Abstract
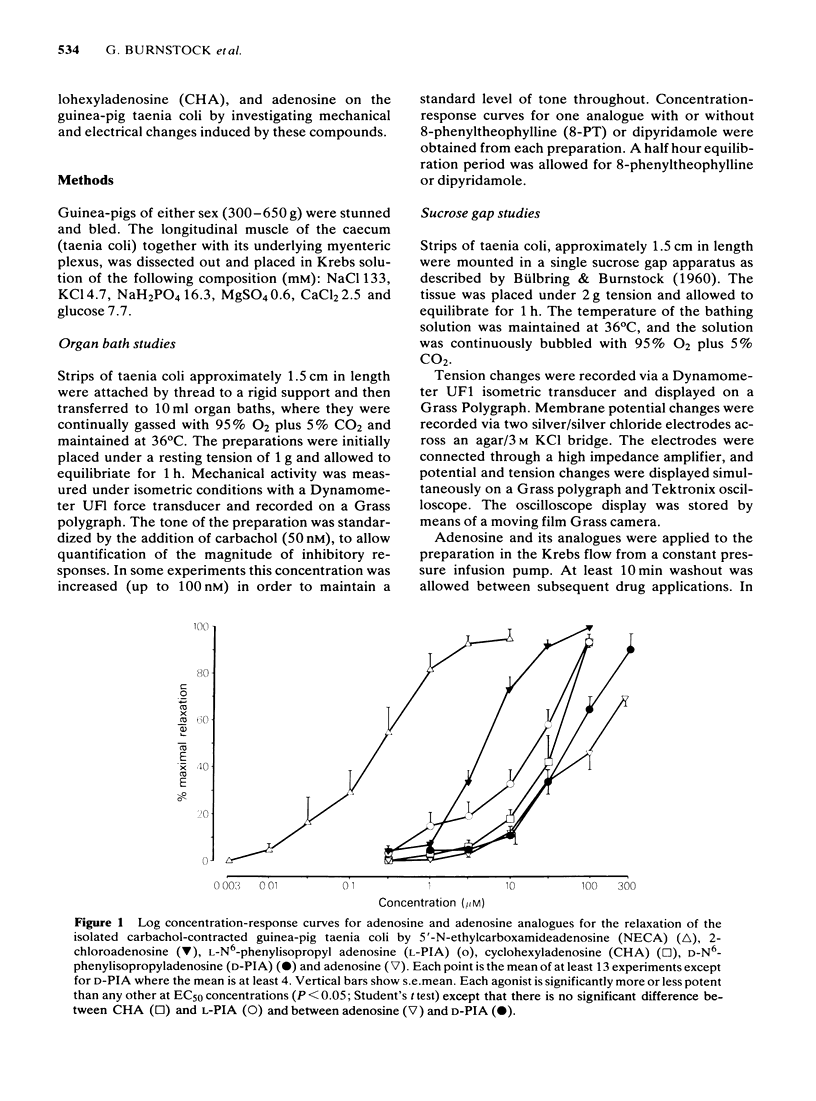
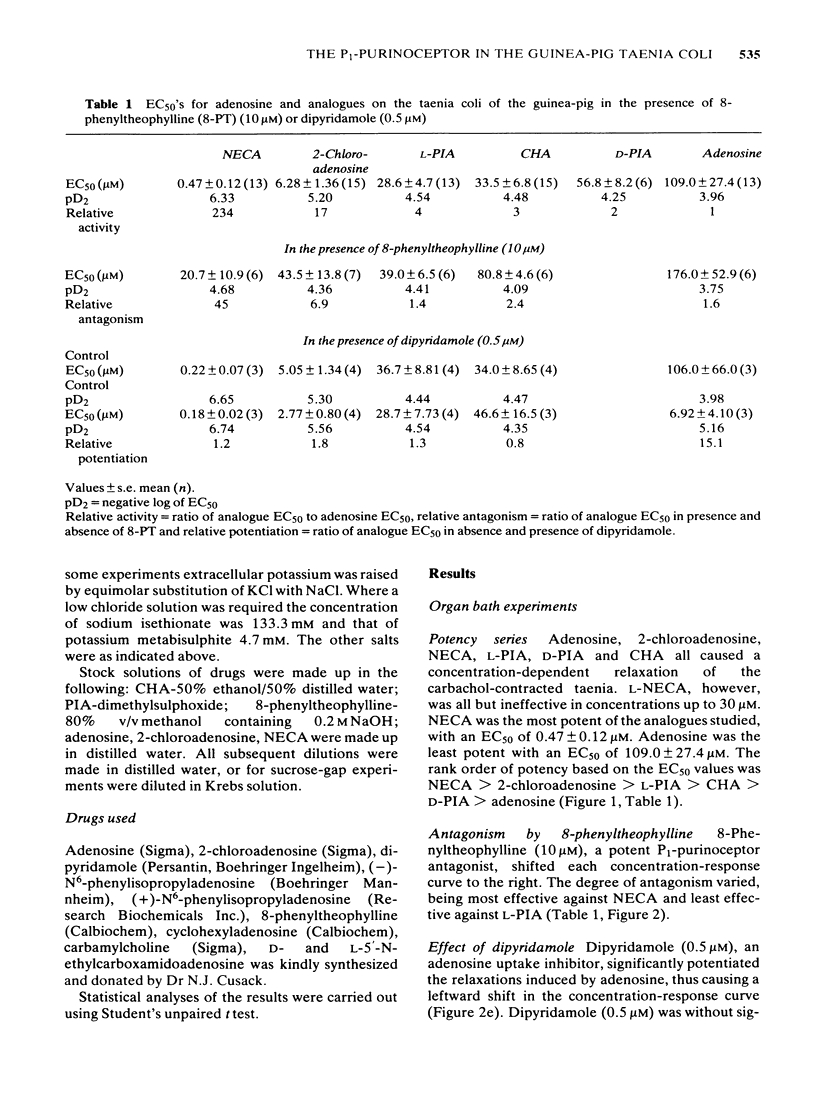
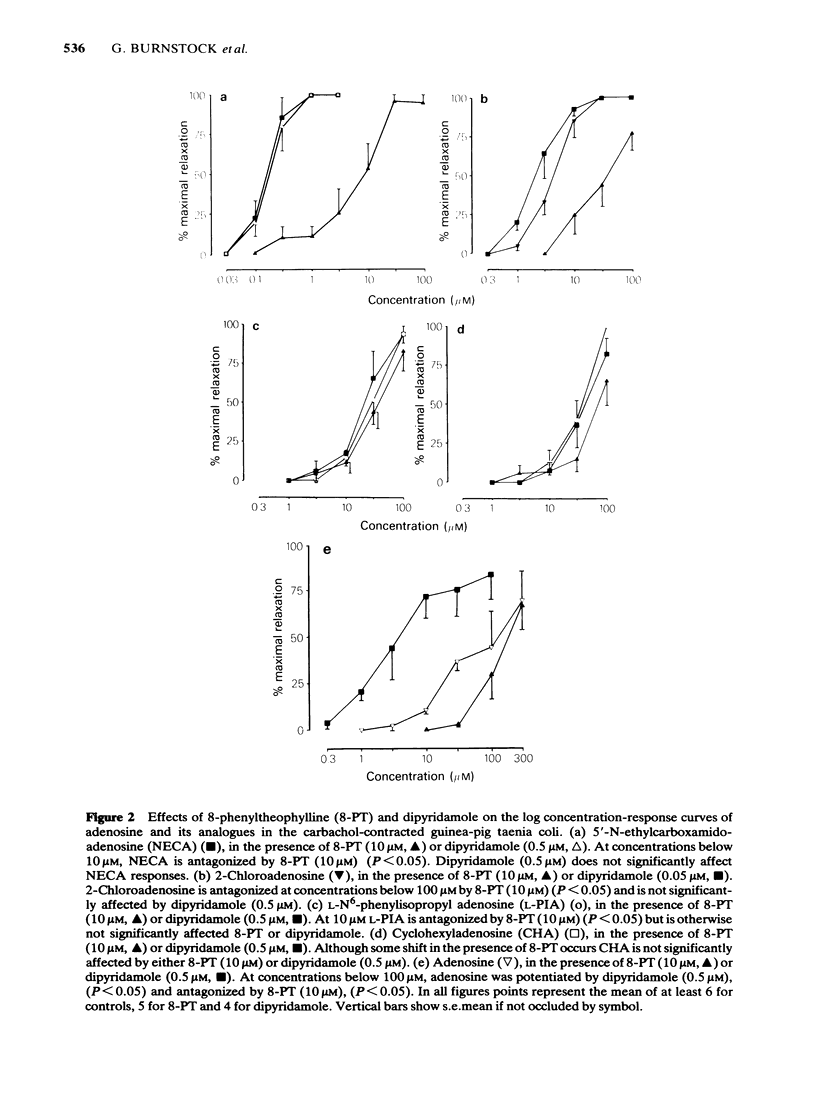
The effects of 5'-N-ethylcarboxamidoadenosine (NECA), L-NECA, 2-chloroadenosine, N6-phenylisopropyladenosine (L-PIA and D-PIA), cyclohexyladenosine (CHA), and adenosine were examined on the guinea-pig taenia coli. All the analogues except L-NECA caused relaxations; the order of potency for the series was: NECA greater than 2-chloroadenosine greater than L-PIA greater than CHA greater than D-PIA greater than adenosine. L-PIA was twice as potent as D-PIA in inducing relaxations of the guinea-pig taenia coli. Adenosine and its analogues that induce relaxation all caused a slow membrane hyperpolarization; differences in the rates of hyperpolarization and latencies were apparent, although not statistically significant. The duration of the response to adenosine was significantly less than that for any adenosine analogue. Ion studies, using the sucrose gap, revealed that responses to the analogues were attenuated in elevated extracellular potassium or reduced extracellular chloride. 8-Phenyltheophylline, a potent P1-purinoceptor antagonist, caused a rightward shift of all the adenosine and analogue concentration-response curves. Dipyridamole, an adenosine uptake inhibitor, potentiated the relaxations to adenosine but had no significant effect on the relaxations induced by the analogues. It is concluded that NECA, 2-chloroadenosine, L-PIA, CHA, D-PIA and adenosine mediate their relaxant effects via an extracellular P1-purinoceptor which displays characteristics of the A2-subtype as determined by the rank order of agonist potency. Electrophysiological analysis of the responses to each of the analogues did not reveal any marked differences in the modes of action even between NECA and L-PIA (preferential A2- and A1-receptor agonists, respectively).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelsson J., Holmberg B. The effects of extracellularly applied ATP and related compounds on electrical and mechanical activity of the smooth muscle taenia coli from the guinea-pig. Acta Physiol Scand. 1969 Jan-Feb;75(1):149–156. doi: 10.1111/j.1748-1716.1969.tb04366.x. [DOI] [PubMed] [Google Scholar]
- Brown C. M., Burnstock G. Evidence in support of the P1/P2 purinoceptor hypothesis in the guinea-pig taenia coli. Br J Pharmacol. 1981 Jul;73(3):617–624. doi: 10.1111/j.1476-5381.1981.tb16796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. M., Collis M. G. Evidence for an A2/Ra adenosine receptor in the guinea-pig trachea. Br J Pharmacol. 1982 Jul;76(3):381–387. doi: 10.1111/j.1476-5381.1982.tb09231.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruns R. F. Adenosine receptor activation in human fibroblasts: nucleoside agonists and antagonists. Can J Physiol Pharmacol. 1980 Jun;58(6):673–691. doi: 10.1139/y80-110. [DOI] [PubMed] [Google Scholar]
- Bruns R. F., Daly J. W., Snyder S. H. Adenosine receptors in brain membranes: binding of N6-cyclohexyl[3H]adenosine and 1,3-diethyl-8-[3H]phenylxanthine. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5547–5551. doi: 10.1073/pnas.77.9.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collis M. G. Evidence for an A1-adenosine receptor in the guinea-pig atrium. Br J Pharmacol. 1983 Jan;78(1):207–212. doi: 10.1111/j.1476-5381.1983.tb09381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusack N. J., Hourani S. M. 5'-N-ethylcarboxamidoadenosine: a potent inhibitor of human platelet aggregation. Br J Pharmacol. 1981 Mar;72(3):443–447. doi: 10.1111/j.1476-5381.1981.tb10995.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly J. W. Adenosine receptors: targets for future drugs. J Med Chem. 1982 Mar;25(3):197–207. doi: 10.1021/jm00345a001. [DOI] [PubMed] [Google Scholar]
- Ferrero J. D., Frischknecht R. Different effector mechanisms for ATP and adenosine hyperpolarization in the guinea-pig taenia coli. Eur J Pharmacol. 1983 Jan 28;87(1):151–154. doi: 10.1016/0014-2999(83)90063-8. [DOI] [PubMed] [Google Scholar]
- Griffith S. G., Meghji P., Moody C. J., Burnstock G. 8-phenyltheophylline: a potent P1-purinoceptor antagonist. Eur J Pharmacol. 1981 Oct 15;75(1):61–64. doi: 10.1016/0014-2999(81)90346-0. [DOI] [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Schlegel W., Rodbell M. Adenosine analogs inhibit adipocyte adenylate cyclase by a GTP-dependent process: basis for actions of adenosine and methylxanthines on cyclic AMP production and lipolysis. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5362–5366. doi: 10.1073/pnas.75.11.5362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Wolff J. Subclasses of external adenosine receptors. Proc Natl Acad Sci U S A. 1980 May;77(5):2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah H. D., Daly J. W. Adenosine-dependent formation of cyclic AMP in brain slices. Pharmacol Res Commun. 1976 Feb;8(1):65–79. doi: 10.1016/0031-6989(76)90030-8. [DOI] [PubMed] [Google Scholar]
- Paton D. M. Structure-activity relations for presynaptic inhibition of noradrenergic and cholinergic transmission by adenosine: evidence for action on A1 receptors. J Auton Pharmacol. 1981 Sep;1(4):287–290. doi: 10.1111/j.1474-8673.1981.tb00457.x. [DOI] [PubMed] [Google Scholar]
- Reddington M., Lee K. S., Schubert P. An A1-adenosine receptor, characterized by [3H] cyclohexyladenosine binding, mediates the depression of evoked potentials in a rat hippocampal slice preparation. Neurosci Lett. 1982 Mar 5;28(3):275–279. doi: 10.1016/0304-3940(82)90070-2. [DOI] [PubMed] [Google Scholar]
- Satchell D. G., Burnstock G. Comparison of the inhibitory effects on the guinea-pig taenia coli of adenine nucleotides and adenosine in the presence and absence of dipyridamole. Eur J Pharmacol. 1975 Jun-Jul;32(02):324–328. doi: 10.1016/0014-2999(75)90299-x. [DOI] [PubMed] [Google Scholar]
- Satchell D. G., Maguire M. H. Evidence for separate receptors for ATP and adenosine in the guinea-pig taenia coli. Eur J Pharmacol. 1982 Jul 30;81(4):669–672. doi: 10.1016/0014-2999(82)90358-2. [DOI] [PubMed] [Google Scholar]
- Schrader J., Rubio R., Berne R. M. Inhibition of slow action potentials of guinea pig atrial muscle by adenosine: a possible effect on Ca2+ influx. J Mol Cell Cardiol. 1975 Jun;7(6):427–433. doi: 10.1016/0022-2828(75)90048-6. [DOI] [PubMed] [Google Scholar]
- Schütz W., Steurer G., Tuisl E. Functional identification of adenylate cyclase-coupled adenosine receptors in rat brain microvessels. Eur J Pharmacol. 1982 Nov 19;85(2):177–184. doi: 10.1016/0014-2999(82)90463-0. [DOI] [PubMed] [Google Scholar]
- Siggins G. R., Schubert P. Adenosine depression of hippocampal neurons in vitro: an intracellular study of dose-dependent actions on synaptic and membrane potentials. Neurosci Lett. 1981 Apr 9;23(1):55–60. doi: 10.1016/0304-3940(81)90186-5. [DOI] [PubMed] [Google Scholar]
- Silinsky E. M. On the calcium receptor that mediates depolarization-secretion coupling at cholinergic motor nerve terminals. Br J Pharmacol. 1981 Jun;73(2):413–429. doi: 10.1111/j.1476-5381.1981.tb10438.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smellie F. W., Daly J. W., Dunwiddie T. V., Hoffer B. J. The dextro and levorotatory isomers of N-phenylisopropyladenosine: stereospecific effects on cyclic AMP-formation and evoked synaptic responses in brain slices. Life Sci. 1979 Nov 12;25(20):1739–1748. doi: 10.1016/0024-3205(79)90477-6. [DOI] [PubMed] [Google Scholar]
- Stone T. W. The effects of 4-aminopyridine on the isolated vas deferens and its effects on the inhibitory properties of adenosine, morphine, noradrenaline and gamma-aminobutyric acid. Br J Pharmacol. 1981 Jul;73(3):791–796. doi: 10.1111/j.1476-5381.1981.tb16817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Calker D., Müller M., Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979 Nov;33(5):999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]


