Abstract
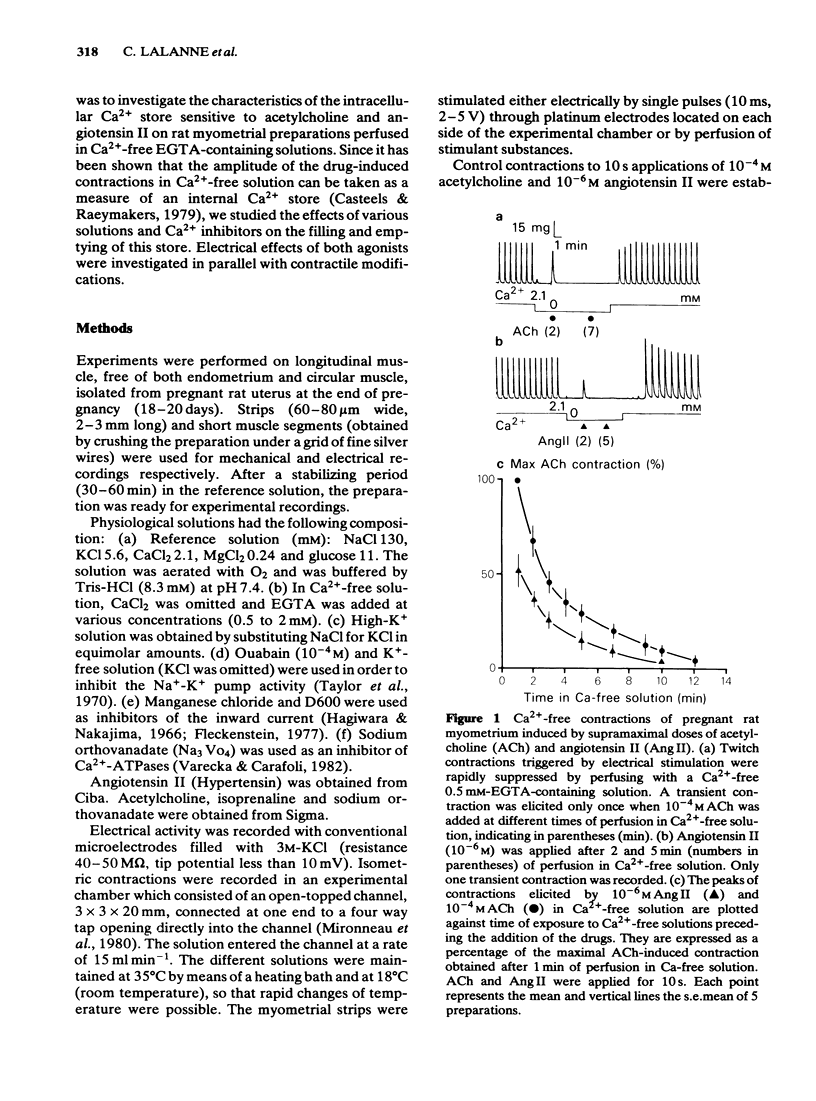
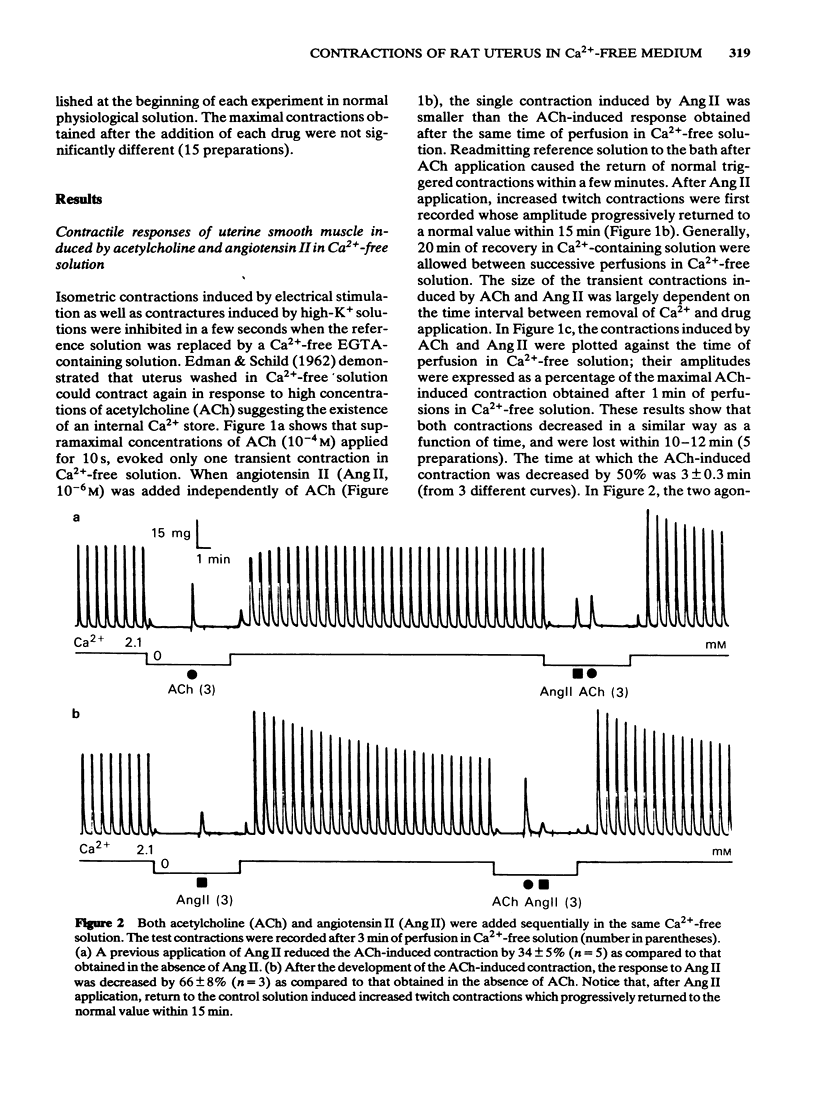
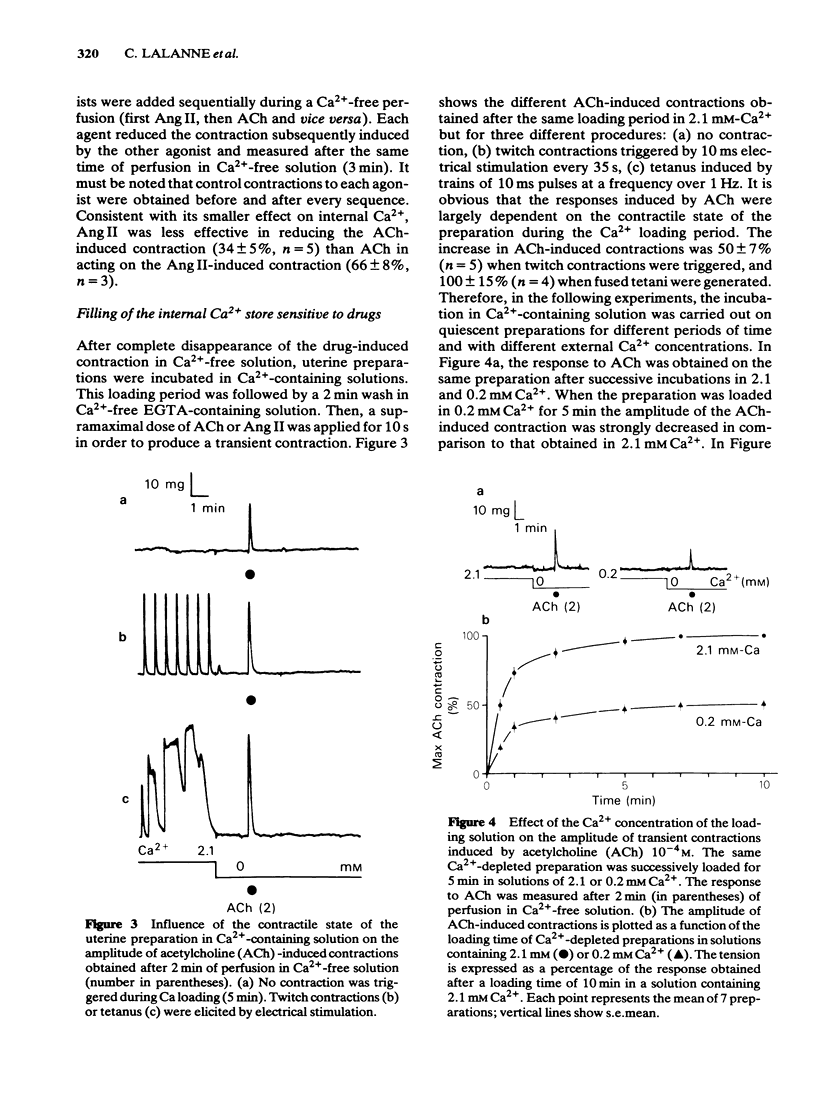
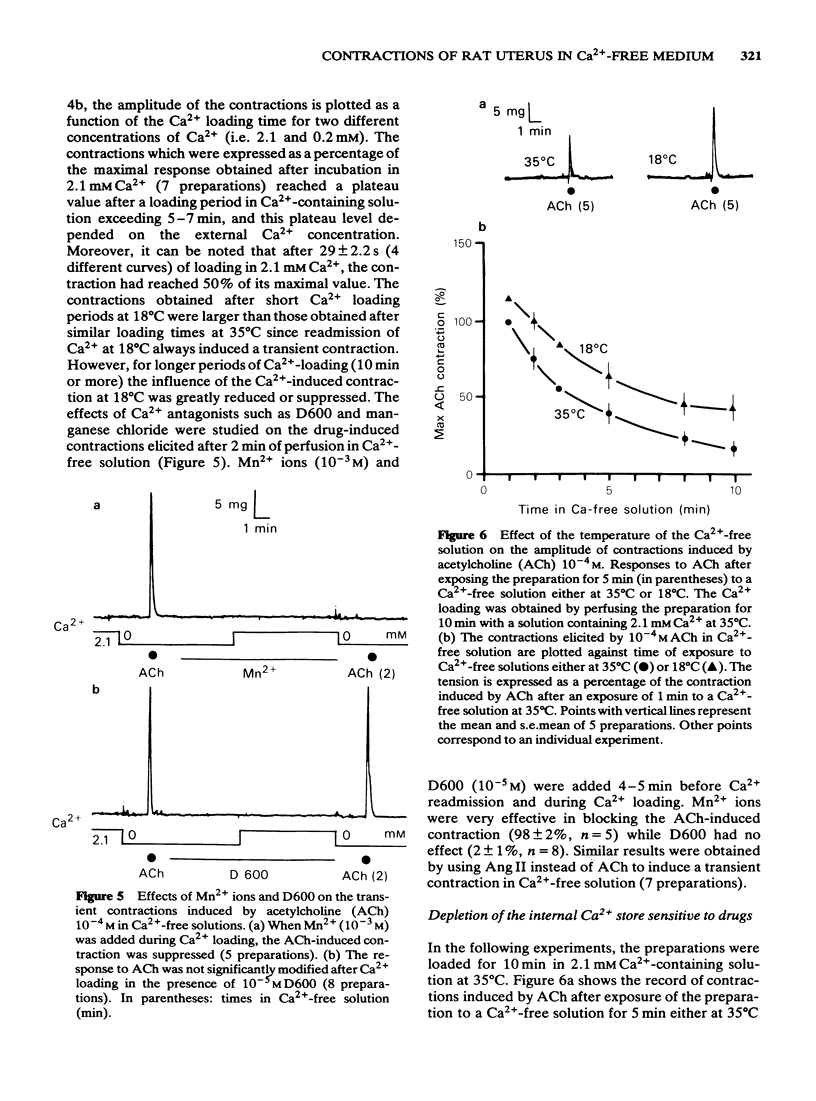
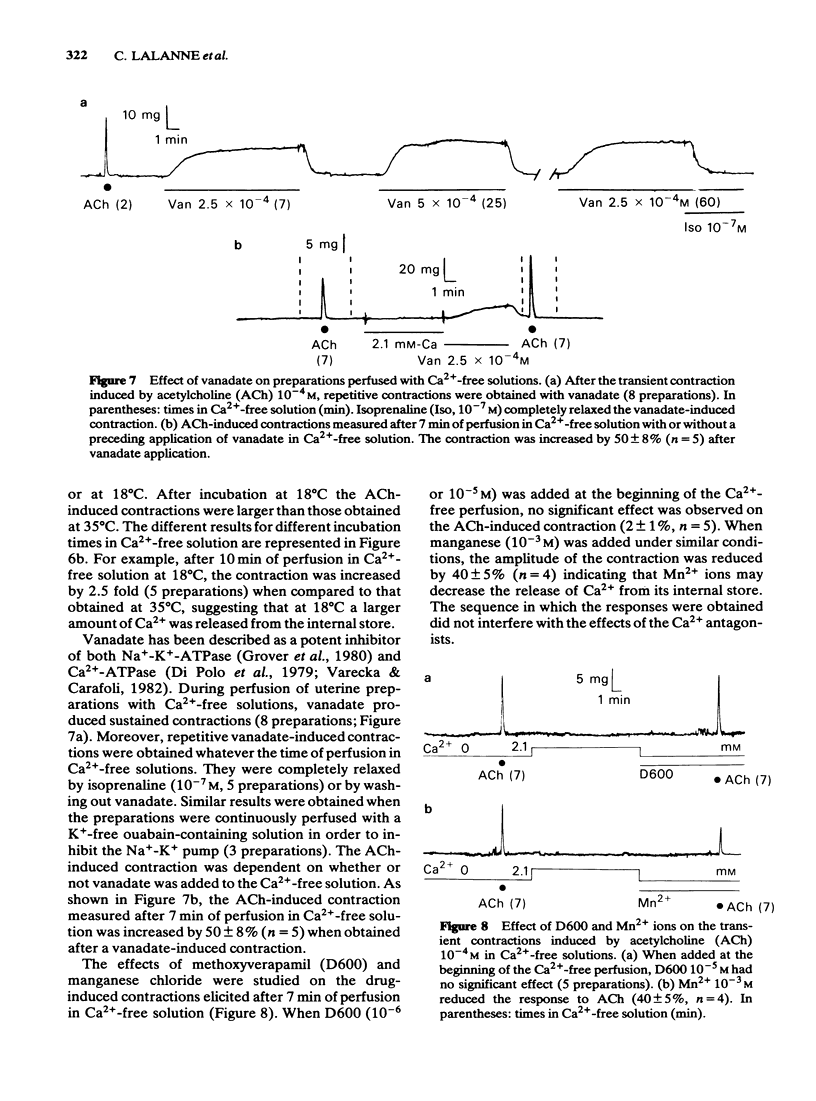
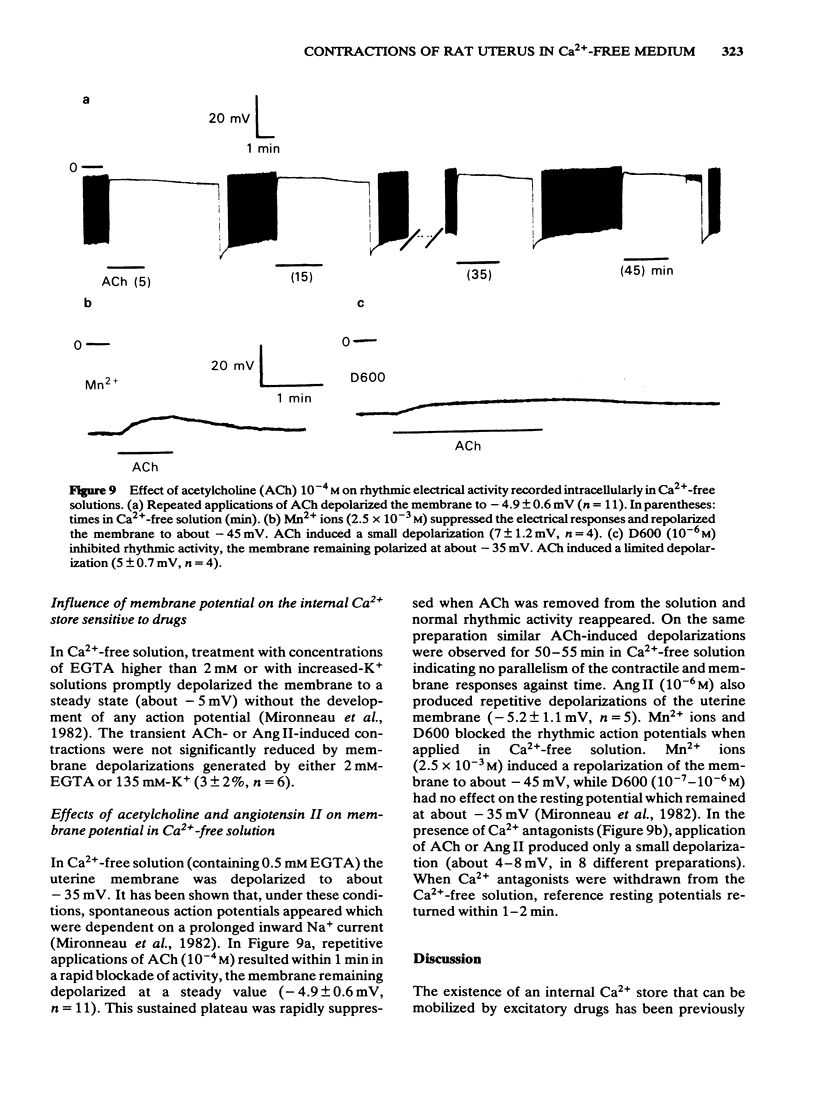
The effects of acetylcholine (ACh, 10(-4)M) and angiotensin II (Ang II, 10(-6) M) have been studied on the mechanical and electrical activities of rat myometrial strips perfused in Ca2+-free EGTA-containing solutions. Both ACh and Ang II produced transient contractions, the amplitude of which can be taken as a measurement of the amount of Ca2+ present in a drug-sensitive Ca2+ store. The degree of filling of this store depended on the external Ca2+ concentration, and on the presence of contractile responses during the Ca2+ loading period. The existence of two pathways (either direct or transcytoplasmic) is suggested for Ca2+ uptake into the internal Ca2+ store. The rate of filling of the Ca2+ store in 2.1 mM-Ca2+-containing solution was faster (time to half-maximal response, t 1/2 = 29 +/- 2.2 s, n = 4) than the rate of depletion in Ca2+-free solution (t 1/2 = 3 +/- 0.3 min, n = 3). The gradual depletion of this store was much slower at 18 degrees C than at 35 degrees C, and in the presence of vanadate which is known to inhibit Ca2+-ATPases. Methoxyverapamil (D600, 10(-6)-10(-5) M) had no appreciable effect on the direct Ca2+ uptake or on the release of Ca2+ from the store by ACh and Ang II. Mn2+ (10(-3) M) completely inhibited the direct pathway to the internal Ca2+ store and also reduced the release of Ca2+. ACh and Ang II induced repetitive depolarizations close to zero potential which did not parallel the transient contractions as a function of the time of perfusion in Ca2+-free solution. Applications of 2 mM EGTA, 135 mM K+ or Ca2+ antagonists which suppressed or reduced the drug-induced depolarizations did not affect appreciably the drug-induced contractions. These results suggest that myometrial cells have an intracellular Ca2+ store sensitive to different stimulus substances. This store is not affected by depolarization of the plasma membrane and is certainly different from that described in voltage-clamp experiments.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bolton T. B., Clark J. P., Kitamura K., Lang R. J. Evidence that histamine and carbachol may open the same ion channels in longitudinal smooth muscle of guinea-pig ileum. J Physiol. 1981 Nov;320:363–379. doi: 10.1113/jphysiol.1981.sp013955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B., Kitamura K. Evidence that ionic channels associated with the muscarinic receptor of smooth muscle may admit calcium. Br J Pharmacol. 1983 Feb;78(2):405–416. doi: 10.1111/j.1476-5381.1983.tb09405.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton T. B. Mechanisms of action of transmitters and other substances on smooth muscle. Physiol Rev. 1979 Jul;59(3):606–718. doi: 10.1152/physrev.1979.59.3.606. [DOI] [PubMed] [Google Scholar]
- Brading A. F., Burnett M., Sneddon P. The effect of sodium removal on the contractile response of the guinea-pig taenia coli to carbachol. J Physiol. 1980 Sep;306:411–429. doi: 10.1113/jphysiol.1980.sp013404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A. F., Sneddon P. Evidence for multiple sources of calcium for activation of the contractile mechanism of guinea-pig taenia coli on stimulation with carbachol. Br J Pharmacol. 1980 Oct;70(2):229–240. doi: 10.1111/j.1476-5381.1980.tb07928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bülbring E., den Hertog A. The action of isoprenaline on the smooth muscle of the guinea-pig taenia coli. J Physiol. 1980 Jul;304:277–296. doi: 10.1113/jphysiol.1980.sp013324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Droogmans G. Exchange characteristics of the noradrenaline-sensitive calcium store in vascular smooth muscle cells or rabbit ear artery. J Physiol. 1981 Aug;317:263–279. doi: 10.1113/jphysiol.1981.sp013824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Raeymaekers L. The action of acetylcholine and catecholamines on an intracellular calcium store in the smooth muscle cells of the guinea-pig taenia coli. J Physiol. 1979 Sep;294:51–68. doi: 10.1113/jphysiol.1979.sp012914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deth R., van Breemen C. Agonist induced release of intracellular Ca2+ in the rabbit aorta. J Membr Biol. 1977 Jan 28;30(4):363–380. doi: 10.1007/BF01869677. [DOI] [PubMed] [Google Scholar]
- Devine C. E., Somlyo A. V., Somlyo A. P. Sarcoplasmic reticulum and excitation-contraction coupling in mammalian smooth muscles. J Cell Biol. 1972 Mar;52(3):690–718. doi: 10.1083/jcb.52.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPolo R., Rojas H. R., Beaugé L. Vanadate inhibits uncoupled Ca efflux but not Na--Ca exchange in squid axons. Nature. 1979 Sep 20;281(5728):229–230. doi: 10.1038/281228a0. [DOI] [PubMed] [Google Scholar]
- Droogmans G., Casteels R. Temperature-dependence of 45Ca fluxes and contraction in vascular smooth muscle cells of rabbit ear artery. Pflugers Arch. 1981 Sep;391(3):183–189. doi: 10.1007/BF00596168. [DOI] [PubMed] [Google Scholar]
- Droogmans G., Raeymaekers L., Casteels R. Electro- and pharmacomechanical coupling in the smooth muscle cells of the rabbit ear artery. J Gen Physiol. 1977 Aug;70(2):129–148. doi: 10.1085/jgp.70.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EDMAN K. A., SCHILD H. O. The need for calcium in the contractile responses induced by acetylcholine and potassium in the rat uterus. J Physiol. 1962 May;161:424–441. doi: 10.1113/jphysiol.1962.sp006897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebashi S., Endo M. Calcium ion and muscle contraction. Prog Biophys Mol Biol. 1968;18:123–183. doi: 10.1016/0079-6107(68)90023-0. [DOI] [PubMed] [Google Scholar]
- FILO R. S., BOHR D. F., RUEGG J. C. GLYCERINATED SKELETAL AND SMOOTH MUSCLE: CALCIUM AND MAGNESIUM DEPENDENCE. Science. 1965 Mar 26;147(3665):1581–1583. doi: 10.1126/science.147.3665.1581. [DOI] [PubMed] [Google Scholar]
- Fleckenstein A. Specific pharmacology of calcium in myocardium, cardiac pacemakers, and vascular smooth muscle. Annu Rev Pharmacol Toxicol. 1977;17:149–166. doi: 10.1146/annurev.pa.17.040177.001053. [DOI] [PubMed] [Google Scholar]
- Grover A. K., Jones T. R., Daniel E. E. Effect of vanadate on rat myometrium plasma membrane enzyme activities. Can J Physiol Pharmacol. 1980 Oct;58(10):1247–1250. doi: 10.1139/y80-189. [DOI] [PubMed] [Google Scholar]
- Haeusler G., Richards J. G., Thorens S. Noradrenaline contractions in rabbit mesenteric arteries skinned with saponin. J Physiol. 1981 Dec;321:537–556. doi: 10.1113/jphysiol.1981.sp014001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara S., Nakajima S. Differences in Na and Ca spikes as examined by application of tetrodotoxin, procaine, and manganese ions. J Gen Physiol. 1966 Mar;49(4):793–806. doi: 10.1085/jgp.49.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamon G., Worcel M. Electrophysiological study of the action of angiotensin II on the rat myometrium. Circ Res. 1979 Aug;45(2):234–243. doi: 10.1161/01.res.45.2.234. [DOI] [PubMed] [Google Scholar]
- Katz A. M. Regulation of cardiac muscle contractility. J Gen Physiol. 1967 Jul;50(6 Suppl):185–196. doi: 10.1085/jgp.50.6.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironneau J., Eugene D., Mironneau C. Sodium action potentials induced by calcium chelation in rat uterine smooth muscle. Pflugers Arch. 1982 Nov 11;395(3):232–238. doi: 10.1007/BF00584815. [DOI] [PubMed] [Google Scholar]
- Mironneau J. Excitation-contraction coupling in voltage clamped uterine smooth muscle. J Physiol. 1973 Aug;233(1):127–141. doi: 10.1113/jphysiol.1973.sp010301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironneau J., Mironneau C., Grosset A., Hamon G., Savineau J. P. Action of angiotensin II on the electrical and mechanical activity of rat uterine smooth muscle. Eur J Pharmacol. 1980 Dec 5;68(3):275–285. doi: 10.1016/0014-2999(80)90525-7. [DOI] [PubMed] [Google Scholar]
- Mironneau J. Voltage clamp analysis of the ionic currents in uterine smooth muscle using the double sucrose gap method. Pflugers Arch. 1974;352(3):197–120. doi: 10.1007/BF00590485. [DOI] [PubMed] [Google Scholar]
- Rubányi G., Balogh I., Kovách A. G., Somogyi E., Sótonyi P. Ultrastructure and localization of calcium in uterine smooth muscle. Acta Morphol Acad Sci Hung. 1980;28(3):269–279. [PubMed] [Google Scholar]
- Sakai K., Higuchi K., Yamaguchi T., Uchida M. Oxytocin-induced Ca-free contraction of rat uterine smooth muscle: effects of preincubation with EGTA and drugs. Gen Pharmacol. 1982;13(5):393–400. doi: 10.1016/0306-3623(82)90104-5. [DOI] [PubMed] [Google Scholar]
- Schirar A., Capponi A., Catt K. J. Elevation of uterine angiotensin II receptors during early pregnancy in the rat. Endocrinology. 1980 May;106(5):1521–1527. doi: 10.1210/endo-106-5-1521. [DOI] [PubMed] [Google Scholar]
- Taylor G. S., Paton D. M., Daniel E. E. Characteristics of electrogenic sodium pumping in rat myometrium. J Gen Physiol. 1970 Sep;56(3):360–375. doi: 10.1085/jgp.56.3.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varecka L., Carafoli E. Vanadate-induced movements of Ca2+ and K+ in human red blood cells. J Biol Chem. 1982 Jul 10;257(13):7414–7421. [PubMed] [Google Scholar]
- Vassort G. Voltage-clamp analysis of transmembrane ionic currents in guinea-pig myometrium: evidence for an initial potassium activation triggered by calcium influx. J Physiol. 1975 Nov;252(3):713–734. doi: 10.1113/jphysiol.1975.sp011167. [DOI] [PMC free article] [PubMed] [Google Scholar]


