Abstract
Embryonic chick retinal pigment epithelial (RPE) cells can undergo transdifferentiation upon appropriate stimulation. For example, basic fibroblast growth factor (bFGF) induces intact RPE tissue younger than embryonic day 4.5 (E4.5) to transdifferentiate into a neural retina. NeuroD, a gene encoding a basic helix-loop–helix transcription factor, triggers de novo production of cells that resemble young photoreceptor cells morphologically and express general neuron markers (HNK-1/N-CAM and MAP2) and a photoreceptor-specific marker (visinin) from cell cultures of dissociated E6 RPE (Yan & Wang, 1998). The present study examined whether bFGF will lead to the same transdifferentiation phenomenon as neuroD when applied to dissociated, cultured E6 RPE cells, and whether interplay exists between the two factors under the culture conditions. Dissociated E6 RPE cells were cultured in the presence or absence of bFGF, and with or without the addition of retrovirus expressing neuroD. Gene expression was analyzed with immunocytochemistry and in situ hybridization. Unlike neuroD, bFGF did not induce the expression of visinin, or HNK-1/N-CAM and MAP2. However, bFGF elicited the expression of RA4 immunogenicity; yet, many of these RA4-positive cells lacked a neuronal morphology. Addition of bFGF to neuroD-expressing cultures did not alter the number of visinin-expressing cells; misexpression of neuroD in bFGF-treated cultures did not change the number of RA4-positive cells, suggesting the absence of interference or synergistic interaction between the two factors. Our data indicated that bFGF and neuroD induced the expression of different genes in cultured RPE cells.
Keywords: RPE, Cell culture, Transdifferentiation, bFGF, NeuroD
Introduction
The retinal pigment epithelium (RPE) and the neural retina are derived from the same neuroepithelial tissue—the optic vesicle. The optic vesicle begins as an evagination of the neural tube. Subsequently, the optic vesicle invaginates to form the optic cup. Cells of the inner and outer layers of the cup adopt different fates. Cells in the inner layer proliferate for several days and give rise to the complex neural retina. On the other hand, cells in the outer layer of the optic cup cease division early and compose the simple looking RPE, a monolayer structure of cells with no apparent heterogeneity.
Unlike retinal neurons, which are terminally differentiated, RPE cells from several species, including human, can transdifferentiate into cells of other types when stimulated (Eguchi, 1986; Reh et al., 1987; Dutt et al., 1993; Zhao et al., 1995; Crisant & Guidry, 1995; Zhao et al., 1997; Araki et al., 1998). Transdifferentiated phenotypes include a lens-like structure (Eguchi, 1986), mesenchyme (Crisant & Guidry, 1995), and, most intriguingly, a neural retina. Orts-Ilorca and Genis-Galvez (1960) first observed RPE transdifferentiation into a neural retina after separating the RPE and the retina of stage 10 chick embryos with a thread. Later, Coulombre and Coulombre (1965) reported the same transdifferentiation phenomenon following an incomplete surgical removal of the retina. Further investigation led to the discovery that bFGF is the stimulus for this phenomenal transdifferentiation (Park & Hollenberg, 1989). Subsequent studies have shown that, when cultured as a sheet, RPE can undergo the same transdifferentiation in vitro in the presence of bFGF (Pittack et al., 1991; Opas & Dziak, 1994; Pittack et al., 1997) or aFGF (Guillemot & Cepko, 1992). Nevertheless, transdifferentiation into a neural retina is strictly age-dependent and can only take place in RPE younger than embryonic day 4.5 (E4.5) in the chick (Coulombre & Coulombre, 1965; Pittack et al., 1991). Furthermore, under in vitro conditions, the transdifferentiation occurs only when RPE is cultured as explant or sheet, and does not occur when cultured as dissociated cells (Pittack et al., 1991).
Histologically, the neural retina generated from RPE resembles the endogenous retina with three nuclear layers: an outer nuclear layer, an inner nuclear layer, and a ganglion cell layer. These layers are orientated with a revered polarity, the outer nuclear layer facing the vitreous side (Coulombre & Coulombre, 1965; Park & Hollenberg, 1989). Thus, the transdifferentiated retina contains many different types of cells. Population analysis of dissociated cells several days after exposing RPE tissue to aFGF (or bFGF) indicated that as much as 80% of the cells in the transdifferentiated RPE express antigens normally present in retinal ganglion cells (Guillemot & Cepko, 1992). Since ganglion cells are the first cell type generated during retinal neurogenesis, this observation has led to the suggestion that conversion of RPE to a retinal fate is directly or indirectly mediated by induction of retinal ganglion cells (Guillemot & Cepko, 1992). It is unclear whether and how transdifferentiation of RPE can be coaxed toward a single neuronal cell type.
We have recently demonstrated that cell cultures derived from dissociated E6 chick RPE can be induced to transdifferentiate into photoreceptor cells by ectopic expression of neuroD. NeuroD encodes a protein belonging to the basic helix-loop–helix family of transcription factors and was originally isolated from mouse and frog (Lee et al., 1995). Microinjection of neuroD mRNA into Xenopus embryos causes ectopic production of neurons at the expense of epidermis (Lee et al., 1995). In the chick, neuroD is expressed in developing photoreceptor cells and their precursors during retinal neurogenesis (Yan & Wang, 1998). Misexpression of neuroD in the retinal neuroepithelium through retroviral transduction produces extra photoreceptor cells, while the number of other major types of retinal cells remains unchanged (Yan & Wang, 1998). When ectopically expressed in cultured E6 RPE cells, neuroD triggers de novo production of cells that express MAP2, an early neural marker (Tucker & Matus, 1987), and visinin, an early marker for cone photoreceptor cells (Yamagata et al., 1990). Morphologically, the transdifferentiated cells appear similar to cultured photoreceptor cells at various different developmental stages; some even have inner-segment-like processes (Yan & Wang, 1998). The fact that the transdifferentiated population does not express markers that are normally expressed by non-photoreceptor retinal neurons suggests that visinin-expressing cells are the major, if not the only, cell type present in E6 RPE cultures under the induction of neuroD. These results raise two important points. First, the fate of presumptive RPE cells could be guided toward a certain neuronal type by using a specific factor(s) deterministic for that cell type. Second, both older (E6, not younger than E4.5) and dissociated RPE cells (not RPE sheets) can be induced to transdifferentiate by neuroD. A single gene-induced dissociated RPE cell transdifferentiation has also been reported recently by Toy et al. (1998). They detected the presence of chx10-expressing cells and visinin-expressing cells in E7 RPE cultures after transfection with a plasmid harboring Optx2, a homeobox gene widely expressed in the retina in both neuroblasts and differentiated neurons of the three nuclear layers.
In this study, we have examined the effect of bFGF to induce gene expression in dissociated RPE cell cultures derived from day 6 chick embryos, in comparison with the induction of neuroD under the same culture conditions. Even though bFGF has reportedly failed to induce RPE older than E4.5 to transdifferentiate into neural retina (Pittack et al., 1991), it induced ectopic expression of RA4 antigenicity in dissociated RPE cell cultures. bFGF did not induce the expression of visinin in E6 RPE cell cultures, although its expression was abundantly detected with forced expression of neuroD. NeuroD did not alter the number of RA4-positive cells induced by bFGF, and bFGF did not change the number of cells expressing visinin induced by neuroD. Our results indicated that under our experimental conditions bFGF and neuroD induced the expression of distinctive genes, without apparently interfering with or stimulating one another.
Methods
RPE cell cultures
Pathogen-free fertilized chicken eggs (White Leghorn) were purchased from Spafas (Preston, CT) and incubated in a Petersime egg incubator. RPE was dissected free from other ocular tissues of day 5 or day 6 chick embryos as previously described (Yan & Wang, 1998). RPE cells were dissociated with trypsin-EDTA, seeded onto 35-mm dishes, and cultured with Medium 199 plus 10% fetal calf serum at 37°C with 5% CO2. At 50–70% confluence, bFGF (at a final concentration of 25 μg/ml) was added and the cells were cultured for various lengths of time with medium changed every 2 days. Alternatively, replication-competent retrovirus RCAS B/P(A) (Hughes et al., 1987) expressing neuroD, or green fluorescent protein (GFP) as a control, was added to the culture at 50–70% confluence to drive the expression of neuroD or GFP in RPE cells. Cells were fixed 8 days or at other time points, as specified, after the addition of the growth factor or retrovirus. Retrovirus stocks were produced as previously described (Yan & Wang, 1998).
To evaluate the possible interaction between the two agents, bFGF and retrovirus expressing neuroD were added to the same dishes at the same time, or bFGF was added 4 days after the addition of the neuroD retrovirus. Dishes were fixed 8 days after the addition of neuroD retrovirus.
In situ hybridization
Digoxigenin-labeled antisense RNA probes against visinin and chx10 were prepared as described previously (Yan & Wang, 1998). Fixed RPE cultures were sequentially treated with 1% Triton X-100 for 20 min, acetic anhydride (0.3% in 0.1 M triethanolamine, pH 8.0) for 10 min, and 50% formamide in 2 × SSC (1 × SSC; 150 mM NaCl, 15 mM Na3Citrate) for 30 min at 63°C. Dishes were washed with phosphate-buffered saline (PBS) between each step. Hybridization was carried out at 63°C overnight with 200 ng/ml of digoxigenin-labeled RNA probes in 50% formamide, five times SSC, 250 μg/ml tRNA, 50 μ g/ml heparin, and 2% blocking solution (Boehringer Mannheim, Mannheim, Germany). Subsequently, dishes were washed sequentially with 2 × SSC for 5 min, 50% formamide in 2 × SSC for 1 h, and two times with 2 × SSC. RNase A (7 μ g/ml in 2 × SSC) was added and the dishes were incubated at room temperature for 30 min, followed by three 2 × SSC washes with the last one at 37°C. The final wash was with 0.1 × SSC at 63°C for 1 h. Immunodetection for digoxigenin was performed following the manufacturer’s instructions (Boehringer Mannheim, Mannheim, Germany).
Immunocytochemistry
We used standard procedures for immunocytochemistry using an ABC kit (Vector Laboratories, Burlingame, CA) with 3-amino-9-ethylcarbazole as a chromogen. Monoclonal antibody RA4 was generously provided by Dr. Steve McLoon. Monoclonal antibodies against HNK-1/N-CAM and MAP2 were purchased from Sigma (St. Louis, MO). Polyclonal antibody against viral protein p27 was purchased from Spafas. Monoclonal antibody against Islet-1 was developed by Thomas Jessell (Columbia University) and was obtained from Developmental Studies Hybridoma Bank, University of Iowa. For double-labeling experiments, cells were subjected to in situ hybridization first, followed by standard immunocytochemistry.
Statistical analysis
The number of positive cells from each dish was counted from 66 view areas under a 10× objective. The total number of positive cells under the cover slip (22 mm × 22 mm) was calculated by multiplying the average of the 66 view areas by 121, the total number of view areas. In each experiment, three dishes were used for a single marker at a single time point. The number of positive cells is presented as the mean with an error bar representing the value of the standard deviation (S.D.). Means, S.D.s, and P values were calculated with a computer program (Microcal Origin, Version 4.1). All comparisons were made within the same experiment. All experiments were completely repeated, from dissection through counting, at least three times.
Results
RPE cells remained unpigmented in the presence of bFGF
Dissociated E6 RPE cells lost their pigmentation after 1 or 2 days in culture, but regained it after approximately 8–10 days in culture. Cultures forced to express neuroD through retroviral transduction appeared to have the same level of pigmentation as either the control with no particular agent added (Fig. 1), or the control infected with retrovirus expressing GFP (data not shown). In the presence of bFGF, however, E6 RPE cultures remained unpigmented (Fig. 1).
Fig. 1.

Pigmentation of E6 RPE cultures under varied culture conditions. RPE cultures treated with bFGF lacked pigmentation, while the control and neuroD-expressing culture became pigmented. “CK” denotes control dishes with no particular agent added.
bFGF induced the expression of RA4 immunogenicity
Immunocytochemical staining with monoclonal antibody RA4, which in the retina labels ganglion cells (Waid & McLoon, 1995), detected thousands of positive cells in E6 RPE cultures treated with 25 mg/ml of bFGF (Fig. 2; Table 1). The induction of RA4-immunogenicity expression by bFGF was in contrast to the basal level of RA4-positive cells in the neuroD-expressing dishes (Table 1; Fig. 2). Morphologically, many of those RA4-positive cells did not look like neurons (Fig. 2); some, however, assumed a neural-like morphology (Fig. 5). In control dishes, a small number of RA4-positive cells (usually in the low hundreds) was found under our experimental conditions. These RA4-positive cells were probably the result of growth factor(s) such as bFGF in the serum-containing culture medium, and thus were regarded as background.
Fig. 2.
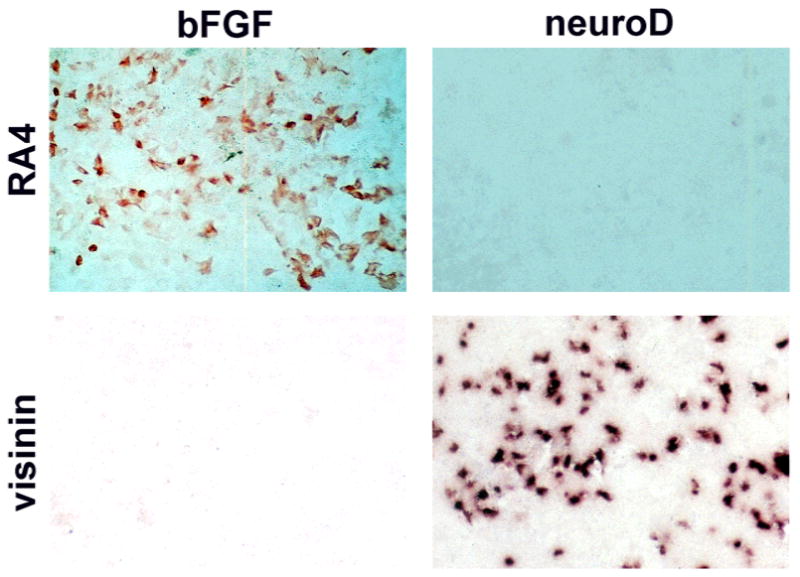
Expression of RA4 and visinin in E6 RPE cultures treated with bFGF or forced to express neuroD. Cells positive for RA4 were detected in bFGF-treated dishes with immunocytochemistry, while cells expressing visinin were detected in dishes ectopically expressing neuroD with in situ hybridization.
Table 1.
The expression of markersa in cultured E6 RPE treated with bFGF or forced to express neuroD
| Markers
|
||||||
|---|---|---|---|---|---|---|
| Treatment | RA4 | visinin | MAP2 | HNK-1/N-CAM | chx10 | Islet-1 |
| Control | + | − | − | + | − | − |
| neuroD | + | +++ | +++ | TMTC | − | − |
| bFGF | +++ | − | − | + | − | − |
In the chick retina, RA4 labels ganglion cells, visinin is expressed in cone photoreceptors. MAP2 is present in all major retina cell types except bipolar cells, which express chx10. HNK-1/N-CAM is an early marker for neurons. “+” indicates basal level of expression; “−” indicates no expression; “+++” indicates thousands of cells were positive; “TMTC” denotes that positive cells were too many to count. Control was given no treatment.
Fig. 5.
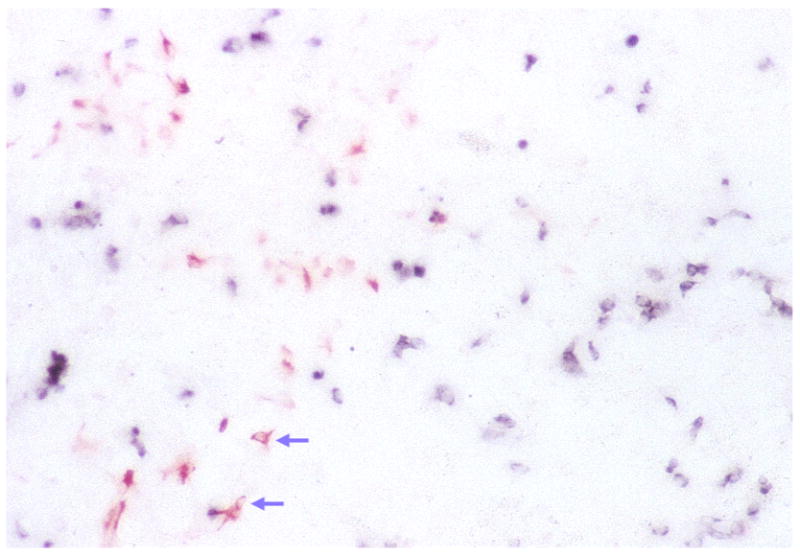
Double labeling of E6 RPE cell cultures under the dual treatment of bFGF and neuroD with RA4 antibody and antisense RNA probes against visinin. RA4-positive cells appear as red and visinin-expressing cells as blue. Arrows indicate RA4-positive cell that assumed a neuronal morphology.
Since RA4 labels retinal ganglion cells (Waid & McLoon, 1995) that also express HNK-1/N-CAM (Andressen et al., 1999; our own observation), MAP2 (Tucker & Matus, 1987), and Islet-1 (Austin et al., 1995), we examined whether these markers were expressed in RPE cell cultures treated with bFGF. Monoclonal antibody against Islet-1 stained the nuclei of ganglion cells in the retina (Austin et al., 1995; our own observation) but did not yield immuno-staining signals when applied to the RPE cultures. The number of cells expressing HNK-1/N-CAM in bFGF-treated dishes remained at a basal level indistinguishable from the control. Anti-MAP2 antibody labeled few cells in the bFGF dishes, indistinguishable from the control (Table 1). Both HNK-1/N-CAM and MAP2 labeled numerous cells in cultures infected with retrovirus expressing neuroD (Table 1; also see Yan & Wang, 1998). These data indicate that bFGF induced a very limited number of genes, and, as a result, those RA4-positive cells are not referred to as retinal ganglion cells.
In situ hybridization with an antisense RNA probe against visinin did not stain any cells in bFGF-treated dishes, but did label numerous cells in dishes inoculated with retrovirus expressing neuroD (Table 1; Fig. 2). No cultures contained cells labeled by the chx10 antisense probe, which, in normal retina, labels bipolar cells (Table 1).
Interplay between neuroD and bFGF
The different effects of bFGF and neuroD on gene expression in dissociated, cultured E6 RPE cells prompted an examination of whether the two agents would interact with each other, leading to an alteration in the expression of visinin or RA4 immunogenicity. bFGF and neuroD were added to the same dishes, either at the same time or with bFGF added 4 days later. Addition of bFGF at a later time was to allow the spreading of retrovirus expressing neuroD into more cells before they were exposed to bFGF. Monitoring infection by immunocytochemistry with a specific antibody against the viral protein, p27, showed that usually >70% of the cells were positive for the viral protein 4 days after the inoculation with the virus.
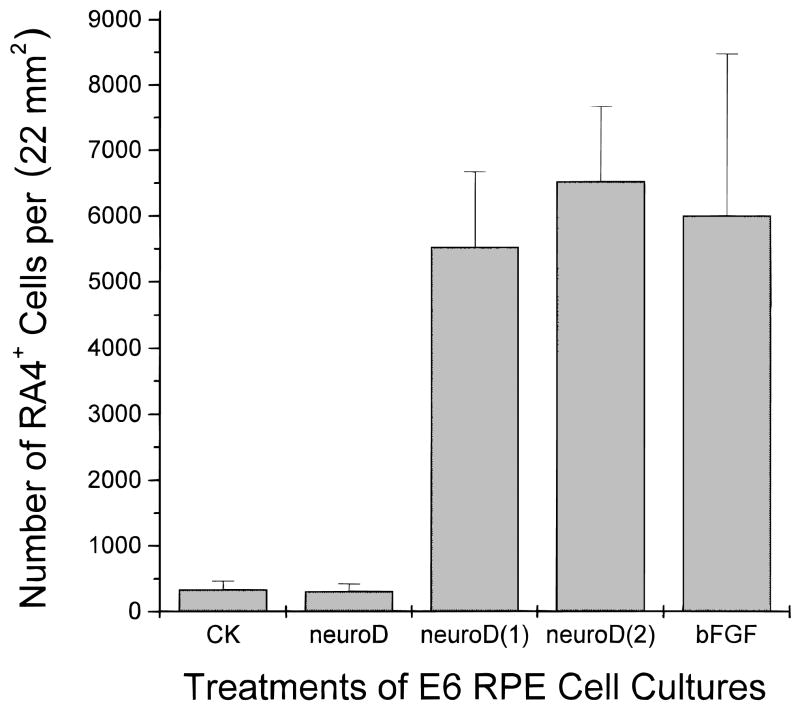
Cells in the dishes were fixed and analyzed 8 days after the addition of the neuroD retrovirus. With both reagents added, many cells became RA4 positive. When bFGF was present with neuroD for 4 days or 8 days, the number of RA4-positive cells was 6525 ± 1141 and 5524 ± 1147, respectively. These numbers are not statistically different (P > 0.1) from those treated with bFGF alone for 8 days (6004 ± 2471; Fig. 3). This indicates that the expression of neuroD did not significantly affect the ability of bFGF to cause RPE cells to express RA4 immunogenicity.
Fig. 3.
Quantification of RA4-positive cells in E6 RPE cultures under different treatments. The number of cells positive for RA4 increased dramatically when RPE cells were cultured in the presence of bFGF alone (bFGF), compared to the control (CK) or to those ectopically expressing neuroD alone (neuroD). In the presence of neuroD and bFGF, either added at the same time [neuroD(1)] or bFGF added 4 days after the addition of neuroD-expressing retrovirus [neuroD(2)], the number of RA4-positive cells was high and not statistically different from that in dishes treated with bFGF alone.
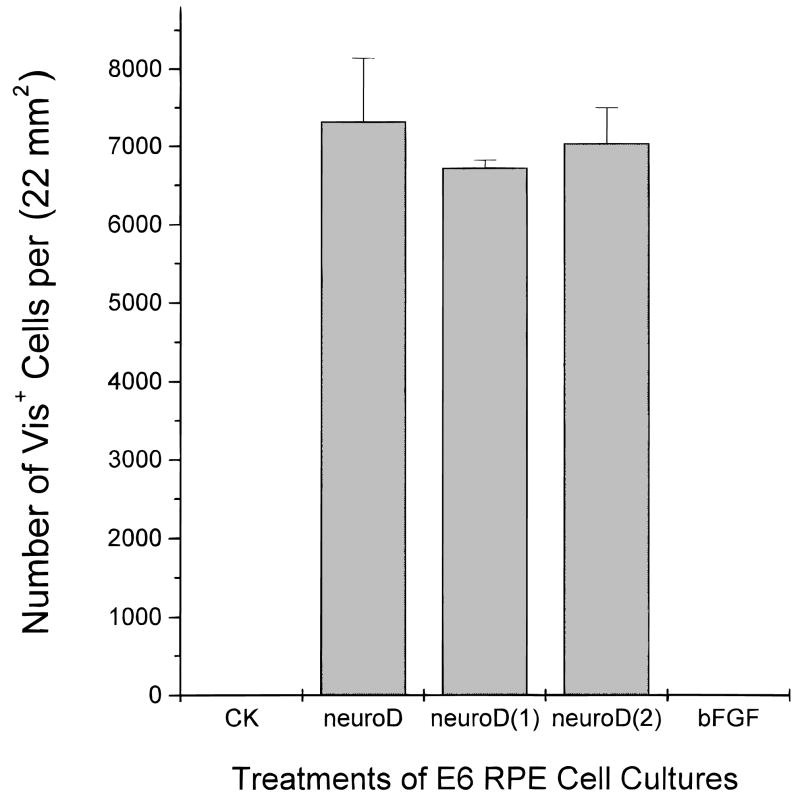
Expression of visinin was examined to see whether neuroD-induced gene expression could be suppressed by bFGF. Visinin-expressing cells were not detected in the control dishes nor the dishes treated with bFGF alone (Fig. 4). But many cells in cultures forced to express neuroD became positive for visinin expression regardless of the presence of bFGF: 6716 ± 101 when bFGF was added the same time as neuroD-expressing virus, 7036 ± 459 when bFGF was added 4 days later, and 7320 ± 817 when no bFGF was added. These numbers are not statistically different (P > 0.15), suggesting that bFGF had little or no effect on neuroD’s induction of visinin expression. Morphologically, the RA4-positive and visinin-expressing cells in the double-agent dishes appeared similar to those with either bFGF or neuroD used alone: most of the RA4-positive cells lacked neuronal morphologies, while visinin-expressing cells were typical of young photoreceptor cells (Fig. 5).
Fig. 4.
Quantification of cells expressing visinin in E6 RPE cultures with different treatments. No cells were found to express visinin in control dishes (CK) and in dishes treated with bFGF alone (bFGF), while such cells were detected in abundance when neuroD was ectopically expressed (neuroD). Addition of bFGF to dishes infected with retrovirus expressing neuroD at the same time [neuroD(1)] or 4 days later [neuroD(2)] did not cause statistically significant changes in the number of visinin-expressing cells.
To address the question whether the visinin and RA4 pathways were independent or mutually exclusive under our experimental conditions, we studied whether visinin and RA4 immunogenicity were co-expressed under the dual induction of bFGF and neuroD. Double-labeling experiments showed that, by far, the majority of cells were singly labeled (Fig. 5) and cells expressing both markers were extremely rare (less than 0.1% of either marker alone). This is not a total surprise since morphologically most cells labeled by RA4 were not neuronal and cells expressing visinin were typical young photoreceptor neurons.
Time course of RA4-immunogenicity expression induced by bFGF
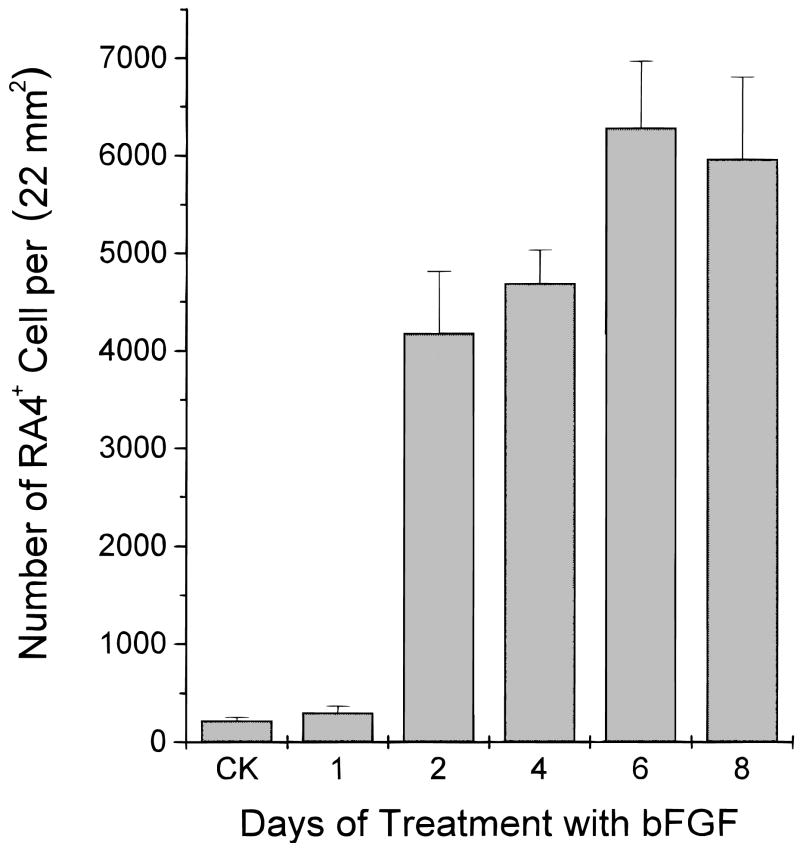
We asked whether the induction of RA4 immunogenicity by bFGF was an instantaneous (or primary) event. Cells were fixed at different time points, either 1 day (24 h), 2 days, 4 days, 6 days, or 8 days after the addition of bFGF, and the number of RA4-positive cells were counted. If expression of RA4 immunogenicity was a primary event of bFGF induction, we would expect a high number of RA4-positive cells soon after the addition of bFGF. However, we observed no statistically significant increase in the number of cells positive for RA4 24 h after the addition of bFGF (297 ± 71; control 218 ± 33; P = 0.16). Only after two days of exposure to bFGF did the number increase dramatically (4178 ± 639; Fig. 6). The number of RA4-positive cells increased and reached a peak 6 days after the addition of bFGF (6279 ± 687). These results suggested that expression of RA4 immunogenicity might be secondary to some primary induction events by bFGF.
Fig. 6.
The number of RA4-positive cells at different time points after the addition of bFGF to E5 RPE cell cultures. A dramatic increase in the number of RA4-positive cells was detected only after 48 h of bFGF treatment. No statistically significant increase in the number of RA4-positive cells was detected when the culture was treated with bFGF for 24 h before fixation as compared to control (CK).
neuroD did not induce the expression of RA4
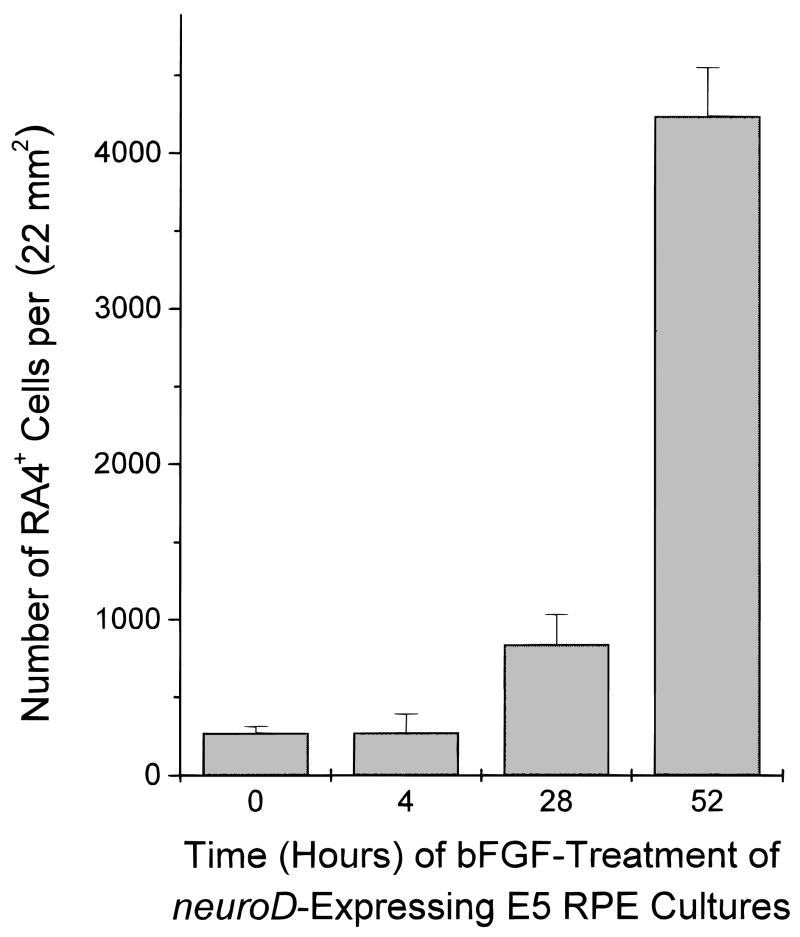
We also addressed the possibility that the scarcity of RA4-positive cells in neuroD dishes was due to the death of newly produced RA4-positive cells. RPE cultures were inoculated with neuroD retrovirus for 8 days before fixation of the cells; on days 6, 7, or 8 the culture was treated with bFGF (52, 28, or 4 h before the fixation, respectively). Thousands of cells were positive for RA4 in dishes treated with bFGF for 52 h (4235 ± 313; Fig. 7). When bFGF treatment was limited to 28 h, significant fewer cells were labeled by RA4 (841 ± 192; P < 10−4). This represents only 20% of that of the 52 h treatment. Dishes treated for 4 h with bFGF contained a basal level of RA4-positive cells, not significantly different from the control (273 ± 119 and 270 ± 43, respectively; P = 0.97). Since many bFGF-treated cells expressed RA4, even in the presence of neuroD, we believe that neuroD does not alter the survival of RA4-positive cells, but simply fails on its own to induce RA4 expression.
Fig. 7.
The number of RA4-positive cells in neuroD-expressing E5 RPE cultures treated with bFGF for various lengths of time. A 52-h treatment gave a high number of RA4-positive cells, a 28-h treatment produced a small yet significant increase, and a 4-h treatment produced no significant increase (P = 0.97) when compared to cultures not treated with bFGF (0).
Discussion
Treatment of E6 RPE cell cultures with bFGF resulted in the expression of RA4 immunogenicity. The majority of the RA4-positive cells we saw in bFGF-treated dishes lacked the typical neuronal morphology and did not express general neuron markers (HNK-1/N-CAM and MAP2) and another ganglion cell marker (Islet-1). The lack of neuronal morphology and the lack of expression of markers additional to RA4 suggest that, under the culture conditions of dissociated E6 RPE cells, bFGF induced limited gene expression, insufficient to induce cells to become characteristic young neurons. It is possible that the E6 RPE cells in our cultures have lost some factors as a result of dissociation process by trypsin/EDTA, which can be damaging to proteins of the plasma membrane (von Recum et al., 1999), or aging (it is known that RPE older than E4.5 cannot be induced to transdifferentiate into a neural retina by bFGF). These factors or genes may be needed for those RA4-positive cells to differentiate into ganglion cells. Additionally, other factors, such as cell–cell contacts or communications that exist in RPE tissue but are absent in dissociated cells, may also be required for ganglion cells to develop. Consistent with this scenario is that RA4 immunogenicity is expressed extremely early, as early as 15 min after terminal mitosis and before the migration of young ganglion cells to their final anatomical location next to the vitreal surface (Waid & McLoon, 1995), while the other markers tested are expressed relatively late (Tucker & Matus, 1987; Austin et al., 1995; Andressen et al., 1999).
Published studies have shown that bFGF, or its receptor, is involved in the production (McFarlane et al., 1998), survival (Fontaine et al., 1998), maturation (Gargini et al., 1999), and death (Gao & Hollyfield, 1995) of photoreceptor cells. Under our experimental conditions, bFGF did not promote the expression of visinin. It is possible that cells in our cultures were defective in the pathway from bFGF signaling to photoreceptor production or gene expression. The deficit could be inherited (as the cells were of RPE origin) or acquired during the culture process.
NeuroD, on the other hand, can trigger transdifferentiation of cultured RPE cells into cells that not only express neural markers (HNK-1/N-CAM and MAP2) and a cone marker (visinin) but also assume morphologies typical of photoreceptor cells at different developmental stages (Yan & Wang, 1998). Since morphologic changes may involve a number of genes, these results suggest that, unlike bFGF, neuroD can trigger a cascade of gene-expression events leading to cellular development and differentiation under our cell culture conditions. Further studies have shown that neuroD can induce the expression of an array of photoreceptor genes in RPE cultures (Yan & Wang, 2000).
In a previous study (Yan & Wang, 1998), we analyzed neuroD-induced RPE transdifferentiation by examining the expression of visinin, RA4, chx10, MAP2, and pax6 (a marker for amacrine cells; Belecky-Adams et al., 1997). No cells were found to express chx10 or pax6 in cultures forced to express neuroD. The number of RA4-positive cells remained at a background level, while the number of cells expressing MAP2 was close, but never exceeded the number of visinin-expressing cells, suggesting that photoreceptor cells are the major products of neuroD-induced RPE transdifferentiation. When we found that RA4-positive cells were abundant in bFGF-treated cultures, we wondered whether the lack of RA4-positive cells in neuroD-expressing cultures was due to a lack of survival of newly emerged RA4-positive cells in the absence of bFGF. We addressed this concern by treating neuroD-expressing cultures with bFGF for a various lengths of time (4, 28, or 52 h). No increase in the number of RA4-positive cells was observed with the 4-h treatment, and the number jumped dramatically when the treatment lasted for 52 h. With 28 h of treatment, the number of RA4-positive cells was only 20% of what it would be in another 24 h. These results argue that neuroD simply does not induce the expression of RA4 in RPE monolayer cultures.
We observed that only a fraction of cells in a dish was responsive to the induction of bFGF to express RA4 immunogenicity, or neuroD to express visinin, raising the possibility of RPE cell heterogeneity under the culture conditions. Expression of visinin triggered by neuroD was not affected by bFGF treatment; expression of RA4 immunogenicity induced by bFGF was not altered by ectopic expression of neuroD. Two plausible explanations are (1) the RA4 and visinin were two independent pathways and (2) cells labeled by RA4 and cells expressing visinin came from distinctive populations of RPE cells. If differential responses to bFGF and neuroD existed among the RPE cells, then the two factors, either alone or in combination, acted upon their constituents and their effects might appear independent. In this case, a cell would express either visinin if it were responsive to neuroD, or RA4 immunogenicity if responsive to bFGF, but not both. On the other hand, if the visinin-expressing cells and the RA4-positive cells were from the same pool of precursors and the two pathways were independent of each other, then we would expect the presence of cells expressing both markers. Double-labeling experiments demonstrated that RA4-positive cells lacked visinin expression and vice versa, suggesting that the RA4 and visinin pathways were mutually exclusive. Taken together, our data support the presumption that RPE cells were heterogeneous under our culture conditions. The heterogeneity could be intrinsic or secondary to induction events by bFGF or neuroD. Expression of RA4 immunogenicity (or some primary events yet to be identified), or the generation of young photoreceptor cells elicited by neuroD, could modify their microenvironment, leading to changes in the properties of neighboring cells. Early-born cells influencing the differentiation of late-born cells has been demonstrated experimentally (Waid & McLoon, 1998). Another possible source of the induced heterogeneity, should it exist, is the expression of neuroD, which has been shown to promote the expression of Delta, the ligand of lateral inhibition mediated by Notch/Delta (Chitnis & Kintner, 1996). Activation of the Notch/Delta pathway is known to inhibit the expression of neurogenic genes such as Xash-3 and neurogenin (Chitnis & Kintner, 1996; Ma et al., 1998). The function of neuroD, however, has been shown to be relatively insensitive to inhibition by Notch/Delta (Chitnis & Kintner, 1996).
In summary, bFGF and neuroD triggered the expression of different genes. bFGF promoted the expression of RA4 immunogenicity, yet most of these cells lacked the characteristic properties of young neurons. NeuroD, on the other hand, did not induce the expression of RA4, but did induce the expression of HNK-1/N-CAM, MAP2, and visinin under monolayer cell culture conditions. Neither agent induced the appearance of chx10-expressing cells. Thus, the expression of RA4 immunogenicity is a characteristic response of dissociated, cultured E6 RPE cells to bFGF and the expression of visinin is a characteristic of transdifferentiation induced by neuroD. Our data suggest the feasibility of inducing different genes in cultured RPE cells by different factors.
Acknowledgments
The authors thank Dr. Steven McLoon for RA4 antibody, Dr. C.-M. Li for discussion during the course of this work, Ms. B. Busbey for administrative assistance, and Ms. J. Macke for critical reading of the manuscript. This work is supported by NIH/NEI Grant EY11640 and by Research to Prevent Blindness.
References
- Andressen C, Arnhold S, Ashwell K, Mai JK, Addicks K. Stage specific glycosylation pattern for lactoseries carbohydrates in the developing chick retina. Histochemical Journal. 1999;31:331–338. doi: 10.1023/a:1003722102996. [DOI] [PubMed] [Google Scholar]
- Araki M, Yamao M, Tsudzuki M. Early embryonic interaction of retinal pigment epithelium and mesenchymal tissue induces conversion of pigment epithelium to neural retinal fate in the silver mutation of the Japanese quail. Development Growth and Differentiation. 1998;40:167–176. doi: 10.1046/j.1440-169x.1998.00006.x. [DOI] [PubMed] [Google Scholar]
- Austin CP, Feldman DE, Ida JA, Cepko CL. Vertebrate retinal ganglion cells are selected from competent progenitors by the action of Notch. Development. 1995;121:3637–3650. doi: 10.1242/dev.121.11.3637. [DOI] [PubMed] [Google Scholar]
- Belecky-Adams T, Tomarev S, Li HS, Ploder L, McInnes RR, Sundin O, Adler R. Pax-6, Prox 1, and Chx10 homeobox gene expression correlates with phenotypic fate of retinal precursor cells. Investigative Ophthalmology and Visual Science. 1997;38:1293–1303. [PubMed] [Google Scholar]
- Chitnis A, Kintner C. Sensitivity of proneural genes to lateral inhibition affects the pattern of primary neurons in Xenopus embryos. Development. 1996;122:2295–2301. doi: 10.1242/dev.122.7.2295. [DOI] [PubMed] [Google Scholar]
- Coulombre JL, Coulombre AJ. Regeneration of neural retina from the pigmented epithelium in the chick embryo. Developmental Biology. 1965;12:79–92. doi: 10.1016/0012-1606(65)90022-9. [DOI] [PubMed] [Google Scholar]
- Crisant S, Guidry C. Transdifferentiation of retinal pigment epithelial cells from epithelial to mesenchymal phenotype. Investigative Ophthalmology and Visual Science. 1995;36:391–405. [PubMed] [Google Scholar]
- Dutt K, Scott M, Sternberg PP, Linser PJ, Srinivasan A. Transdifferentiation of adult human pigment epithelium into retinal cells by transfection with an activated H-ras proto-oncogene. DNA and Cell Biology. 1993;12:667–673. doi: 10.1089/dna.1993.12.667. [DOI] [PubMed] [Google Scholar]
- Eguchi G. Instability in cell commitment of vertebrate pigmented epithelial cells and their transdifferentiation into lens cells. In: Moscona AA, Monroy A, editors. Current Topics in Developmental Biology. New York: Academic Press, Inc; 1986. pp. 21–37. [DOI] [PubMed] [Google Scholar]
- Fontaine V, Kinkl N, Sahel J, Dreyfus H, Hicks D. Survival of purified rat photoreceptors in vitro is stimulated directly by fibroblast growth factor-2. Journal of Neuroscience. 1998;18:9662–9672. doi: 10.1523/JNEUROSCI.18-23-09662.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H, Hollyfield JG. Basic fibroblast growth factor in retinal development: Differential levels of bFGF expression and content in normal and retinal degeneration (rd) mutant mice. Developmental Biology. 1995;169:168–184. doi: 10.1006/dbio.1995.1135. [DOI] [PubMed] [Google Scholar]
- Gargini C, Belfiore MS, Bisti S, Cervetto L, Valter K, Stone J. The impact of basic fibroblast growth factor on photoreceptor function and morphology. Investigative Ophthalmology and Visual Science. 1999;40:2088–2099. [PubMed] [Google Scholar]
- Guillemot F, Cepko CL. Retinal fate and ganglion cell differentiation are potentiated by acidic FGF in an in vitro assay of early retinal development. Development. 1992;114:743–754. doi: 10.1242/dev.114.3.743. [DOI] [PubMed] [Google Scholar]
- Hughes SH, Greenhouse JJ, Petropoulos CJ, Sutrave P. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. Journal of Virology. 1987;61:3004–3012. doi: 10.1128/jvi.61.10.3004-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JE, Hollenberg SM, Snider L, Turner DL, Lipnick N, Weintraub H. Conversion of Xenopus ectoderm into neurons by NeuroD, a basic helix-loop-helix protein. Science. 1995;268:836–844. doi: 10.1126/science.7754368. [DOI] [PubMed] [Google Scholar]
- Ma Q, Chen Z, del Barco Barrantes I, de la Pompa JL, Anderson DJ. Neurogenin1 is essential for the determination of neuronal precursors for proximal cranial sensory ganglia. Neuron. 1998;20:469–482. doi: 10.1016/s0896-6273(00)80988-5. [DOI] [PubMed] [Google Scholar]
- McFarlane S, Zuber ME, Holt CE. A role for the fibroblast growth factor receptor in cell fate decisions in the developing vertebrate retina. Development. 1998;125:3967–3975. doi: 10.1242/dev.125.20.3967. [DOI] [PubMed] [Google Scholar]
- Opas M, Dziak E. bFGF-induced transdifferentiation of RPE to neuronal progenitors is regulated by the mechanical properties of the substratum. Developmental Biology. 1994;161:440–454. doi: 10.1006/dbio.1994.1043. [DOI] [PubMed] [Google Scholar]
- Orts-Ilorca F, Genis-Galvez JM. Experimental production of retinal septa in the chick embryo. Differentiation of pigment epithelium into neural retina. Acta Anatomica. 1960;42:31–70. doi: 10.1159/000141635. [DOI] [PubMed] [Google Scholar]
- Park CM, Hollenberg MJ. Basic fibroblast growth factor induces retinal regeneration in vivo. Developmental Biology. 1989;134:201–205. doi: 10.1016/0012-1606(89)90089-4. [DOI] [PubMed] [Google Scholar]
- Pittack C, Grunwald GB, Reh TA. Fibroblast growth factors are necessary for neural retina but not pigmented epithelium differentiation in chick embryos. Development. 1997;124:805–816. doi: 10.1242/dev.124.4.805. [DOI] [PubMed] [Google Scholar]
- Pittack C, Jones M, Reh TA. Basic fibroblast growth factor induces retinal pigment epithelium to generate neural retina in vitro. Development. 1991;113:577–588. doi: 10.1242/dev.113.2.577. [DOI] [PubMed] [Google Scholar]
- Reh TA, Nagy T, Gretton H. Retinal pigmented epithelial cells induced to transdifferentiate to neurons by laminin. Nature. 1987;330:68–71. doi: 10.1038/330068a0. [DOI] [PubMed] [Google Scholar]
- Toy J, Yang JM, Leppert GS, Sundin OH. The optx2 homeobox gene is expressed in early precursors of the eye and activates retina-specific genes. Proceedings of National Academy of Sciences of the USA. 1998;95:10643–10648. doi: 10.1073/pnas.95.18.10643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker RP, Matus AI. Developmental regulation of two microtubule-associated proteins (MAP2 and MAP5) in the embryonic avian retina. Development. 1987;101:535–546. doi: 10.1242/dev.101.3.535. [DOI] [PubMed] [Google Scholar]
- von Recum HA, Okano T, Kim SW, Bernstein PS. Maintenance of retinoid metabolism in human retinal pigment epithelium cell culture. Experimental Eye Research. 1999;69:97–107. doi: 10.1006/exer.1999.0682. [DOI] [PubMed] [Google Scholar]
- Waid DK, McLoon SC. Immediate differentiation of ganglion cells following mitosis in the developing retina. Neuron. 1995;14:117–124. doi: 10.1016/0896-6273(95)90245-7. [DOI] [PubMed] [Google Scholar]
- Waid DK, McLoon SC. Ganglion cells influence the fate of dividing retinal cells in culture. Development. 1998;125:1059–1066. doi: 10.1242/dev.125.6.1059. [DOI] [PubMed] [Google Scholar]
- Yamagata K, Goto K, Kuo CH, Kondo H, Miki N. Visinin: A novel calcium binding protein expressed in retinal cone cells. Neuron. 1990;4:469–476. doi: 10.1016/0896-6273(90)90059-o. [DOI] [PubMed] [Google Scholar]
- Yan RT, Wang SZ. NeuroD induces photoreceptor cell overproduction in vivo and de novo generation in vitro. Journal of Neurobiology. 1998;36:485–496. [PMC free article] [PubMed] [Google Scholar]
- Yan R-T, Wang S-Z. Expression of an array of photoreceptor genes in chick embryonic retinal pigment epithelium cell cultures under the induction of neuroD. Neuroscience Letters. 2000 doi: 10.1016/s0304-3940(99)01003-4. (in press) [DOI] [PubMed] [Google Scholar]
- Zhao S, Rizzolo LJ, Barnstable CJ. Differentiation and transdifferentiation of the retinal pigment epithelium. International Review of Cytology. 1997;171:225–265. doi: 10.1016/s0074-7696(08)62589-9. [DOI] [PubMed] [Google Scholar]
- Zhao S, Thornquist SC, Barnstable CJ. In vitro transdifferentiation of embryonic rat pigment epithelium to neural retina. Brain Research. 1995;677:300–310. doi: 10.1016/0006-8993(95)00163-k. [DOI] [PubMed] [Google Scholar]