Abstract
Decision-making deficits are a robust cognitive correlate of substance abuse, but few studies have addressed the long-term differential associations of cocaine use and marijuana (MJ) use on decision-making. This study utilized the Iowa Gambling Task (IGT), a widely used measure of decision-making, to investigate the relationship between cocaine and MJ use and IGT learning. We analyzed between and within group differences across two consecutive testing sessions in abstinent users of either MJ or cocaine. We assessed long-term correlates of the use of these drugs by evaluating users after 25 days of enforced abstinence. Results showed that both cocaine users and MJ users performed worse than controls on the total IGT net score. All groups showed learning between Session 1 and Session 2, but the cocaine users showed the smallest increase in performance. The pattern of learning from the beginning to the end (block × block) of the IGT (Session 2) was different for the drug groups, with the cocaine group showing more learning than the MJ group. Dose-related measures of cocaine use (grams/week) and MJ use (joints/week) predicted IGT performance (the heavier the drug use the lower the performance). Differential correlates of cocaine use and MJ use on decision-making learning may have important implications for the development of novel treatment interventions.
Keywords: Cocaine, Marijuana, Decision-making, Learning, Iowa Gambling Task, Repeated Testing
1. Introduction
The prevalence of illicit drug abuse in the United States has reached overwhelming numbers. In 2002, an estimated 14.0 million Americans reported using marijuana (MJ) within the past month. An estimated 1.2 million Americans reported using cocaine: 265,000 of these were crack-cocaine users (National Household Survey on Drug Abuse, 2002). Because use of these illicit drugs is so pervasive, it is imperative to ascertain what adverse effects these substances have on individuals. Previous research has measured the effects of illegal drug use on cognition, suggesting that heavy use may be related to observed decrements in psychomotor speed, memory, executive functioning, and decision-making (Block & Ghoneim, 1993; Bolla et al., 2002; Grant et al., 2000; Pope et al., 2001; Solowij et al., 2002; Whitlow et al., 2004). However, the majority of studies conducted have been interested in a cross-sectional comparison between the functioning of substance users versus controls during acute withdrawal (or short-term abstinence) rather than the persistent correlates of heavy use over a longer period of time (Haney et al., 1999; Tucker et al., 2004). Although it is important to assess cognitive deficits associated with each stage of abstinence, assessment during short-term abstinence presents interpretive problems. Specifically, decrements in functioning may be due to anything from alterations in the brain, residues of the drug in the brain, or the withdrawal symptoms themselves. Reports of cognitive functioning in drug users with longer-term abstinence have been mixed (Bolla et al., 2002; Pope et al., 2001). For example, Pope et al. (2001) found that after seven days of abstinence, cognitive deficits in MJ users appeared to be reversed, whereas our group found that decrements in cognitive functioning persisted after 28 days of abstinence. We concluded that the difference was due to discrepancies in the demographics of the groups including a dose-effect. Our group appeared to have heavier drug use and thus larger decrements in functioning when compared to the MJ users studied by Pope et al. (2001). A similar controversy can be found in cocaine neurocognitive studies (DiSclafani et al., 2002; Selby & Azrin, 1998; Toomey et al., 2003). Therefore, the long-term detrimental correlates of substance abuse on cognitive functioning need further clarification.
Decision-making is defined as the ability to select the most adaptive course of action for the organism from a set of possible alternative behaviors (Bechara et al., 2000). Decision-making appears to be one area that is hindered by heavy drug use. This is evidenced by the users’ persistent use of substances despite the potential negative physical, psychological, social, and legal consequences with which they are faced. This continued use may be attributable to damage to specific neural networks involved in decision-making and performance monitoring (Volkow & Fowler, 2000). Previous Positron Emission Tomography (PET) activation studies have shown that cocaine and marijuana (MJ) users have different brain activation patterns than a non-drug using control group (Bolla et al., 2003, 2005; Eldreth et al., 2004). Furthermore, there is a dose-related effect, such that heavier use is related to greater deviations in brain functioning (Bolla et al., 2002, 2005). Specifically, the right orbital frontal cortex (OFC) does not appear to be as efficient in MJ and cocaine users as in controls (Bolla et al., 2003, 2005). A similar pattern of OFC dysfunction during decision-making performance has been revealed by functional Magnetic Resonance Imaging (fMRI) in methamphetamine users (Paulus et al., 2002, 2003). It is possible that such compromised decision-making could be attributable to heavy drug use, may result in continued drug use, and may contribute to failed attempts at abstinence (Bolla et al., 2003; Paulus et al., 2005). Abnormal decision-making may also predispose a given individual to the onset of drug use and to becoming a substance abuser.
The Iowa Gambling task (IGT) is a neurocognitive task designed to tap into real-life decision-making that requires the individual to weigh simultaneously the costs and benefits of their decisions. The task was developed originally to test patients with lesions in the ventromedial prefrontal cortex (VMPFC) (Bechara et al., 1994). The VMPFC patients consistently scored lower than controls (Bechara et al., 2000, 1997, 1994). Cocaine and stimulant abusers also performed worse than controls (Bartzokis et al., 2000; Bolla et al., 2003; Grant et al., 2000; Verdejo-García et al., 2006). Unfortunately, less research has been conducted in MJ users, especially with regard to the long-term effects of the drug on decision-making performance. This is unfortunate as both animal and human studies indicate that impaired decision-making may be a robust cognitive correlate of the use of MJ (Whitlow et al., 2003, 2004). For example, MJ intoxication was associated with detrimental effects on risk taking behavior (Lane et al., 2006). In addition, short-term MJ withdrawal (12 hours) was also related to poorer IGT performance of MJ users as compared to controls (Whitlow et al., 2004). Still yet, another study reported lower IGT performance in a group of MJ polysubstance users abstinent for a mean of 15 days (Lamers et al., 2006). However, 73% of the MJ users included in this study tested positive for THC on the day of testing, and therefore we cannot draw conclusions about long-term effects. Bolla et al. (2005) found decision-making deficits in a 28-days abstinent MJ group using the IGT. In contrast, Quednow et al. (2007) failed to find decision-making deficits in a group of pure MJ users who had been abstinent for a mean of seven days in this task. However, this study revealed decision-making deficits in a group of MDMA users with a co-use of MJ. Discrepancies between findings may be related to several variables, including length of abstinence, amount of drug use and co-use of other drugs (Bolla et al., 2002, 2005). We will attempt to address these limitations in the present study by including documented, relatively pure users of cocaine and MJ, with 25 days of controlled abstinence, and by specifically analyzing dose-related effects of drug use on decision-making performance.
It is interesting to note that unlike patients with lesions in the VMPFC, substance abusers (including users of cocaine and MJ) tend to show an adaptive shift in decision-making performance towards the end of the IGT (see Bechara et al., 2001, Bechara & Damasio, 2002; Lamers et al., 2006; Verdejo-García et al., 2006). That is, their decision-making impairment is often characterized better by a strategy learning lag rather than an inability to learn from task contingencies (Bechara et al., 2001). This notion may have important implications for rehabilitation strategies. For example, if substance abusers can actually learn over repeated decision trials it may be effective to train them using cognitive restoration on decision-making skills. However, no studies to date have analyzed learning effects between consecutive testing sessions of the IGT in substance abuser groups.
Another unresolved issue is whether different drugs of abuse are differentially associated with brain functioning and decision-making performance. Previous evidence suggests that cocaine and other stimulants may be linked to more severe deficits than opiate use on neurocognitive measures of cognitive impulsivity and decision-making (Ersche et al., 2006; Rogers et al., 1999; Verdejo-García et al., 2006). Furthermore, PET studies have shown that cocaine and MJ appear to be associated with different correlates on brain functioning. For example, during actual decision-making performance, cocaine users showed greater activation in the right OFC and less activation in the right dorsolateral prefrontal cortex (DLPFC) and left medial prefrontal cortex (MPFC) than controls (Bolla et al., 2003). This finding suggests that the cocaine abusers may have a heightened sensitivity to the reward contingencies of the task. In contrast, using the same paradigm, MJ users showed less activation of the right OFC and the right DLPFC and greater activation in the cerebellum (Bolla et al., 2005) than controls. Furthermore, heavier MJ use was associated with increased activation in the parahippocampus, posterior cingulate/precuneus, and cerebellum, suggesting additional involvement of a working memory process. However, to date, no studies have directly compared the performance of cocaine and MJ users on the IGT.
We conducted the current study to determine the differential correlates of cocaine use and MJ use on learning, between and within consecutive testing sessions, on the IGT. We administered the IGT twice to 25-day abstinent cocaine and MJ users and matched-healthy controls. We examined between and within group differences on a number of outcome measures including: total net IGT score; amount of learning between two consecutive testing sessions (Session 1 and 2); and the amount of learning within the different phases of the task during each session (block-by-block). We also examined if performance and rate of learning were dose-related to the amount of cocaine or MJ used, and if performance and rate of learning were related to the duration of cocaine or MJ used, and to the age of onset of cocaine or MJ use. We hypothesized: (1) that cocaine and MJ users would have poorer performance than healthy controls on the total IGT score (Session 1 + Session 2); (2) that cocaine and MJ users would show decreased learning from Session 1 to Session 2 of the IGT as compared with controls; (3) that cocaine and MJ users would also show decreased learning within different phases of the task (block-by-block performance) on each session. Due to the differential correlates of cocaine and MJ on the brain, we predicted these groups to have different patterns of decision-making performance and learning, both between sessions and within blocks on each session; and (4) that there would be a dose-related association between cocaine and MJ use and IGT performance.
2. Methods
2.1. Participants
We recruited participants through newspaper advertisements. To control for any medical, neurological or psychiatric conditions, participants received full medical and psychiatric screenings. Psychological screening consisted of drug use and psychological history using the Drug Use Survey Questionnaire (DUSQ) (Smith, 1991), Addiction Severity Index (ASI) (McLellan et al., 1980), and the Psychiatric Diagnostic Interview Schedule (DIS) (Robins et al., 1981). Medical screening consisted of complete physical and neurological examinations, including urine toxicology. All participants were men, right-handed, spoke English as their native language, and had estimated IQs of ≥ 80 as assessed by the Shipley Institute of Living Scale (Zachary, 1991). We used the Shipley to estimate intelligence because we empirically determined that it correlated with performance on more tests in our neurobehavioral test battery than other tests that are commonly used to estimate pre-morbid intellectual functioning (i.e., the WRAT reading subtest or WAIS-R Vocabulary subtest). Therefore, we believe that the Shipley is the best estimate of intellectual functioning in our specific population of drug abusers. None of the participants had Axis I or II co-morbid disorders. All participants also had MRI scans that were read as within normal limits by a neuroradiologist. The Institutional Review Boards of the National Institute on Drug Abuse - Intramural Research Program (NIDA-IRP), the Johns Hopkins Medical Institutions, Joint Committee on Clinical Investigation, and the Johns Hopkins Bayview Medical Institutional Review Board, approved this study. All participants provided informed consent and received remuneration.
2.1.1. Control Group
Control participants qualified if they consumed less than 12 alcoholic drinks/week and reported no current use of any illicit drug. A urine toxicology screen prior to testing revealed no use of any illicit drug for all participants in the control group. We excluded individuals if they met Diagnostic and Statistical Manual of Mental Disorders- IV (DSM-IV) criteria for past dependence on any drug including MJ or alcohol. No participant in the control group reported using MJ in the past 3 months.
2.1.2. Cocaine Group
The cocaine group claimed that cocaine was their drug of choice, used cocaine by any route for at least 2 years, self-administered cocaine at least four times/month, had two urine toxicology screens (at least 48 hours apart) that were positive for cocaine metabolites, and reported alcohol consumption of fewer than 12 alcoholic drinks/week (except for one participant) and marijuana use less than 8 days/month. The positive screens confirmed cocaine use during the 24 to 72 h prior to admission into the inpatient unit and ensured that all participants were abstinent for a uniform period. We excluded individuals if they met the DSM – IV criteria as assessed on the DIS for current or past dependence on any other psychoactive substance other than cocaine, including alcohol, or if their urine toxicology screen was positive for substances other than cocaine and its metabolites.
2.1.3. Marijuana Group
The MJ group consisted of individuals who claimed MJ as their drug of choice, smoked MJ for at least 2 years, currently smoked MJ at least four times/week, had two positive urine toxicology screens for MJ and its metabolites (at least 48 hours apart) to verify self-reports of marijuana use, and consumed fewer than 12 alcoholic drinks/week. We excluded participants if they met the DSM- IV criteria using the DIS indicating current or past dependence on any other psychoactive substance other than MJ, including alcohol, or if their urine toxicology screen was positive for substances other than MJ and its metabolites.
2.1.4. Exclusion Criteria for All Participants
We excluded volunteers if they had past or current Axis I/II disorders other than nicotine or marijuana dependence by DSM-IV criteria using the DIS (e.g., post-traumatic stress disorder, major depressive disorder). We excluded individuals with Axis I and Axis II (i.e., antisocial personality disorder) disorders because both types of disorders have been associated with pre-frontal lobe dysfunction and would most likely affect performance on the IGT independent of drug use. We excluded volunteers if: there was a past or current history of neurological illness (e.g., head trauma resulting in loss of consciousness, seizure disorder, and stroke); the neurological examination was abnormal; English was not the first language; or they were left-handed. We excluded volunteers if they reported current or past use of psychoactive medication (e.g., anti-depressants) or other drugs, including MDMA, heroin, opiates, amphetamines, and barbiturates.
2.2. Data Collection
At the initial visit to the Clinical Inpatient Research Unit (CIRU) at NIDA-IRP, all participants had a full medical screening. We then admitted the cocaine and MJ users to the CIRU for approximately 25 days. We admitted the Control group to the General Clinical Research Center (GCRC) for 3 days. We collected random drug screens to ensure abstinence. We gave no treatment or medications for drug abuse over the 25-day stay. Participants were instructed to abstain from smoking cigarettes and drinking caffeinated beverages for 3 hours before the study. Of note, our smokers were not heavy smokers (less than 3 packs/week) and reported low levels of addiction to nicotine on the Fagerstrom Test for Nicotine Dependence (Fagerstrom, 1978).
2.3. Iowa Gambling Task (IGT)
This task evaluates decision-making by measuring the participant’s ability to choose between disadvantageous decks of cards yielding high gains with a risk of extremely high losses (negative net score), and advantageous decks of cards yielding low gains with a risk of smaller losses (positive net score). In this study, participants performed the task inside a PET scanner, as part of a brain imaging protocol, results of which have been reported elsewhere (Bolla et al., 2003, 2005). We administered the IGT on two different occasions for each participant (Session 1 and Session 2). We used the standard version of the IGT (Bechara et al., 1994) for both sessions, which used the same predetermined order of cards, payoffs and losses. These sessions were separated by an interval of approximately 25 minutes. During this time interval, participants remained inside the PET scanner and performed a control task for the PET paradigm (for details see Bolla et al., 2003). In both sessions, we instructed the participants to win as much money as possible by picking one card at a time from each of the 4 decks (A, B, C, and D) in any order until the computer instructed them to stop (after the selection of the 100th card). We also told them that some decks are worse than others are and that they would win if they avoided the bad decks. To motivate participants to perform well on this task, they were informed that for each ‘game dollar’ they won, we would pay them one-cent ‘real money’ equaling a possible $20.00 per session and maximum total of $40.00 for both sessions. While performing the task, the computer displayed the amount of money the participant had remaining after each card was selected. Participants selected on average 20 cards per minute.
A net global outcome score (net score) was calculated by subtracting the total number of cards selected from the disadvantageous decks (A+B) from the total number of cards selected from the advantageous decks (C+D) in Session 1 and Session 2 and then deriving a sum score for both sessions. We also calculated net scores for each block of 20 trials to analyze learning within the different phases of the IGT (e.g., block 1 equals net scores for trials 1 to 20, block 2 equals net scores for trials 21 to 40 and so forth; see Bechara et al., 1997).
2.4. Statistical Analysis
We used Analysis of Variance (ANOVA) and mixed effects regression models for data analyses. For each set of analysis, we first examined data distributional properties and removed outlier cases. The Alpha level was set at p < 0.05 (two-tailed) for all analyses.
3. Results
3.1. Demographic characteristics
Table 1 shows the demographic and drug use characteristics of the control, cocaine, and MJ groups. We used one-way ANOVAs and pairwise Bonferroni tests to ascertain group differences. We only selected men for this report because we have reported highly significant sex-related differences on the IGT (Bolla et al., 2005). There were no significant group differences for years of education, maternal education, Shipley IQ, and Hollingshead Index of socioeconomic status, race, alcohol use, or proportion of cigarette smokers. Cocaine users were older than the MJ and the control participants, F (2, 34) = 12.51, p < 0.05 (Table 1). However, age was not significantly correlated with IGT performance (r = −0.05, p = 0.74 for the total net score) and thus it was not included as a covariate in the group comparisons.
TABLE 1.
Demographic characteristics of control group, cocaine abusers, and marijuana abusers
| Characteristics | Control group (n = 14) | Cocaine group (n = 12) | MJ group (n = 11) |
|---|---|---|---|
| Age, years | 30.9 ± 5.9 (22–43) | 36.8 ± 5.1 (28–43) | 25.7 ± 4.9 (21–35) |
| Education, years | 13.6 ± 2.6 (10–19) | 13.3 ± 1.7 (12–18) | 13.0 ± 1.7 (10–16) |
| Mother’s education, years | 12.9 ± 2.4 (9–17) | 11.8 ± 3.1 (8–20) | 13.6 ± 3.1 (10–20) |
| Shipley IQ | 100.0 ± 9.8 (83–118) | 98.6 ± 10.7 (80–113) | 102.1 ± 7.8 (92–115) |
| Hollingshead SES | |||
| Sex M/F | 14/0 | 12/0 | 11/0 |
| Race, A.A./C | 8/5 1 Asian | 8/3 1 Asian | 7/2 1 Hispanic
1 Other |
| Cocaine use | |||
| Days/week | 0 | 4.8 ± 1.4 (3–7) | 0 |
| Grams/week | 0 | 3.2 ± 2.3 (.9–7.2) | 0 |
| Duration | 0 | 8.5 ± 5.8 (2–17) | 0 |
| Route of administration | 100% | ||
| Smoke | |||
| Age of first use | 26.3 ± 7.8 (16–38) | ||
| MJ use | |||
| Days/week | 0 | .42 ± .90 (0–3) | 6.3 ± 1.4 (3–7) |
| Joints/week | 0 | 1.6 ± 4.3 (0–15) | 40.1 ± 22.3 (8–84) |
| Duration | 0 | 9.7 ± 9.4 (0–29) | 7.9 ± 5.6 (2–29) |
| Age of first use | 15 ± 1.3 (12–16) | ||
| Alcohol use | |||
| Drinks/week | .79 ± 1.6 (0–6) | 8.3 ± 16.9 (0–60)* | 2.4 ± 3.9 (0–12) |
We ran the analyses with and without the one participant who drank >12 drinks/week (60 drinks) and the results did not change. Thus, we did not exclude this participant from any of the analyses.
3.2. Between Group Differences on the IGT (Total Net Score)
Group differences on Total IGT net score (Session 1 + Session 2) were analyzed using a one-way ANOVA and pairwise Bonferroni tests. We found a significant effect of drug type on total net score, F (2, 34) = 3.89, p < 0.05. Specifically, pairwise Bonferroni tests revealed that cocaine users (Mnet score = 8.83; SD = 54.36) performed significantly worse than controls (Mnet score = 48.09; SD = 25.81) (p = 0.04 Cohen’s d=0.94). MJ users also performed worse than controls (Mnet score =16.73; SD = 28.92) but this effect was not significant.
3.3. Group by Session Differences (Cocaine, Marijuana and Control groups by Sessions 1 and 2)
Next, we conducted a 3 Group (cocaine vs. MJ vs. controls) by 2 Session (Session 1 vs. Session 2) mixed-design ANOVA to evaluate group differences in learning between sessions on the IGT net scores (hypothesis 2). We used post-hoc one-way ANOVAs and Bonferroni pairwise tests to determine group differences on each session separately (Session 1 and Session 2). Figure 1 shows main effects of session (Session 1 vs. Session 2), F (1, 33) = 22.69, p < 0.001, and group (cocaine vs. MJ vs. controls), F (2, 33) = 6.32, p < 0.01, and a marginal effect of the “session × group” interaction (p = 0.1). Post-hoc ANOVAs for each individual session showed significant group differences only on the second session, F (2, 33) = 5.48, p < 0.01.
Figure 1.
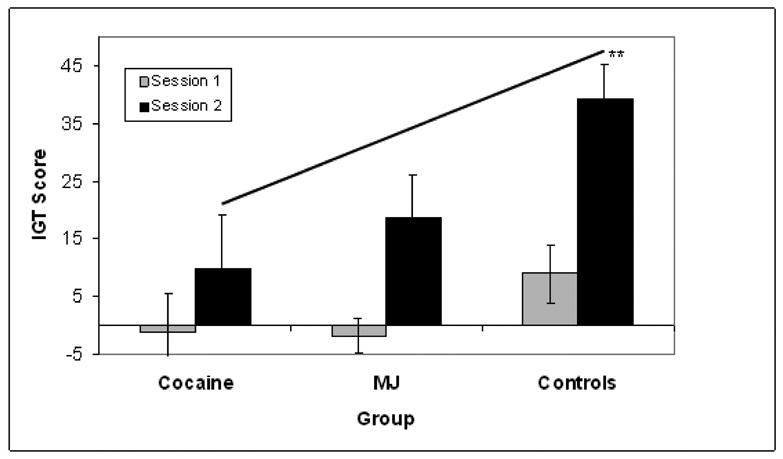
These results indicate that all groups learned between sessions. However, the rate of learning differed for the groups. The effect sizes for learning (session 2 – session 1, Cohen’s d) were 1.45 for healthy controls, 1.18 for MJ users, and 0.44 for cocaine users. Pairwise Bonferroni tests indicated that within session group differences on session 2 were mainly due to lower performance of the cocaine group than the controls (p = 0.008, Cohen’s d=1.27) (Figure 1). MJ users also had lower performance than controls on session 2, but this discrepancy was not significant (p = 0.16, Cohen’s d=0.89).
3.4. Group by IGT Block Differences (Cocaine, Marijuana and Control groups by blocks within each session)
Next, we performed two separate 3 Group (cocaine vs. MJ vs. controls) × 5 Block (IGT block) mixed-design ANOVAs on the IGT scores for Session 1 and 2 to examine differential learning effects within blocks on each session (hypothesis 3). We used post-hoc one-way ANOVAs to explore group differences on each individual block. Results from Session 1 showed a main effect of block but no effects of group or the “block × group” interaction. Figure 2 displays results from Session 2. We removed two participants (1 cocaine user and 1 MJ user) from this analysis due to outlier performance across blocks. Therefore, 11 cocaine users, 10 MJ users and 14 controls entered the analysis. Results showed a main effect of group, F (2,30) = 5.40, p < 0.01, and a marginal effect of the “block × group” interaction (quadratic), F(2,30) = 2.92, p = 0.06. Post-hoc univariate ANOVAs and pairwise contrasts showed controls performed better than the cocaine and MJ groups. Specifically, the cocaine group showed lower performance on blocks 2 (p = 0.03), 3 (p = 0.03), and 4 (p = 0.004); and the MJ group showed lower performance on blocks 3 (p = 0.03) and 4 (p = 0.04), compared to controls. When the cocaine group was compared to the MJ group, cocaine users showed the lowest performance on block 2, and both groups were equally impaired on blocks 3 and 4 (Figure 2). Additionally, as the learning curves in Figure 2 show, both the cocaine and MJ groups exhibited a slower learning curve than controls within session 2, with MJ users showing a flat learning curve (block 5 – block 1, Cohen’s d = 0.26), and cocaine users showing more learning over blocks (block 5 – block 1, Cohen’s d = 0.97). Separate repeated-measures ANOVAs for each group showed an effect of block in the cocaine users, F(4,8) = 3.97, p < 0.05, but not in the MJ users, F(4,7) = 0.24, p = 0.91. However, since the cocaine group showed the worse performance initially, this effect could be reflecting “regression towards the mean”.
Figure 2.
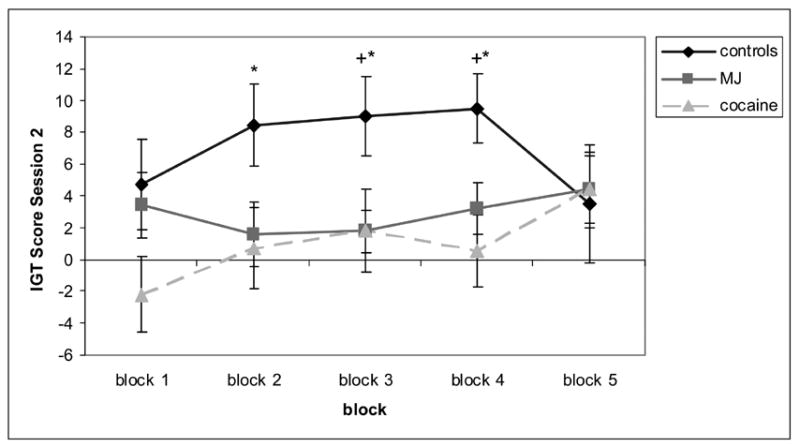
A detailed analysis of the pattern of card choices among the different groups demonstrated that several controls (9/14) used up all the cards from the advantageous decks before the end of the task (during blocks 4 and 5). Therefore, they were forced to choose from the disadvantageous decks during the fifth block, which explains their counter-intuitive pattern of decreased performance towards the end of the task. The cocaine users showed a mixed pattern of using up decks during the 4th and 5th blocks, with 4 cocaine users using up the advantageous decks and 4 users using up the disadvantageous decks. Only 1/10 MJ user used up an advantageous deck. To test if the fact that the participants had run out of cards may have affected results, we re-analyzed the data using only blocks 1–4 for all subjects. Re-analysis of the data had no effect on initial results. There was a main effect of group, F (2,30) = 6.62, p < 0.01, and a marginal effect of the “block × group” interaction (quadratic), F(2,30) = 1.88, p = 0.1. Post-hoc univariate ANOVAs and pairwise contrasts for group comparisons also replicated initial results.
3.5. Dose-Effects Relationship between Cocaine and MJ use on IGT Performance
We first tested exploratory hierarchical regression models to examine the relative influence of age and Shipley IQ on IGT scores. Results indicated that these variables did not contribute significantly to IGT performance (R2 adj. = −0.009, p = 0.44), while addition of the drug use variables (dose and duration) resulted in a significant change in F value (p = 0.001) and significant prediction of IGT score (R2 adj. = 0.35, p = 0.003).
Next, we carried out mixed effects regression analyses that took into account the repeated assessment of individual effects over the two testing sessions (Pinheiro & Bates, 2000) to simultaneously examine the relation between cocaine and MJ dose-related measures and IGT performance (hypothesis 4). Variables included in the analysis were dose of cocaine (grams/week), dose of MJ (joints/week), and session (Session 1 vs. Session 2). The dependent variable was total IGT score (Session 1 + Session 2). The model contained random effects at two levels: the effects for the individual participant and the effects for the session of testing within each participant. Table 2 shows the main effects for both dose of cocaine and dose of MJ. Higher doses of cocaine and MJ were associated with lower IGT performance scores (see Figure 3). There was also a main effect for session, such that performance during Session 1 was lower than during Session 2 (Table 2). Higher doses of cocaine and MJ were associated with lower IGT performance scores (see Figure 3). There were no significant interactions between drugs or between drug dose and session and no nonlinear drug dose effects were found; these terms were therefore excluded from the final model. We also analyzed the relationship between lifetime duration of drug use and IGT performance. Variables included in the analysis were duration of cocaine use, duration of MJ use, and Session. Results showed both main effects of cocaine duration, and MJ duration. Greater duration of MJ and cocaine use was associated with lower IGT performance. There was also a main effect for session, such that performance during Session 1 was lower than during Session 2 (Table 2). Finally, we analyzed the relationship between age of onset of drug use and decision-making performance. Results showed that ages of onset of MJ/cocaine use were not significantly associated with IGT performance.
TABLE 2.
Mixed regression analyses evaluating combined effects of dose (marijuana and cocaine) and testing session on total IGT performance.
| Dependent variable | Predictor variables | t | p |
|---|---|---|---|
| IGT Total net score | Marijuana (joints/week) | −2.47 | 0.01 |
| Marijuana Duration (years) | −4.12 | 0.0001 | |
| Session (1 versus 2) | −4.46 | 0.0001 | |
| Cocaine (grams/week) | −3.29 | 0.002 | |
| Cocaine Duration (years) | −3.37 | 0.001 | |
| Session (1 versus 2) | −3.85 | 0.0001 |
Figure 3.
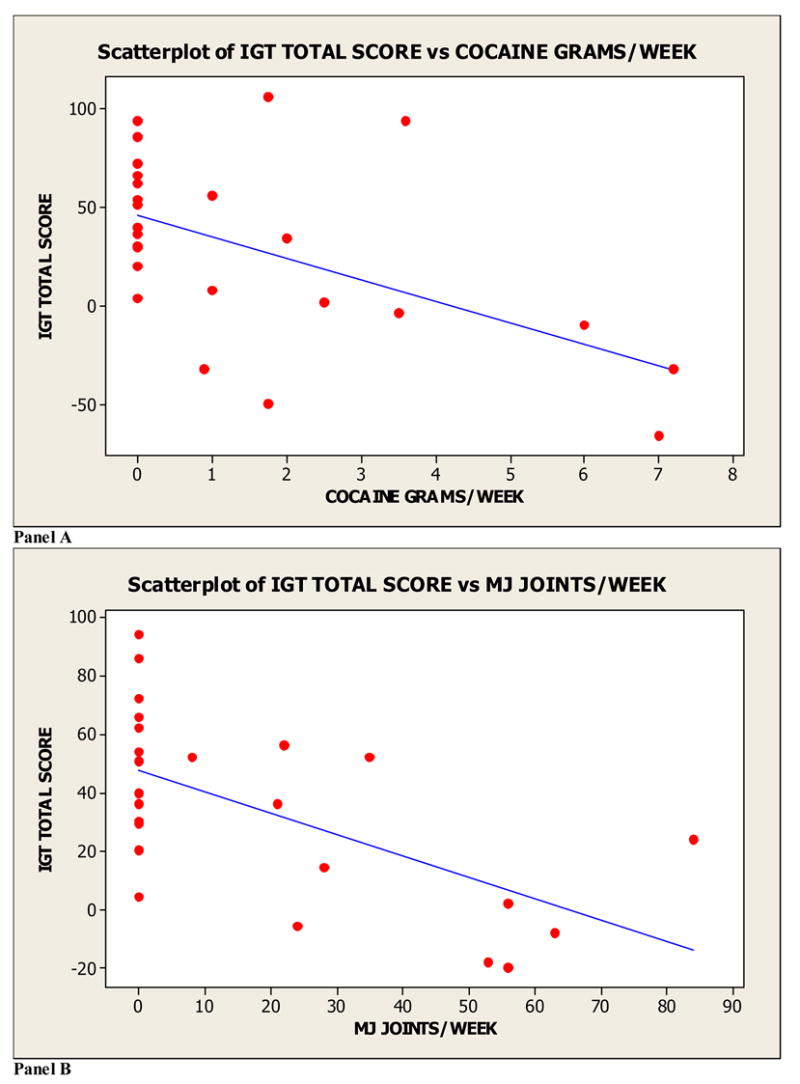
In order to explore further specific drug effects for each drug, separate sets of analyses were conducted for the cocaine group and the MJ group, both compared to the healthy controls (see Table 3). These analyses included terms for drug dose, session, and blocks of trials within sessions as predictor variables. These models included random effects at three levels: the individual participant, the sessions within each participant, and the blocks of trials within each session. The dependent variable was again the total IGT score. The analyses found that both cocaine and MJ, considered independently, showed linear dose-response effects on IGT performance (Table 3). Both the number of joints smoked and the grams of cocaine smoked per week prior to the period of enforced abstinence were negatively related to IGT performance. Participants in the cocaine group, but not the MJ group, showed a positive relationship between trial block and IGT performance, suggesting an impaired ability to learn the task contingencies within a session for those in the MJ group.
TABLE 3.
Separate mixed regression analyses for cocaine and MJ users evaluating combined effects of dose, testing session and blocks within session on total IGT performance.
| Dependent variable | Predictor variables | Cocaine | MJ | ||
|---|---|---|---|---|---|
| t | p | t | p | ||
| IGT Total net score | Drug dose | −2.99 | 0.006 | −3.33 | 0.003 |
| Block | 2.18 | 0.03 | 0.54 | 0.58 | |
| Session (1 versus 2) | 3.52 | 0.002 | 4.44 | 0.002 | |
5. Discussion
This study was successful in clarifying the differential correlates of cocaine use and MJ use on decision-making performance over repeat testing with the IGT. First, both cocaine users and MJ users show poorer performance than healthy controls on the total IGT net score even after 25 days of enforced abstinence. However, this difference is statistically significant only in the cocaine group. Second, although all groups show learning between Session 1 and Session 2, the control group shows the largest increase in performance, followed by the MJ users (although MJ and controls were not statistically different), then cocaine users, who show the smallest increase in performance. When we examine each session separately, no significant group differences are found for Session 1. However, the cocaine group shows significantly poorer performance than the control group in Session 2. Third, both the cocaine and MJ groups show less learning over the course of the IGT (block by block) than controls. Not surprising, the pattern of learning over blocks is different for the cocaine and MJ users. Specifically, the cocaine group shows more learning from the beginning (block 1) of the task to the end (block 5) of the task while the MJ group shows little learning between the beginning and the end. A detailed inspection of deck choices revealed that the counter-intuitive pattern of decreased performance by the control group from block 4 to 5 was the result of using up all the cards from the advantageous decks during the 4th or 5th block, thus forcing them to pick cards from the disadvantageous decks. Neither the cocaine nor MJ group showed a similar pattern of card use. Therefore, the observed discrepancies between cocaine and MJ users across blocks appear to be related to differences in decision-making learning, and not forced-drawing. In support, a post-hoc analysis using only blocks 1 to 4 left the “group by block” results unchanged. Finally, dose-related measures of cocaine use (grams per week) and MJ use (joints per week) predict IGT performance. The heavier the drug use, the lower the performance.
This is the first study to contrast decision-making performance in two distinct drug-using groups over repeated testing with the IGT. Cocaine users show lower scores than the MJ users or controls on the Total Net Score (Session 1 + Session 2). We believe that group discrepancies on the IGT can be largely related to the use of these specific drugs rather than to differences in group demographics. We were able to match the cocaine group, MJ group, and control group on several demographic variables that may affect cognitive performance including education and estimated Shipley IQ. Furthermore, both drug groups include relatively pure users of each drug since we exclude users with current or past dependence on any other substance. We also exclude individuals who had urine toxicology screens that are positive for drugs other than cocaine or MJ.
The main effect found between sessions (Session 1 versus Session 2) indicates that cocaine users learned less between sessions than the MJ or control groups (effect sizes; cocaine = <0.5; MJ = 1.18; and controls = 1.45). This confirms the relevance of examining decision-making processes over repeated testing. When we examined each session separately, most of the significance between group differences were evident on session 2 but not session 1. Specifically, within session 2, MJ users have the most difficulty switching their decision-making strategy towards advantageous choices across the different blocks of the second IGT session. MJ users therefore show a lower effect size (d=0.26) than cocaine users (d=0.97) when learning within blocks of the task during Session 2. Interestingly, Ernst et al., (2003) reported similar findings in adolescents. Adolescents with behavior disorders performed worse than healthy controls only in the second testing session of the IGT. These results suggest that adolescents with behavior disorders have a limited ability to improve decision-making performance across repeated testing. Noteworthy, behavior disorders during adolescence have been consistently associated with subsequent drug use (Tarter et al., 2003). A similar finding in substance abusers suggests that their learning deficit on decision-making performance could be more profound than previously acknowledged, and may have important implications for treatment failure and relapse.
Our results also demonstrate a strong dose-related association between cocaine and MJ use and IGT performance across both testing sessions. These results are consistent with previous reports showing that heavier use of cocaine and MJ is associated with greater neurocognitive impairment (Bolla et al., 1999, 2002, 2005). Our results also showed a significant association between duration of cocaine and MJ use and IGT performance. It is biologically plausible that prolonged use of these drugs may induce persistent damage to the brain regions responsible for decision-making processes (Franklin et al., 2002; Matochik et al., 2003, 2005). Indeed, decision-making deficits are evident in our sample even after 25-days of controlled abstinence. Previous studies had failed to reveal decision-making deficits in MJ users during abstinence. For example Quednow et al. (2007) did not find decision-making deficits in a group of pure MJ users. However, the same study showed decision-making alterations in a group of MDMA users with co-use of MJ. The most important difference between both studies is that we found a significant association between dose and duration of MJ use and decision-making dysfunction, whereas in the Quednow et al. (2007) study, poorer IGT performance was associated with MDMA but not MJ use. This is reasonable, since MJ users included in our study had greater amount and duration of MJ use than those included in the pure MJ and the MDMA+MJ groups from the Quednow et al. study. Furthermore, these effects are unlikely to be related to co-morbid mood or personality alterations, since we specifically excluded those drug abusers with Axis I/II disorders. Although heavy use of cocaine and MJ can potentially lead to alterations in brain functioning, we are aware that dose-related association does not prove that heavy drug use causes brain damage. It is possible that individuals with altered brain functioning are predisposed to use more cocaine or MJ. The present data cannot determine where drug use and decision-making, respectively, fall in the causal pathway. The strong relationship between cocaine use and MJ use and IGT performance seems to indicate that poorer performance of the drug using groups across testing sessions is actually a correlate of drug use and not a consequence of lack of effort or reduced motivation. Furthermore, we specifically attempted to increase participants’ motivation by giving them a proportional amount of real money contingent to the play money they gained in the task. Nevertheless, since level of effort or motivation was not controlled experimentally or statistically, we cannot completely rule out their impact.
These reported discrepancies between cocaine use and MJ use on IGT performance may be associated with the differential correlates of both drugs on brain regions and cognitive processes involved in decision-making. Previous PET activation studies have highlighted different brain deficits in cocaine and MJ users during performance on the IGT. Specifically, cocaine users showed hyperactivation of the orbitofrontal cortex (OFC) and ventral striatum (Bolla et al., 2003; Ersche et al., 2005), which are involved in the evaluation of reward related thoughts and events (O’Doherty, 2004; O’Doherty et al., 2001). In contrast, a group of MJ users had decreased OFC activation, whereas the heavy MJ users (greater than 35 joints/week) showed greater recruitment of posterior cingulate and parahippocampal regions argued to be involved in working memory and on-line task monitoring (Burgess, 2002; Peterson et al., 1999). Therefore, poorer performance by the cocaine group on the IGT could be related to enhanced sensitivity to the rewarding contingencies of the task. On the other hand, MJ deficits could be associated with impaired ability to track ongoing changes in task contingencies. This notion is supported by recent studies that have applied computational decision models to map the relationship between separate neuropsychological deficits and impaired performance on complex tasks of decision-making such as the IGT (Yechiam et al., 2005; Stout et al., 2004, 2005). According to these models, cocaine deficits are associated with a higher motivational valuation of reward, or a higher focus on gains, in the IGT and other risk taking paradigms (Lane et al., 2006; Stout et al., 2004, 2005). Conversely, memory/learning deficits (i.e., differences in the learning rate parameter) associated with higher discounting of distant losses could be mainly responsible for the MJ deficits (Lane et al., 2006; Yechiam et al., 2005). Both neuroimaging and decision models findings are consistent with our data. We believe that cocaine users may have more difficulty learning between IGT sessions because they always develop an exaggerated response to reward at the beginning of each session and this emotional evaluation bias their choices (Bechara & Damasio, 2002; Crone et al., 2004). This is consistent with the fact that cocaine users have increased sensitivity to monetary rewards (Bechara, personal communication) or memories/images related to drug use (Bonson et al., 2002; Grant et al., 1996). On the other hand, MJ users could have greater difficulty learning across blocks within each session because they fail to track memories of past losses (i.e., impaired on-line monitoring of task contingencies). This is supported by evidence showing that MJ users are frequently impaired on tasks of memory updating (Bolla et al., 2002; Lamers et al., 2006; Pope et al., 2003).
The pattern of limited learning in cocaine and MJ users over repeated testing may have important implications for treatment strategies for substance abuse. Previous studies indicated that decision-making deficits in substance abusers could be reversed as they become more experienced with the task (Bechara et al., 2001; Ernst et al., 2003). Our results support this notion, although both cocaine and MJ users learned slower than controls. This learning lag should be taken into account by specific treatments aimed to improve decision-making skills in substance abusers. Specific treatments aimed to recover discrete processes involved in complex decision-making, such as motivational processes involved in hypersensitivity to reward, or working memory, may be indicated.
Although these findings are in a relatively small sample of MJ and cocaine users, our results are robust and biologically plausible. Effect sizes reported were medium to large effects according to Cohen (>0.50). Furthermore, participants were selected stringently and the groups were well matched on several demographic variables relevant to cognitive functioning. Nonetheless, others need to replicate these findings in larger groups of MJ and cocaine users. An important limitation that restricts generalization of these findings was the non-inclusion of women. In addition, the exclusion of individuals with Axis-II co-morbidity, who are quite prevalent in this population, also limits generalization. Although we did not include these participants in an attempt to avoid methodological confounds, future studies should directly investigate the influence of gender and psychopathology on decision-making and other cognitive correlates of drug use. Finally, the fact that some participants run out of cards before task completion is a problematic issue. However, it is important to note that this issue mainly affected performance of the control group (a drop of performance on block 5) but not that of clinical groups. Furthermore, results were unchanged when we re-analyzed “group by block” performance using only the first four blocks. In any case, researchers should be aware of this limitation of the IGT that can be surpassed using a later version of the task including 60 cards per deck.
Acknowledgments
Supported by NIH grants DA 11426 (KB), the JHBMC-GCRC (MO1 RR02719) and the DHH NIDA Intramural Research Program. We thank the nurses and clinical staff at NIDA-IRP, the Brain Imaging Center, and the Johns Hopkins Bayview GCRC who contributed to this project. We especially thank Debra Hill, B.A. for computer and database support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bartzokis G, Lu PH, Beckson M, Rapoport R, Grant S, Wiseman EJ, London ED. Abstinence from cocaine reduces high-risk responses on a gambling task. Neuropsychopharmacology. 2000;22:102–103. doi: 10.1016/S0893-133X(99)00077-9. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio AR, Damasio H, Anderson SW. Insensitivity to future consequences following damage to human prefrontal cortex. Cognition. 1994;50:7–15. doi: 10.1016/0010-0277(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H, Tranel D, Damasio AR. Deciding advantageously before knowing the advantageous strategy. Science. 1997;28:1293–1295. doi: 10.1126/science.275.5304.1293. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Denburg N, Hindes A, Anderson SW, Nathan PE. Decision-making deficits, linked to a dysfunctional ventromedial prefrontal cortex, revealed in alcohol and stimulant abusers. Neuropsychologia. 2001;39:376–389. doi: 10.1016/s0028-3932(00)00136-6. [DOI] [PubMed] [Google Scholar]
- Bechara A, Damasio H. Decision-making and addiction (part I), impaired activation of somatic states in substance dependent individuals when pondering decisions with negative future consequences. Neuropsychologia. 2002;40:1675–1689. doi: 10.1016/s0028-3932(02)00015-5. [DOI] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H. Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain. 2000;123:2189–2202. doi: 10.1093/brain/123.11.2189. [DOI] [PubMed] [Google Scholar]
- Block RI, Ghoneim MM. Effects of chronic marijuana use on human cognition. Psychopharmacology. 1993;110:219–228. doi: 10.1007/BF02246977. [DOI] [PubMed] [Google Scholar]
- Bolla K, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–1343. doi: 10.1212/01.wnl.0000031422.66442.49. [DOI] [PubMed] [Google Scholar]
- Bolla K, Eldreth D, London ED, Kiehl KA, Mouratidis M, Contoreggi C, Matochik JA, Kurian V, Cadet JL, Kimes AS, Funderburk F, Ernst M. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. NeuroImage. 2003;19:1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolla KI, Rothman RB, Cadet J-L. Dose-related neurobehavioral effects of chronic cocaine use. J Neuropsychiatry Clin Neurosci. 1999;11:361–369. doi: 10.1176/jnp.11.3.361. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Eldreth DA, Matochik JA, Cadet JL. Neural substrates of faulty decision-making in abstinent marijuana users. Neuroimage. 2005;26:480–492. doi: 10.1016/j.neuroimage.2005.02.012. [DOI] [PubMed] [Google Scholar]
- Bonson KR, Grant S, Contoreggi C, Links J, Metalfe J, Wayl HL, Kurian V, Ernst M, London ED. Neural systems and cue-induced cocaine craving. Neuropsychopharmacology. 2002;26:376–386. doi: 10.1016/S0893-133X(01)00371-2. [DOI] [PubMed] [Google Scholar]
- Burgess N. The hippocampus, space, and viewpoints in episodic memory. Q J Exp Psychol. 2002;55:1057–1080. doi: 10.1080/02724980244000224. [DOI] [PubMed] [Google Scholar]
- Crone EA, Somsen RJ, Van BB, van der Molen MW. Heart rate and skin conductance analysis of antecendents and consequences of decision making. Psychophysiology. 2004;41:531–540. doi: 10.1111/j.1469-8986.2004.00197.x. [DOI] [PubMed] [Google Scholar]
- Di Sclafani V, Tolou-Shams M, Price LJ, Fein G. Neuropsychological performance of individuals dependent on crack-cocaine, or crack-cocaine and alcohol, at 6 weeks and 6 months of abstinence. Drug Alcohol Depend. 2002;66:161–171. doi: 10.1016/s0376-8716(01)00197-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldreth DA, Matochik JA, Cadet JL, Bolla KI. Abnormal brain activity in prefrontal brain regions in abstinent marijuana users. Neuroimage. 2004;23:914–920. doi: 10.1016/j.neuroimage.2004.07.032. [DOI] [PubMed] [Google Scholar]
- Ernst M, Grant SJ, London ED, Contoreggi CS, Kimes AS, Spurgeon L. Decision making in adolescents with behavior disorders and adults with substance abuse. Am J Psychiatry. 2003;160:33–40. doi: 10.1176/appi.ajp.160.1.33. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Clark L, London M, Robbins TW, Sahakian BJ. Profile of executive and memory function associated with amphetamine and opiate dependence. Neuropsychopharmacology. 2006;31:1036–1047. doi: 10.1038/sj.npp.1300889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ersche KD, Fletcher PC, Lewis SJ, Clark L, Stocks-Gee G, London M, Deakin JB, Robbins TW, Sahakian BJ. Abnormal frontal activations related to decision-making in current and former amphetamine and opiate dependent individuals. Psychopharmacology (Berl) 2005;180:612–623. doi: 10.1007/s00213-005-2205-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin TR, Acton PD, Maldjian JA, Gray JD, Croft JR, Dackis CA, O’Brien CP, Childress AR. Decreased gray matter concentration in the insular, orbitofrontal, cingulate, and temporal cortices of cocaine patients. Biol Psychiatry. 2002;51:134–142. doi: 10.1016/s0006-3223(01)01269-0. [DOI] [PubMed] [Google Scholar]
- Grant S, London ED, Newlin DB, Villemagne VL, Liu X, Contoreggi C, Phillips RL, Kimes AS, Margolin A. Activation of memory circuits during cue-elicited cocaine craving. Proc Natl Acad Sci. 1996;93:12040–12045. doi: 10.1073/pnas.93.21.12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant S, Contoreggi C, London ED. Drug abusers show impaired performance in a laboratory test of decision-making. Neuropsychologia. 2000;38:1180–1187. doi: 10.1016/s0028-3932(99)00158-x. [DOI] [PubMed] [Google Scholar]
- Haney M, Ward AS, Comer SD, Foltin RW, Fischman MW. Abstinence symptoms following smoked marijuana in humans. Psychopharmacology. 1999;141:395–404. doi: 10.1007/s002130050849. [DOI] [PubMed] [Google Scholar]
- Knutson B, Cooper JC. Functional magnetic resonance imaging of reward prediction. Curr Opin Neurol. 2005;18:411–417. doi: 10.1097/01.wco.0000173463.24758.f6. [DOI] [PubMed] [Google Scholar]
- Lamers CT, Bechara A, Rizzo M, Ramaekers JG. Cognitive function and mood in MDMA/THC users, THC users and non-drug using controls. J Psychopharmacol. 2006;20:302–311. doi: 10.1177/0269881106059495. [DOI] [PubMed] [Google Scholar]
- Lane SD, Yechiam E, Busemeyer JR. Application of a computational decision model to examine acute drug effects on human risk taking. Exp Clin Psychopharmacol. 2006;14:254–264. doi: 10.1037/1064-1297.14.2.254. [DOI] [PubMed] [Google Scholar]
- Matochik J, London ED, Eldreth D, Cadet JL, Bolla K. Frontal cortical tissue composition in abstinent cocaine abusers, A magnetic resonance imaging study. Neuroimage. 2003;19:1095–1102. doi: 10.1016/s1053-8119(03)00244-1. [DOI] [PubMed] [Google Scholar]
- Matochik JA, Eldreth DA, Cadet JL, Bolla KI. Altered brain tissue composition in heavy marijuana users. Drug Alcohol Depend. 2005;77:23–30. doi: 10.1016/j.drugalcdep.2004.06.011. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Luborsky L, Woody GE, O’Brien CP. An improved evaluation instrument for substance abuse patients, the Addiction Severity Index. J Nerv Ment Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- National Household Survey on Drug Abuse. US Department of Health and Human Services Substance Abuse and Mental Health Services Administration Office of Applied Studies. Washington, D.C: U.S. Government Printing Office; 2002. [Google Scholar]
- O’Doherty J, Kringelbach ML, Rolls ET, Hornak J, Andrews C. Abstract reward and punishment representations in the human orbitofrontal cortex. Nature Neurosci. 2001;4:95–102. doi: 10.1038/82959. [DOI] [PubMed] [Google Scholar]
- O’Doherty JP. Reward representations and reward-related learning in the human brain, insights from neuroimaging. Curr Opin Neurobiol. 2004;14:769–776. doi: 10.1016/j.conb.2004.10.016. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Schuckit MA. Neural activation patterns of methamphetamine-dependent subjects during decision making predict relapse. Arch Gen Psychiatry. 2005;62:761–768. doi: 10.1001/archpsyc.62.7.761. [DOI] [PubMed] [Google Scholar]
- Peterson BS, Skudlarski P, Gatenby JC, Zhang H, Anderson AW, Gore JC. An fMRI study of Stroop word-color interference, evidence for cingulate subregions subserving multiple distributed attentional systems. Biol Psychiatry. 1999;45:1237–1258. doi: 10.1016/s0006-3223(99)00056-6. [DOI] [PubMed] [Google Scholar]
- Pinheiro JC, Bates DM. Mixed effect models in S and S-Plus. New York: Springer; 2000. [Google Scholar]
- Pope HG, Gruber AJ, Hudson JI, Huestis M, Yurgelun-Todd D. Neuropsychological performance in long-term cannabis users. Arch Gen Psychiatry. 2001;58:909–915. doi: 10.1001/archpsyc.58.10.909. [DOI] [PubMed] [Google Scholar]
- Pope HG, Gruber AJ, Hudson JI, Cohane G, Huestis MA, Yurgelun-Todd D. Early-onset cannabis use and cognitive deficits, what is the nature of the association? Drug Alcohol Depend. 2003;69:303–310. doi: 10.1016/s0376-8716(02)00334-4. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Kuhn KU, Hoppe C, Westheide J, Maier W, Daum I, Wagner M. Elevated impulsivity and impaired decision-making cognition in heavy users of MDMA (“Ecstasy”) Psychopharmacology (Berl) 2007;189:517–530. doi: 10.1007/s00213-005-0256-4. [DOI] [PubMed] [Google Scholar]
- Robins LN, Helzer JE, Croughan J, Ratcliff KS. National Institute of Mental Health Diagnostic Interview Schedule, Its history, characteristics, and validity. Arch Gen Psychiatry. 1981;38:381–389. doi: 10.1001/archpsyc.1981.01780290015001. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Everitt BJ, Baldacchino AM, Blackshaw AJ, Swainsom R, Wynne K, Sahakian BJ. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers, Evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999;20:322–339. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- Selby MJ, Azrin RL. Neuropsychological functioning in drug abusers. Drug Alcohol Depend. 1998;50:39–45. doi: 10.1016/s0376-8716(98)00002-7. [DOI] [PubMed] [Google Scholar]
- Smith SS. Addictive drug survey manual. Baltimore: NIDA Addiction Research Center; 1991. [Google Scholar]
- Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, Christiansen K, McRee B, Vendetti J Marijuana Treatment Project Research Group. Cognitive functioning of long-term heavy Cannabis users seeking treatment. J Am Med Assoc. 2002;287:1123–1131. doi: 10.1001/jama.287.9.1123. [DOI] [PubMed] [Google Scholar]
- Stout JC, Busemeyer JR, Lin A, Grant SJ, Bonson KR. Cognitive modeling analysis of decision-making processes in cocaine abusers. Psychon Bull Rev. 2004;11:742–747. doi: 10.3758/bf03196629. [DOI] [PubMed] [Google Scholar]
- Stout JC, Rock SL, Campbell MC, Busemeyer JR, Finn PR. Psychological processes underlying risky decisions in drug abusers. Psychol Addict Behav. 2005;19:148–157. doi: 10.1037/0893-164X.19.2.148. [DOI] [PubMed] [Google Scholar]
- Tarter RE, Kirisci L, Mezzich A, Cornelius JR, Pajer K, Pajer K, Vanyukov M, Gardner W, Blackson T, Clark D. Neurobehavioral disinhibition in childhood predicts early age at onset of substance use disorder. Am J Psychiatry. 2003;160:1078–1085. doi: 10.1176/appi.ajp.160.6.1078. [DOI] [PubMed] [Google Scholar]
- Toomey R, Lyons MJ, Eisen SA, Xian H, Chantarujikapong S, Seidman LJ, Faraone SY, Tsuang MT. A twin study of the neuropsychological consequences of stimulant abuse. Arch Gen Psychiatry. 2003;60:303–310. doi: 10.1001/archpsyc.60.3.303. [DOI] [PubMed] [Google Scholar]
- Tucker KA, Browndyke JN, Gottschalk PC, Cofrancesco AT, Kosten TR. Gender-specific vulnerability for rCBF abnormalities among cocaine abusers. Neuroreport. 2004;15:797–801. doi: 10.1097/00001756-200404090-00011. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia A, Rivas-Perez C, Vilar-Lopez R, Perez-Garcia M. Strategic self-regulation, decision-making and emotion processing in poly-substance abusers in their first year of abstinence. Drug Alcohol Depend. 2006;86:139–146. doi: 10.1016/j.drugalcdep.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Verdejo-Garcia AJ, Perales JC, Perez-Garcia M. Cognitive impulsivity in cocaine and heroin polysubstance abusers. Addict Behav. 2006 doi: 10.1016/j.addbeh.2006.06.032. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS. Addiction, a disease of compulsion and drive, Involvement of the orbitofrontal cortex. Cereb Cortex. 2000;10:318–325. doi: 10.1093/cercor/10.3.318. [DOI] [PubMed] [Google Scholar]
- Whitlow CT, Freedland CS, Porrino LJ. Functional consequences of the repeated administration of Delta9-tetrahydrocannabinol in the rat. Drug Alcohol Depend. 2003;71:169–177. doi: 10.1016/s0376-8716(03)00135-2. [DOI] [PubMed] [Google Scholar]
- Whitlow CT, Liguori A, Livengood LB, Hart SL, Mussat-Whitlow BJ, Lamborn CM, Laurienti PJ, Porrino LJ. Long-term heavy marijuana users make costly decisions on a gambling task. Drug Alcohol Depend. 2004;76:107–111. doi: 10.1016/j.drugalcdep.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Yechiam E, Busemeyer JR, Stout JC, Bechara A. Using cognitive models to map relations between neuropsychological disorders and human decision-making deficits. Psychol Sci. 2005;16:973–978. doi: 10.1111/j.1467-9280.2005.01646.x. [DOI] [PubMed] [Google Scholar]
- Zachary RA. Shipley Institute of Living Scale, Revised Manual. Los Angeles, CA: Western Psychological Services; 1991. [Google Scholar]


