Abstract
Research on anhedonia in schizophrenia has revealed mixed results, with patients reporting greater anhedonia than healthy controls on self-report measures and semi-structured interviews, but also reporting comparable experiences of positive emotions in response to pleasurable stimuli. Basic science points to the importance of distinguishing between anticipatory and consummatory (or in-the-moment) pleasure experiences, and this distinction may help to reconcile the mixed findings on anhedonia in schizophrenia. In two studies, we tested the hypothesis that anhedonia in schizophrenia reflects a deficit in anticipatory pleasure but not consummatory pleasure. In Study 1, we used experience sampling methodology to assess reported experiences of consummatory and anticipated pleasure among schizophrenia patients and controls. In Study 2, schizophrenia patients and controls completed a self-report trait measure of anticipatory and consummatory pleasure and interviews that assessed negative symptoms, including anhedonia, and community functioning. In both studies, we found evidence for an anticipatory but not a consummatory pleasure deficit in schizophrenia. In addition, anticipatory pleasure was related to clinical ratings of anhedonia and functional outcome. Clinical and research implications of these findings are discussed.
Keywords: schizophrenia, pleasure, anhedonia, anticipation, consummatory, TEPS
1.1 Introduction
Although anhedonia, defined as an inability to experience pleasure, has long been considered a core feature of schizophrenia, recent research raises fundamental questions about the nature of this emotional disturbance. On the one hand, patients report experiencing lower levels of pleasure than controls on self-report trait measures and in semi-structured interviews (see Horan, Kring, & Blanchard, 2006b for a review). On the other hand, patients report experiencing as much pleasant emotion as controls in response to emotionally evocative stimuli (e.g., Berenbaum & Oltmanns, 1992; Kring & Earnst, 1999; Kring & Neale, 1996).
In an effort to reconcile these findings, we have proposed that the nature of the anhedonia deficit in schizophrenia is more circumscribed (Germans & Kring, 2000; Kring, 1999). Drawing on neurobehavioral models that distinguish between components of hedonic experience (e.g., Berridge & Robinson, 2003; Depue & Collins, 1999; Gard, Germans Gard, Kring, & John, 2006; Knutson, Adams, Fong, & Hommer, 2001), we proposed that schizophrenia patients experience normal levels of pleasure when directly engaged in an enjoyable activity, or consummatory pleasure, but experience disturbances in the experience of pleasure related to future activities, or anticipatory pleasure. These aspects of hedonic experience have distinguishable neural circuitry, neurotransmitter involvement, and behavioral sequelae. Anticipatory pleasure appears to rely heavily, though not exclusively, on dopamine and the mesolimbic pathway whereas serotonergic and opioid systems appear to be more centrally involved in consummatory pleasure (Berridge & Robinson, 1998; Schultz, 2002; Wise, 2002). Anticipatory pleasure can be further parsed into two components: (1) predicting the future experience of pleasure, and (2) the concurrent experience of pleasure knowing that a future activity is going to occur - that is, the pleasure experienced in anticipation of things to come. In addition, anticipatory pleasure is linked to motivational processes that promote goal-directed behaviors aimed at achieving desired rewards (Carver, 2001; Dickinson & Balleine, 1995; Schultz, 2002). Thus, to the extent that schizophrenia patients exhibit deficits in anticipatory pleasure, we would expect this to be linked to a decrement in goal-directed behavior.
In the current studies, we used convergent methods to evaluate the hypothesis that schizophrenia patients have an anticipatory but not consummatory pleasure deficit. In Study 1, we examined daily reports of anticipatory and consummatory pleasure in schizophrenia patients and healthy controls over the course of one week. Using the experience sampling method, we assessed participants’ ability to predict future pleasure from both goal-directed and non-goal-directed activities. In Study 2, we administered a battery of self-report and interview-based measures, including a trait measure of anticipatory and consummatory pleasure, to schizophrenia patients and healthy controls. The trait measure used in Study 2 assesses the experience of pleasure in anticipation of future events. To further establish the validity of this pleasure distinction in schizophrenia, we examined the correlates of anticipatory and consummatory pleasure in schizophrenia patients, including other measures of anhedonia, approach motivation, and functional outcome.
2.1 Study 1: Method
2.1.1 Participants
Outpatients with either schizophrenia (n = 10) or schizoaffective disorder (n = 5) participated. Diagnoses were confirmed using the Structured Clinical Interview for DSM-IV (First, Spitzer, Gibbon, & Williams, 1994). Individuals were excluded from the study if they reported current alcohol or drug abuse, a history of head trauma or loss of consciousness, poor fluency in English, or an active mood episode. All patients were taking medication, either typical (n = 10) or atypical (n = 5) antipsychotics1. Healthy controls (n = 12) were recruited via flyers in the community and were screened with a phone interview. Controls were excluded if they reported a personal or family history of mental illness or psychiatric hospitalization, past head trauma or loss of consciousness, current substance abuse or dependence, English fluency problems, or prescription medication that might have affected mood. All participants were paid $50.00 for their participation. This study received IRB approval for all procedures, and all participants gave written informed consent to participate.
Patients and controls did not significantly differ in age t(25) = 1.81, ns, education t(25) = .12, ns, gender χ2(1, N=27) = 1.69, ns, ethnicity χ2(3, N=27) = 2.13, ns, marital status χ2(3, N=27) = 6.63, ns, or employment status χ2(2, N=27) = 4.33, ns (see Table 1).
Table 1.
Demographic and Clinical Characteristics.
| Study 1
|
Study 2
|
|||
|---|---|---|---|---|
| Patient N=15 | Control N=12 | Patient N=51 | Control N=50 | |
| M(SD) | M(SD) | M(SD) | M(SD) | |
| Age | 42.67 (8.33) | 36.33 (9.85) | 48.06 (9.61) | 46.62 (10.27) |
| Education (years) | 12.5(1.96) | 12.42 (1.68) | 13.14 (2.00) | 13.35 (1.02) |
| Gender | N | N | N | N |
| Male | 10 | 5 | 33 | 25 |
| Female | 5 | 7 | 18 | 25 |
| Ethnicity | ||||
| African American | 5 | 4 | 19 | 17 |
| Asian American | 1 | 1 | 3 | 1 |
| Latino | 1 | 3 | 5 | 3 |
| White/Caucasian | 8 | 4 | 23 | 26 |
| Other | 0 | 0 | 1 | 3 |
| Marital Status | ||||
| Never Married | 12 | 5 | 25 | 28 |
| Married | 0 | 3 | 6 | 10 |
| Divorced | 3 | 3 | 19 | 12 |
| Widowed | 0 | 1 | 0 | 0 |
| Diagnosis | ||||
| Schizoaffective | 5 | N/A | 1 | N/A |
| Schizophrenia | 10 | 50 | ||
| Medication Status | ||||
| Typical antipsychotics | 10 | N/A | 8 | N/A |
| Atypical antipsychotics | 5 | 31 | ||
| Both typicals and atypicals | 0 | 8 | ||
| No medication | 0 | 2 | ||
2.1.2 Measures and Procedure
We followed typical experience sampling study procedures by using pagers and booklets for participants to enter information regarding their daily experience. Participants were paged seven times a day for seven days (49 observations per participant). The times of the pages were pseudo-random between 8 a.m. and 10 p.m. such that no two pages occurred within 45 minutes of each other and that pages occurred at least once every 3 hours. Once paged, participants wrote down what they were doing and rated the amount of enjoyment they were experiencing (i.e., their consummatory pleasure) using a 5 point Likert scale (0 = not at all to 5 = very much). Participants rated anticipatory pleasure by indicating what they were looking forward to doing (no time frame was indicated) and the amount of enjoyment they anticipated they would get out of that activity using the same Likert scale. For current and ‘looked forward to’ activities, participants chose from a list of activities that included “nothing.” Responses made more than 15 minutes after a page (less than 2% for each group) were not included in the final analyses given the evidence that such responses are less reliable than earlier responses (e.g., deVries, 1992).
Two undergraduate research assistants coded the reported activities into ‘goal-directed’ and ‘non-goal-directed’ categories, and agreement was nearly 100 percent. Goal-directed activities were part of pursuing a goal (making dinner, doing an errand, en route somewhere, working or studying). Non-goal-directed activities were not directly related to going after a larger goal (eating dinner, watching TV, smoking, sleeping).
2.2 Study 1: Results and Discussion
Data were analyzed using hierarchical linear modeling (HLM; e.g., Bryk & Raudenbush, 1992; Schwartz & Stone, 1998). HLM treats the multiple observations gathered for each participant (enjoyment ratings or reported activities) as ‘Level 1’ data (within subjects) that are nested within individuals at ‘Level 2’ (between subjects variable of group: patient or control). Separate models were estimated for current and ‘looking forward to’ activities, consummatory pleasure, and anticipatory pleasure.
Patients and controls did not significantly differ with respect to the number of pages they responded to, with patients responding to 89.4 percent (SD= 6.6), and controls responding to 86.4 percent (SD=9.0) of pages. Patients and healthy controls did not significantly differ in the amount of time spent engaging in or looking forward to non-goal-directed activities ts (25) = 1.45 & 0.46, ns. However, patients (17 percent) were engaged in goal-directed activities less often than controls (35.7 percent) t (25) = 3.12, p < .01, and patients reported looking forward to goal-directed activities less often than controls (4.8% versus 15.1%) t(25) = 2.71, p<.01. As predicted and shown in Figure 1, patients did not significantly differ from controls in their reported consummatory pleasure. This was true for both non-goal-directed, t (25) = 0.74, ns, and goal-directed activities, t (25) = 1.45, ns. Hence, compared with healthy controls the patients reported similar levels of pleasure from goal-directed activities, but did them less often.
Figure 1.
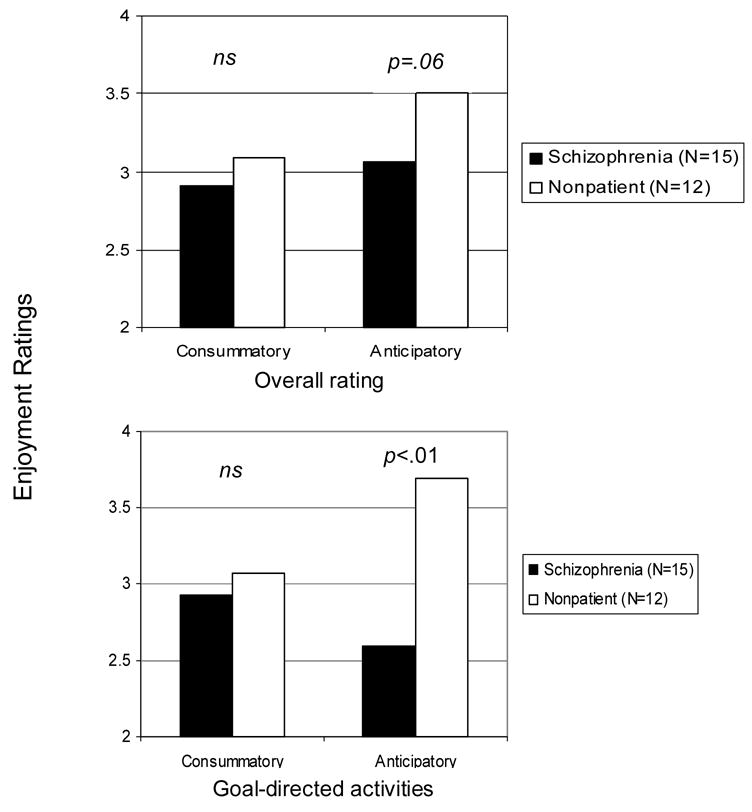
Ratings of anticipatory and consummatory pleasure for schizophrenia patient and healthy control participants for Study 1.
Also shown in Figure 1 is the expected finding that patients reported anticipating less pleasure from future activities compared to controls t(25) = 1.95, p=.06. Moreover, patients reported significantly less anticipatory pleasure for goal-directed activities than controls t(25) = 3.92, p<.01, suggesting a link between anticipatory pleasure of motivated behavior. Patients did not differ from controls in their anticipatory pleasure for non-goal-directed activities.
Consistent with our hypothesis, schizophrenia patients exhibited a deficit in anticipatory pleasure, particularly for goal-directed activities, but did not exhibit a deficit in consummatory pleasure. Furthermore, schizophrenia patients reported engaging in and looking forward to fewer goal-directed activities in daily life than controls. Despite the large number of observations per participant, the small sample size limits our ability to make stronger conclusions based on these data. In Study 2, we include more patients and controls to increase the generalizability of the findings.
3.1 Study 2
In Study 2, participants completed the Temporal Experience of Pleasure Scale (TEPS), a trait measure of pleasure in anticipation and consummatory pleasure (Gard et al., 2006), which measures pleasure experienced in the moment and in anticipation of future activities. We hypothesized that patients would score lower than controls on the anticipatory scale but not the consummatory scale. In addition, we investigated the correlates of anticipatory and consummatory pleasure. We hypothesized that anticipatory pleasure, but not consummatory pleasure would be related to other interview and self-report measures of anhedonia. We expected that decrements in anticipatory pleasure would be linked to decrements in motivational processes. In addition, we examined the relationship between anticipatory and consummatory pleasure and functional outcome in schizophrenia, predicting that a deficit in anticipatory pleasure may have important functional consequences for patients with schizophrenia.
3.2 Study 2: Method
3.2.1 Participants
Participants were recruited from UC Berkeley and the Greater Los Angeles VA. Patients from UC Berkeley were diagnosed with either schizophrenia (n = 21) or schizoaffective disorder (n = 1) and did not have current alcohol or drug abuse, a history of head trauma or loss of consciousness, poor fluency in English, or an active mood episode. Patients from the VA were diagnosed with schizophrenia (n = 29) and did not have current alcohol or drug abuse, identifiable neurological disorder, mental retardation, or seizure disorder. (For additional details on participant selection at the Greater Los Angeles VA, see Horan, Green, Kring, & Nuechterlein, 2006a). At both sites, diagnoses were confirmed using the SCID-IV. Two patients were not taking antipsychotic medication, 8 were taking typical, 31 atypical, and 8 a combination of typical and atypical antipsychotics. Medication information was unavailable for two patients. Healthy controls from UC Berkeley (n = 19) were recruited from the community using the same criteria as in Study 1. Controls from the VA (n = 31) were recruited from the community through notices placed in newspapers, flyers, and websites and were screened with the SCID and SCID–II (First, Gibbon, Spitzer, Williams, & Benjamin, 1996). Controls were excluded if they had a first-degree relative with a psychotic disorder, were currently abusing drugs or alcohol, had a neurological or seizure disorder, mental retardation, mood disorder, borderline, avoidant, or any Cluster A personality disorder. There were no differences between the Berkeley and VA samples for either patients or controls in terms of age, years of education, ethnicity, marital status, patient medication status (i.e., number of patients taking typical or atypical antipsychotics), or gender, except that there were more women controls in the Berkeley sample than the VA sample. This study received IRB approval at both institutions for all procedures, and all participants gave written informed consent to participate.
As shown in Table 1, patients and controls did not significantly differ with respect to age t (98) = 0.72, ns, education t (98) = 0.66, ns, gender χ2 (2.23, N=101) = 1.69, ns, ethnicity χ2 (4, N=101) = 2.79, ns, or marital status χ2 (2, N=100) = 2.75, ns.
3.2.2 Measures and Procedure
All participants completed the Temporal Experience of Pleasure Scale (Gard et al., 2006), an 18-item self-report measure of trait anticipatory and consummatory pleasure. The TEPS has good internal consistency, test-retest reliability, and convergent and discriminant validity (Gard et al., 2006). All participants also completed the Behavioral Inhibition and Behavioral Activation Scales (BIS/BAS; Carver & White, 1994), a 24-item self-report scale that measures trait approach and avoidance motivation. The BIS scale measures sensitivity to punishment and non-reward. The BAS scale measures sensitivity to reward and goal-directed pursuits and contains three subscales: Drive, Fun Seeking, and Reward Responsiveness.
A subset of patients from the VA (n=31) completed the Revised Physical Anhedonia Scale (PAS; Chapman, Chapman, & Raulin, 1976), a 61-item scale of physical pleasure deficits, and the Revised Social Anhedonia Scale (SAS; Eckblad, Chapman, Chapman, & Mishlove, 1982), a 40-item scale of social pleasure deficits. Both scales have been used extensively in schizophrenia studies (Horan et al., 2006b). These patients were also rated for negative symptoms using the Schedule for the Assessment of Negative Symptoms (SANS; Andreasen, 1983) and for functioning in the areas of work productivity, independent living and self care, immediate family networks, and extended social networks using the Role Functioning Scales (RFS; Goodman, Sewell, Cooley, & Leavitt, 1993).
3.3 Study 2: Results and Discussion
Consistent with Study 1 and shown in Figure 2, patients did not significantly differ from controls on the TEPS consummatory scale, t (99) = 1.46, ns. However, patients reported significantly less anticipatory pleasure than controls t (99) = 2.97, p < .01.
Figure 2.
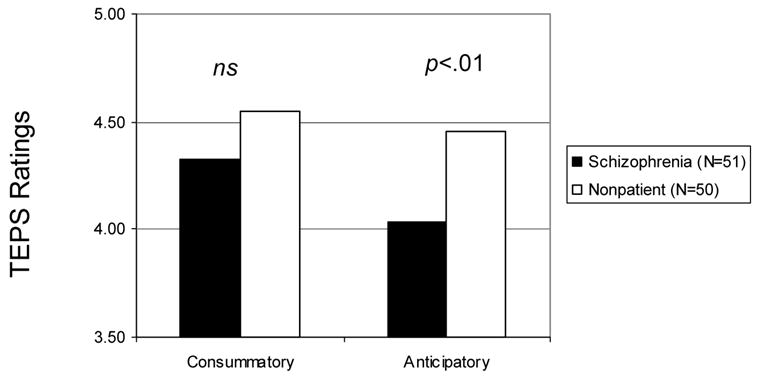
Ratings of anticipatory and consummatory pleasure on the Temporal Experience of Pleasure Scale for schizophrenia patient and healthy control participants for Study 2.
In order to indirectly examine medication influences, we examined TEPS scores based on the types of medications patients were taking. There were no differences in reports of anticipatory or consummatory pleasure between patients taking only typical antipsychotics and those taking only atypical antipsychotics, t’s (37) = 0.79 & 1.17, ns. Although this is not an optimal test of medication effects (cf. Blanchard & Neale, 1992), there were no marked differences among patients taking different types of medications.
The correlates of anticipatory and consummatory pleasure were examined among schizophrenia patients by computing correlations between the TEPS scales and the measures of motivation, anhedonia, and functional outcome (see Table 2). Trait motivational avoidance (BIS) was not significantly related to anticipatory or consummatory pleasure, consistent with previous research (Gard et al., 2006). As predicted, anticipatory pleasure (but not consummatory pleasure) was significantly positively correlated with scores on the BAS-Total scale as well as the drive and reward seeking subscales of the BAS, indicating that anticipatory pleasure was related to key aspects of approach motivation system sensitivity.
Table 2.
Correlates of Anticipatory and Consummatory Pleasure in Schizophrenia
| Measure | TEPS Anticipatory | TEPS Consummatory |
|---|---|---|
| BIS | .17 | .05 |
| BAS total | .42** | .23 |
| Fun-seeking | .19 | .09 |
| Reward Responsiveness | .41** | .18 |
| Drive | .29* | .23 |
| SANS Anhedonia | −.38** | −.17 |
| RFS | ||
| Social | .51** | .21 |
| Family | .50** | .21 |
| Independent Living | .19 | .11 |
| Work | .08 | .33 |
| Physical Anhedonia Scale | −.48** | −.62** |
| Social Anhedonia Scale | −.43** | −.08 |
Note: BIS = Behavioral Inhibition Scale; BAS = Behavioral Activation Scale; SANS = Schedule for the Assessment of Negative Symptoms; RFS = Role Functioning Scale.
indicates p < .01;
indicates p < .05.
Patients’ reports of anticipatory pleasure were also significantly correlated with physical and social anhedonia. In contrast, consummatory pleasure was significantly correlated with physical anhedonia, but not social anhedonia. Since both TEPS’ scales assess physical pleasure, it is not surprising that physical anhedonia was related with both TEPS subscales.
As predicted, SANS ratings of anhedonia were significantly correlated with patients’ reports of anticipatory pleasure, but not consummatory pleasure. Although the SANS asociality/anhedonia scale does not include an anticipatory pleasure rating, these ratings were nonetheless more sensitive to patients’ reports of anticipatory pleasure. Schizophrenia patients’ reports of anticipatory pleasure were also significantly correlated with ratings of family functioning and extended social networks, while reports of consummatory pleasure were unrelated to all four RFS subscales. Thus, anticipatory pleasure was selectively related to how well patients were functioning in their daily lives.
4. General Discussion
Across two studies using complementary methods, schizophrenia patients reported as much pleasure in the moment as controls in their daily lives and on a measure of trait consummatory pleasure. However, schizophrenia patients predicted that future daily life events would be less pleasurable, and they reported experiencing less pleasure in anticipation of future events compared to healthy controls. Further supporting the distinction between anticipatory and consummatory pleasure, clinical ratings of anhedonia were related to anticipatory, but not consummatory pleasure. In addition, ratings of functional outcome spanning family and social functioning were related to patients’ reports of anticipatory, but not consummatory pleasure.
These findings help provide clarity to the discrepancy between experimental studies of emotion and schizophrenia (where no clear pleasure deficits have been found) and self-report and interview studies (where pleasure deficits have been found). Schizophrenia patients may have a deficit in anticipating that things will be pleasurable, but once an enjoyable stimulus is before them, they can and do experience pleasure. This distinction may help uncover some of the mechanisms behind social and emotional functioning deficits in schizophrenia, and it may also lead to more targeted treatments of anhedonia. In Study 2, we found evidence for a linkage between the experience of anticipatory pleasure and family and social network functional outcomes. A deficit in the ability to experience anticipatory pleasure may contribute to interpersonal or social isolation, limited social engagement, and other impoverished environments, though such causal suggestions are clearly beyond the data presented here. Future studies should look more specifically to social anticipatory pleasure to clarify these relationships.
Given the connection between dopamine and anticipatory pleasure, it is possible that the observed anticipatory pleasure deficit in schizophrenia reflects a consequence of medication more than an aspect of the illness. However, other investigations have observed anhedonia among unmedicated patients, using both self-report measures (e.g., Arnfred & Chen, 2004; Gruzelier & Davis, 1995) and clinical ratings (Zhang, Peet, Ramchand, Shah, & Reynolds, 2001), and among first episode patients, many of whom were not taking medication (e.g., Malla et al., 2002). A recent fMRI investigation (Juckel, Schlagenhauf, Koslowski, Wustenberg et al., 2006) also found evidence for an anticipatory deficit among unmedicated patients as indexed by less activation than healthy controls in brain regions associated with reward anticipation (ventral striatum, including nucleus accumbens; cf Knutson et al., 2001) during presentation of reward cues. Furthermore, this reduced activation was associated with more severe negative symptoms, including anhedonia. In a second study using the same reward anticipation task, reduced activation in ventral striatum was found only among patients taking typical neuroleptics (Juckel, Schlagenhauf, Koslowski, Filonov et al., 2006). Most of the patients in Study 2 were taking atypical antipsychotics. In future studies it would be useful to study at risk groups or unmedicated individuals in the schizophrenia spectrum.
Although schizophrenia patients represent emotion in the same two-dimensional structure (valence, arousal) as healthy controls (e.g., Kring, Barrett, & Gard, 2003), demand characteristics may have influenced reports of pleasure. However, there is no reason to believe that demand characteristics would operate more strongly for consummatory pleasure than anticipatory pleasure, or for patients more than controls. Nonetheless, it will be important for future studies to assess anticipatory and consummatory pleasure with additional behavioral and physiological measures.
The anticipatory/consummatory pleasure distinction has been discussed in the depression literature (e.g., Klein, 1984), and cognitive therapy for depression has targeted anticipatory pleasure (e.g., Burns, 1999). Though it remains to be seen if this type of intervention would be beneficial in schizophrenia, such investigations are now underway (e.g., Beck & Rector, 2005; Turkington, Dudley, Warman, & Beck, 2004).
Acknowledgments
We would like to thank Laila Ansari, Phuong Chou, Matthew Goodman, and Barbara Stuart for their help with data entry and collection.
Footnotes
Portions of this research were presented at the annual meeting of the Society for Research in Psychopathology in October, 2000 in Boulder, Colorado and at the Society for Research in Psychopathology in November, 2003 in Toronto, Canada.
Given the small number of patients taking atypical neuroleptics in Study 1, we felt it imprudent to examine patients’ responses based on medication type or dosage. Indeed, the most optimal test for medication effects is to test the same patients both on and off medication (Blanchard & Neale, 1992).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andreasen NC. The scale for assessment of negative symptoms (SANS) Iowa City, IA: University Press; 1983. [Google Scholar]
- Arnfred SM, Chen ACN. Exploration of somatosensory P50 gating in schizophrenia spectrum patients: Reduced P50 amplitude correlates to social anhedonia. Psychiatry Research. 2004;125(2):147–159. doi: 10.1016/j.psychres.2003.12.008. [DOI] [PubMed] [Google Scholar]
- Beck AT, Rector NA. Cognitive Approaches To Schizophrenia: Theory And Therapy. Annual Review of Clinical Psychology. 2005;1(1):577–606. doi: 10.1146/annurev.clinpsy.1.102803.144205. [DOI] [PubMed] [Google Scholar]
- Berenbaum H, Oltmanns TF. Emotional experience and expression in schizophrenia and depression. Journal of Abnormal Psychology. 1992;101(1):37–44. doi: 10.1037//0021-843x.101.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res Brain Res Rev. 1998;28(3):309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE. Parsing reward. Trends Neurosci. 2003;26(9):507–513. doi: 10.1016/S0166-2236(03)00233-9. [DOI] [PubMed] [Google Scholar]
- Blanchard JJ, Neale JM. Medication status of participants in psychopathology research: Selective review of current reporting practices. Journal of Abnormal Psychology. 1992;101(4):732–734. doi: 10.1037//0021-843x.101.4.732. [DOI] [PubMed] [Google Scholar]
- Bryk AS, Raudenbush SW. Hierarchical linear models: Applications and data analysis methods. Thousand Oaks, CA: Sage Publications Inc; 1992. [Google Scholar]
- Burns DD. The feeling good handbook. rev. New York, NY: Plume/Penguin Books; 1999. [Google Scholar]
- Carver CS. Affect and the functional bases of behavior: On the dimensional structure of affective experience. Personality and Social Psychology Review. 2001;5(4):345–356. [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. Journal of Personality and Social Psychology. 1994;67(2):319–333. [Google Scholar]
- Chapman LJ, Chapman JP, Raulin ML. Scales for Physical and Social Anhedonia. Journal of Abnormal Psychology. 1976;85(4) doi: 10.1037//0021-843x.85.4.374. [DOI] [PubMed] [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behavioral & Brain Sciences. 1999;22(3) doi: 10.1017/s0140525x99002046. [DOI] [PubMed] [Google Scholar]
- deVries MW, editor. The experience of psychopathology: Investigating mental disorders in their natural settings. New York, NY, US: Cambridge University Press; 1992. [Google Scholar]
- Dickinson A, Balleine B. Motivational control of instrumental action. Current Directions in Psychological Science. 1995;4(5):162–167. [Google Scholar]
- Eckblad ML, Chapman LJ, Chapman JP, Mishlove M. The Revised Social Anhedonia Scale. 1982 unpublished test. [Google Scholar]
- First MB, Gibbon M, Spitzer RL, Williams JBW, Benjamin L. Structured Clinical Interview for DSM-IV Axis II Personality Disorders. New York: Biometrics Research Department; 1996. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for Axis I DSM-IV Disorders - Patient Edition (SCID-I/P, version 2.0) New York: Biometrics Research Department; 1994. [Google Scholar]
- Gard DE, Germans Gard M, Kring AM, John OP. Anticipatory and Consummatory Components of the Experience of Pleasure: A Scale Development Study. Journal of Research in Personality. 2006;40(6):1086–1102. [Google Scholar]
- Germans MK, Kring AM. Hedonic deficit in anhedonia: Support for the role of approach motivation. Personality and Individual Differences. 2000;28(4):659–672. [Google Scholar]
- Goodman SH, Sewell DR, Cooley EL, Leavitt N. Assessing levels of adaptive functioning: The Role Functioning Scale. Community Mental Health Journal. 1993;29(2):119–131. doi: 10.1007/BF00756338. [DOI] [PubMed] [Google Scholar]
- Gruzelier J, Davis S. Social and physical anhedonia in relation to cerebral laterality and electrodermal habituation in unmedicated psychotic patients. Psychiatry Research. 1995;56(2):163–172. doi: 10.1016/0165-1781(95)02553-4. [DOI] [PubMed] [Google Scholar]
- Horan WP, Green MF, Kring AM, Nuechterlein KH. Does Anhedonia in Schizophrenia Reflect Faulty Memory for Subjectively Experienced Emotions? Journal of Abnormal Psychology. 2006a;115(3):496–508. doi: 10.1037/0021-843X.115.3.496. [DOI] [PubMed] [Google Scholar]
- Horan WP, Kring AM, Blanchard JJ. Anhedonia in schizophrenia: a review of assessment strategies. Schizophrenia Bulletin. 2006b;32(2):259–273. doi: 10.1093/schbul/sbj009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Filonov D, Wustenberg T, Villringer A, et al. Dysfunction of ventral striatal reward prediction in schizophrenic patients treated with typical, not atypical, neuroleptics. Psychopharmacology. 2006;187(2):222–228. doi: 10.1007/s00213-006-0405-4. [DOI] [PubMed] [Google Scholar]
- Juckel G, Schlagenhauf F, Koslowski M, Wustenberg T, Villringer A, Knutson B, et al. Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage. 2006;29(2):409–416. doi: 10.1016/j.neuroimage.2005.07.051. [DOI] [PubMed] [Google Scholar]
- Klein D. Depression and anhedonia. In: Clark DC, Fawcett J, editors. Anhedonia and affect deficit states. New York: PMA Publishing; 1984. pp. 1–34. [Google Scholar]
- Knutson B, Adams CM, Fong GW, Hommer D. Anticipation of increasing monetary reward selectively recruits nucleus accumbens. Journal of Neuroscience. 2001;21(16) doi: 10.1523/JNEUROSCI.21-16-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kring AM. Emotion in schizophrenia: Old mystery, new understanding. Current Directions in Psychological Science. 1999;8(5):160–163. [Google Scholar]
- Kring AM, Barrett LF, Gard DE. On the broad applicability of the affective circumplex: representations of affective knowledge among schizophrenia patients. Psychological Science. 2003;14 (3):207–214. doi: 10.1111/1467-9280.02433. [DOI] [PubMed] [Google Scholar]
- Kring AM, Earnst KS. Stability of emotional responding in schizophrenia. Behavior Therapy. 1999;30:373–388. [Google Scholar]
- Kring AM, Neale JM. Do schizophrenic patients show a disjunctive relationship among expressive, experiential, and psychophysiological components of emotion? Journal of Abnormal Psychology. 1996;105(2):249–257. doi: 10.1037//0021-843x.105.2.249. [DOI] [PubMed] [Google Scholar]
- Malla AK, Takhar JJ, Norman RMG, Manchanda R, Cortese L, Haricharan R, et al. Negative symptoms in first episode non-affective psychosis. Acta Psychiatrica Scandinavica. 2002;105(6):431–439. doi: 10.1034/j.1600-0447.2002.02139.x. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36(2):241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Schwartz JE, Stone AA. Strategies for analyzing ecological momentary assessment data. Health Psychology. 1998;17(1):6–16. doi: 10.1037//0278-6133.17.1.6. [DOI] [PubMed] [Google Scholar]
- Turkington D, Dudley R, Warman DM, Beck AT. Cognitive-behavioral therapy for schizophrenia: A review. Journal of Psychiatric Practice. 2004;10(1):5–16. doi: 10.1097/00131746-200401000-00002. [DOI] [PubMed] [Google Scholar]
- Wise RA. Brain reward circuitry: insights from unsensed incentives. Neuron. 2002;36(2):229–240. doi: 10.1016/s0896-6273(02)00965-0. [DOI] [PubMed] [Google Scholar]
- Zhang ZJ, Peet M, Ramchand CN, Shah S, Reynolds GP. Plasma homovanillic acid in untreated schizophrenia--relationship with symptomatology and sex. J Psychiatr Res. 2001;35(1):23–28. doi: 10.1016/s0022-3956(01)00008-5. [DOI] [PubMed] [Google Scholar]


