracellular mRNA localization: Motors move messages. Trends Genet. 2002;18:636–642. doi: 10.1016/s0168-9525(02)02819-6. [DOI] [PubMed] [Google Scholar]
- Wesolowski, M., Fukuhara, H. The genetic map of transfer RNA genes of yeast mitochondria: Correction and extension. Mol. Gen. Genet. 1979;170:261–275. doi: 10.1007/BF00267059. [DOI] [PubMed] [Google Scholar]