Abstract
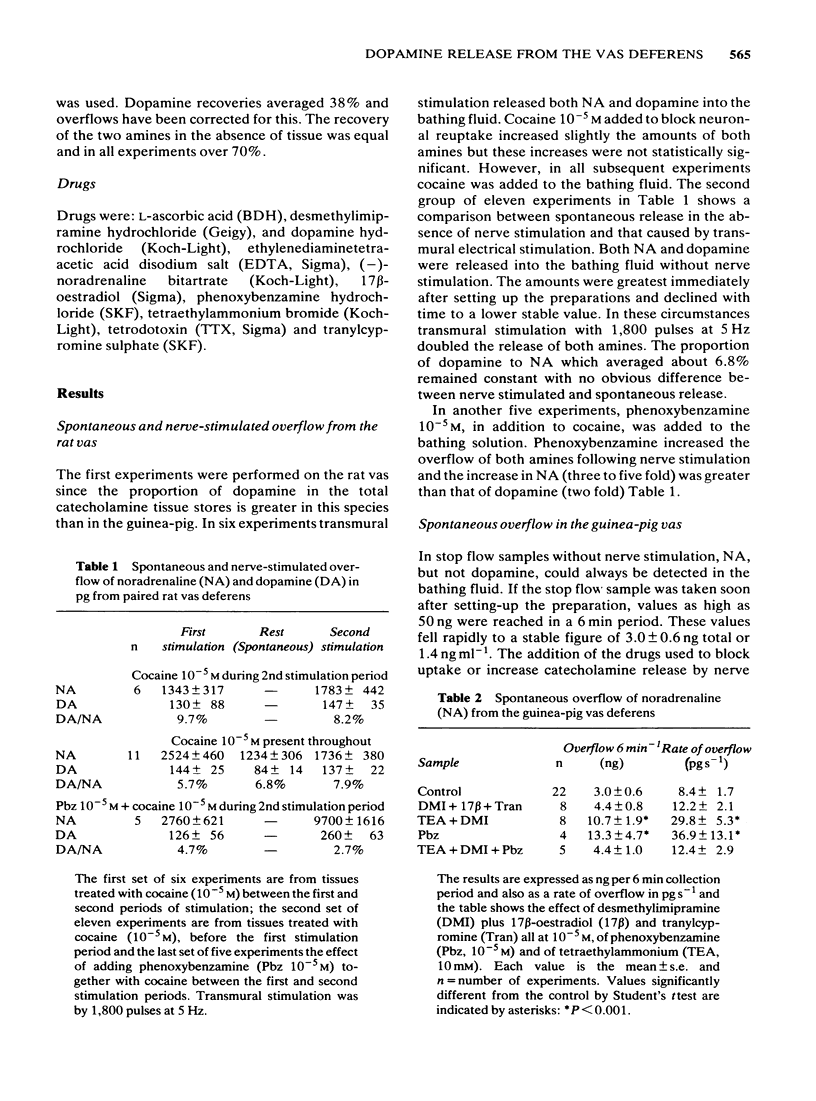
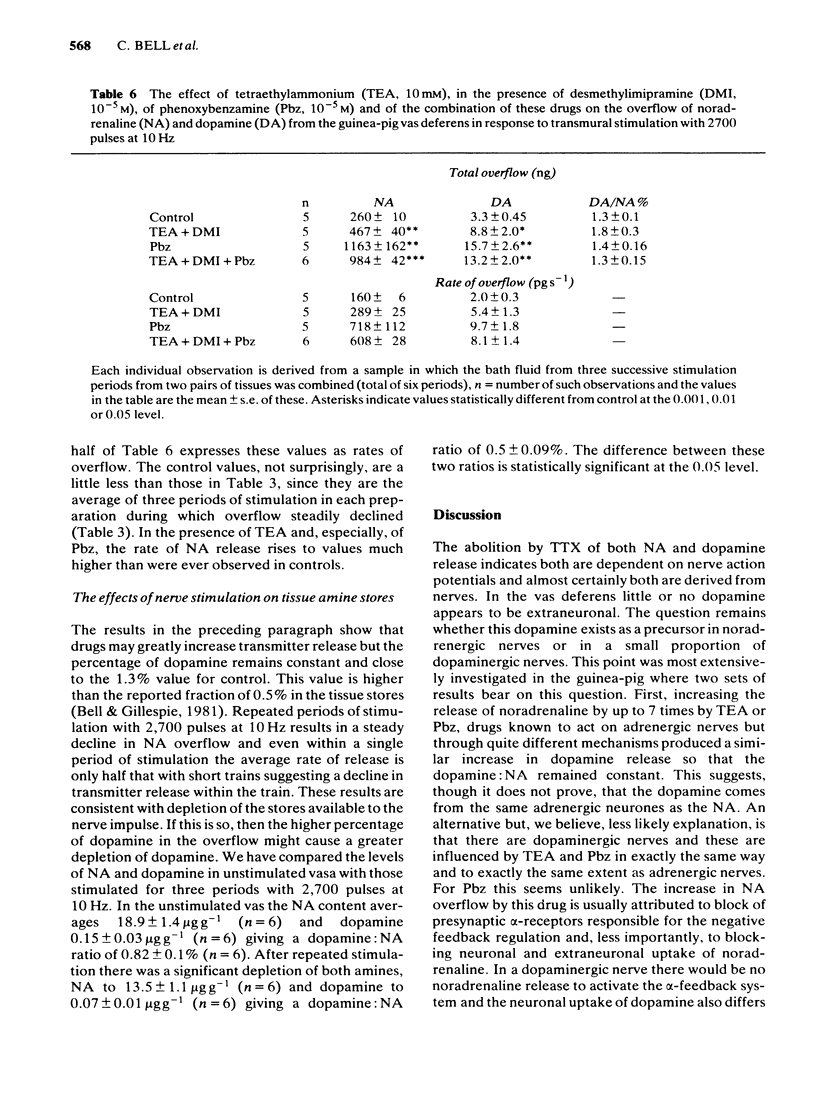
Spontaneous and nerve stimulated release of noradrenaline and dopamine from rat and guinea-pig vas deferens have been measured electrochemically after separation by high performance liquid chromatography (h.p.l.c.). In the absence of nerve stimulation both noradrenaline (NA) and dopamine were released into the bathing fluid in the rat but in the guinea-pig only noradrenaline could be detected. Drugs which block neuronal and extraneuronal uptake of catecholamines had little effect on spontaneous overflow but both tetraethylammonium and phenoxybenzamine increased overflow. Transmural nerve stimulation (5-10 Hz) increased catecholamine overflow in both species and dopamine release was now measurable from the guinea-pig vas. In the rat, the proportion of dopamine to NA was unchanged from that released spontaneously. The release of both amines was little affected by drugs that block neuronal and extraneuronal uptake and a monoamine oxidase inhibitor, but was inhibited by tetrodotoxin 0.2 microgram ml-1. In the guinea-pig tetraethylammonium 10 mM doubled overflow and phenoxybenzamine 10(-5)M increased it by five times but the dopamine percentage remained constant and equal to the control. Following nerve stimulation the amount of dopamine released expressed as a percentage of total catecholamine release was 6% for the rat and 1.3% for the guinea-pig. These values were considerably higher than the comparable figures for dopamine: NA content of the two tissues (2% and 0.5% respectively).(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell C. Dopamine as a postganglionic autonomic neurotransmitter. Neuroscience. 1982 Jan;7(1):1–8. doi: 10.1016/0306-4522(82)90147-6. [DOI] [PubMed] [Google Scholar]
- Bell C. Effects of dopamine on adrenergic neuromuscular transmission in the guinea-pig vas deferens. Br J Pharmacol. 1980 Mar;68(3):505–512. doi: 10.1111/j.1476-5381.1980.tb14565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell C., Gillespie J. S. Dopamine and noradrenaline levels in peripheral tissues of several mammalian species. J Neurochem. 1981 Feb;36(2):703–706. doi: 10.1111/j.1471-4159.1981.tb01645.x. [DOI] [PubMed] [Google Scholar]
- Callingham B. A., Barrand M. A. Catecholamines in blood. J Pharm Pharmacol. 1976 Apr;28(4 Suppl):356–360. doi: 10.1111/j.2042-7158.1976.tb04179.x. [DOI] [PubMed] [Google Scholar]
- Christensen N. J., Mathias C. J., Frankel H. L. Plasma and urinary dopamine: studies during fasting and exercise and in tetraplegic man. Eur J Clin Invest. 1976 Sep 10;6(5):403–409. doi: 10.1111/j.1365-2362.1976.tb00535.x. [DOI] [PubMed] [Google Scholar]
- Holman M. E., Surprenant A. An electrophysiological analysis of the effects of noradrenaline and alpha-receptor antagonists on neuromuscular transmission in mammalian muscular arteries. Br J Pharmacol. 1980;71(2):651–661. doi: 10.1111/j.1476-5381.1980.tb10986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvetnansky R., Sun C. L., Lake C. R., Thoa N., Torda T., Kopin I. J. Effect of handling and forced immobilization on rat plasma levels of epinephrine, norepinephrine, and dopamine-beta-hydroxylase. Endocrinology. 1978 Nov;103(5):1868–1874. doi: 10.1210/endo-103-5-1868. [DOI] [PubMed] [Google Scholar]
- Musacchio J. M., Fischer J. E., Kopin I. J. Subcellular distribution and release by sympathetic nerve stimulation of dopamine and alpha-methyl dopamine. J Pharmacol Exp Ther. 1966 Apr;152(1):51–55. [PubMed] [Google Scholar]


