Abstract
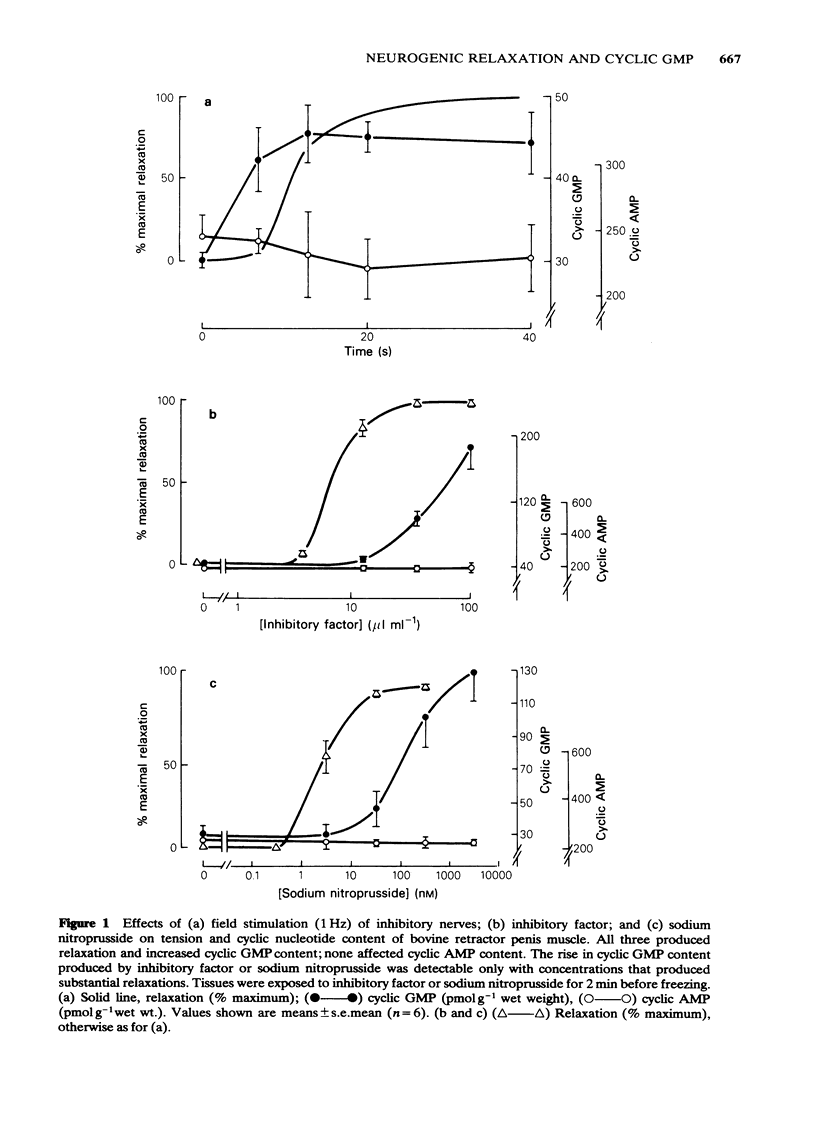
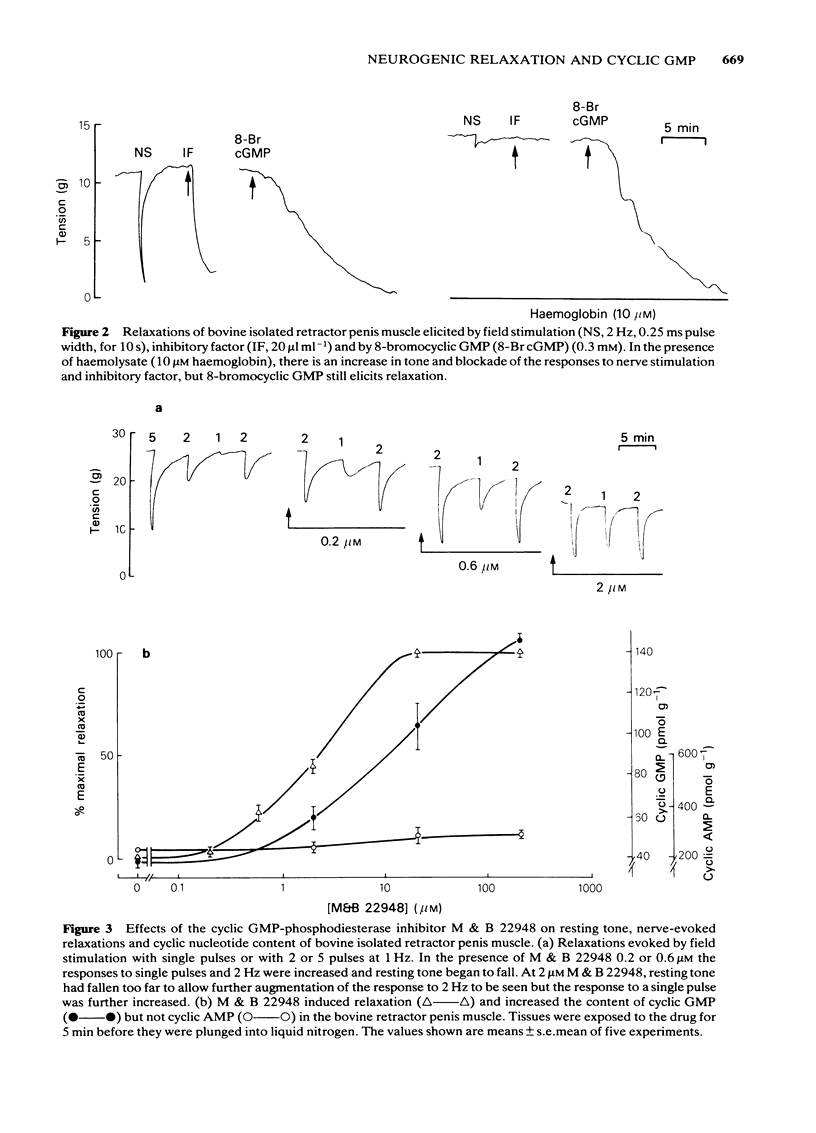
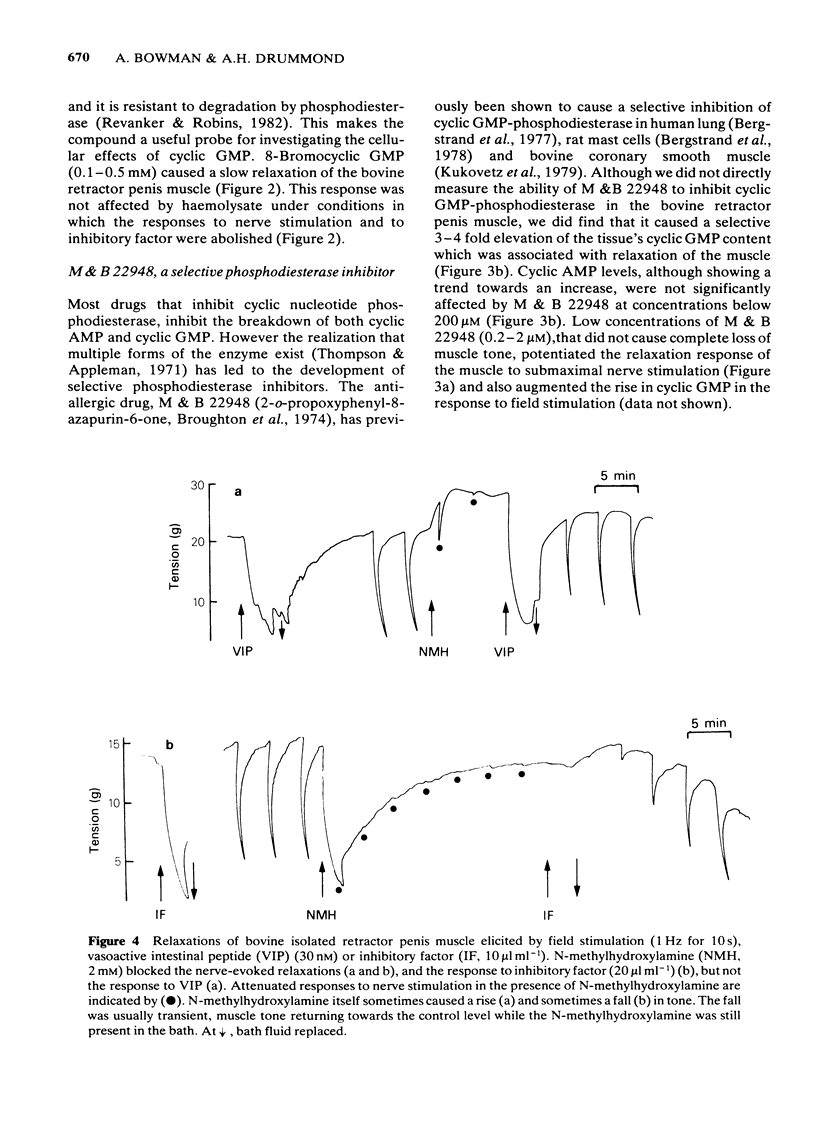
Field stimulation of the non-adrenergic, non-cholinergic inhibitory nerves to the bovine isolated retractor penis muscle evoked a relaxation that was preceded by a rise in the tissue content of cyclic GMP. There was no change in the content of cyclic AMP. The selective cyclic GMP phosphodiesterase inhibitor, 2-o- propoxyphenyl -8- azapurin -6-one (M&B 22948), elevated the tissue's cyclic GMP content, and potentiated both the relaxation and the rise in cyclic GMP produced by inhibitory nerve stimulation. Sodium nitroprusside and an inhibitory factor extracted from the bovine retractor penis muscle mimicked the effects of inhibitory nerve stimulation in that they each produced relaxation associated with a selective rise in cyclic GMP concentration. Haemoglobin (in the form of erythrocyte haemolysate) and N- methylhydroxylamine , which are known to block guanylate cyclase, blocked the relaxation and the rise in cyclic GMP content produced by inhibitory nerve stimulation, inhibitory factor and sodium nitroprusside. Haemoglobin itself caused a rise in muscle tone and at the same time reduced the cyclic GMP content of the tissue. 8-Bromocyclic GMP, a permeant derivative of cyclic GMP, produced a relaxation of the muscle that, as expected, was not blocked by haemoglobin. Vasoactive intestinal polypeptide, prostaglandin E1 and forskolin each produced relaxation associated with a selective rise in cyclic AMP content. Their effects were not blocked by haemoglobin or N- methylhydroxylamine . It is concluded that inhibitory nerve stimulation in the bovine retractor penis muscle produces a relaxation that is mediated by cyclic GMP, although some substances relax the muscle without affecting cyclic GMP levels. The results are also compatible with the view that the extracts of muscle contain the inhibitory neurotransmitter.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambache N., Killick S. W., Aboo Aar M. Extraction from ox retractor penis of an inhibitory substance which mimics its atropine-resistant neurogenic relaxation. Br J Pharmacol. 1975 Jul;54(3):409–410. doi: 10.1111/j.1476-5381.1975.tb07585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergstrand H., Kristoffersson J., Lundquist B., Schurmann A. Effects of antiallergic agents, compound 48/80, and some reference inhibitors on the activity of partially purified human lung tissue adenosine cyclic 3',5'-monophosphate and guanosine cyclic 3',5'-monophosphate phosphodiesterases. Mol Pharmacol. 1977 Jan;13(1):38–43. [PubMed] [Google Scholar]
- Bergstrand H., Lundquist B., Schurmann A. Rat mast cell high affinity cyclic nucleotide phosphodiesterases: separation and inhibitory effects of two antiallergic agents. Mol Pharmacol. 1978 Sep;14(5):848–855. [PubMed] [Google Scholar]
- Bowman A., Gillespie J. S. Block of some non-adrenergic inhibitory responses of smooth muscle by a substance from haemolysed erythrocytes. J Physiol. 1982 Jul;328:11–25. doi: 10.1113/jphysiol.1982.sp014250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman A., Gillespie J. S., Martin W. The inhibitory material in extracts from the bovine retractor penis muscle is not an adenine nucleotide. Br J Pharmacol. 1979 Nov;67(3):327–328. doi: 10.1111/j.1476-5381.1979.tb08683.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman A., Gillespie J. S. Neurogenic vasodilatation in isolated bovine and canine penile arteries. J Physiol. 1983 Aug;341:603–616. doi: 10.1113/jphysiol.1983.sp014827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowman A., Gillespie J. S., Pollock D. Oxyhaemoglobin blocks non-adrenergic non-cholinergic inhibition in the bovine retractor penis muscle. Eur J Pharmacol. 1982 Nov 19;85(2):221–224. doi: 10.1016/0014-2999(82)90470-8. [DOI] [PubMed] [Google Scholar]
- Broughton B. J., Chaplen P., Knowles P., Lunt E., Pain D. L., Wooldridge K. R., Ford R., Marshall S., Walker J. L., Maxwell D. R. New inhibitor of reagin-mediated anaphylaxis. Nature. 1974 Oct 18;251(5476):650–652. doi: 10.1038/251650a0. [DOI] [PubMed] [Google Scholar]
- Deguchi T., Saito M., Kono M. Blockade by N-methylhydroxylamine of activation of guanylate cyclase and elevations of guanosine 3',5'-monophosphate levels in nervous tissues. Biochim Biophys Acta. 1978 Nov 15;544(1):8–19. doi: 10.1016/0304-4165(78)90204-0. [DOI] [PubMed] [Google Scholar]
- Diamond J. Lack of correlation between cyclic GMP elevation and relaxation of nonvascular smooth muscle by nitroglycerin, nitroprusside, hydroxylamine and sodium azide. J Pharmacol Exp Ther. 1983 May;225(2):422–426. [PubMed] [Google Scholar]
- Drummond A. H., Baguley B. C., Staehelin M. Beta adrenergic regulation of glycogen phosphorylase activity and adenosine cyclic 3', 5'-monophosphate accumulation in control and desensitized C-6 astrocytoma cells. Mol Pharmacol. 1977 Nov;13(6):1159–1169. [PubMed] [Google Scholar]
- Echlin F. Experimental vasospasm, acute and chronic, due to blood in the subarachnoid space. J Neurosurg. 1971 Dec;35(6):646–656. doi: 10.3171/jns.1971.35.6.0646. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Gillespie J. S., Hunter J. C., Martin W. Some physical and chemical properties of the smooth muscle inhibitory factor in extracts of the bovine retractor penis muscle. J Physiol. 1981 Jun;315:111–125. doi: 10.1113/jphysiol.1981.sp013736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie J. S., Martin W. A smooth muscle inhibitory material extracted from the bovine retractor penis and rat anococcygeus muscles [proceedings]. J Physiol. 1978 Jul;280:45P–46P. [PubMed] [Google Scholar]
- Gillespie J. S., Martin W. A smooth muscle inhibitory material from the bovine retractor penis and rat anococcygeus muscles. J Physiol. 1980 Dec;309:55–64. doi: 10.1113/jphysiol.1980.sp013493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper J. F., Brooker G. Femtomole sensitive radioimmunoassay for cyclic AMP and cyclic GMP after 2'0 acetylation by acetic anhydride in aqueous solution. J Cyclic Nucleotide Res. 1975;1(4):207–218. [PubMed] [Google Scholar]
- Katsuki S., Arnold W. P., Murad F. Effects of sodium nitroprusside, nitroglycerin, and sodium azide on levels of cyclic nucleotides and mechanical activity of various tissues. J Cyclic Nucleotide Res. 1977 Aug;3(4):239–247. [PubMed] [Google Scholar]
- Katsuki S., Arnold W., Mittal C., Murad F. Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J Cyclic Nucleotide Res. 1977 Feb;3(1):23–35. [PubMed] [Google Scholar]
- Katsuki S., Murad F. Regulation of adenosine cyclic 3',5'-monophosphate and guanosine cyclic 3',5'-monophosphate levels and contractility in bovine tracheal smooth muscle. Mol Pharmacol. 1977 Mar;13(2):330–341. [PubMed] [Google Scholar]
- Klinge E., Sjöstrand N. O. Contraction and relaxation of the retractor penis muscle and the penile artery of the bull. Acta Physiol Scand Suppl. 1974;420:1–88. [PubMed] [Google Scholar]
- Kukovetz W. R., Holzmann S., Pöch G. Function of cyclic GMP in acetylcholine-induced contraction of coronary smooth muscle. Naunyn Schmiedebergs Arch Pharmacol. 1982 Apr;319(1):29–33. doi: 10.1007/BF00491474. [DOI] [PubMed] [Google Scholar]
- Kuo J. F., Kuo W. N., Shoji M., Davis C. W., Seery V. L., Donnelly T. E., Jr Purification and general properties of guanosine 3':5'-monophosphate-dependent protein kinase from guinea pig fetal lung. J Biol Chem. 1976 Mar 25;251(6):1759–1766. [PubMed] [Google Scholar]
- Kuo J. F., Miyamoto E., Reyes P. Activation and dissociation of adenosine 3'-5'-monophosphate-dependent and guanosine 3'-5'-monophosphate-dependent protein kinases by various cyclic nucleotide analogs. Biochem Pharmacol. 1974 Jul 15;23(14):2011–2021. doi: 10.1016/0006-2952(74)90260-3. [DOI] [PubMed] [Google Scholar]
- Langley J. N., Anderson H. K. The Innervation of the Pelvic and adjoining Viscera: Part II. The Bladder. Part III. The External Generative Organs. Part IV. The Internal Generative Organs. Part V. Position of the Nerve Cells on the Course of the Efferent Nerve Fibres. J Physiol. 1895 Dec 30;19(1-2):71–139. doi: 10.1113/jphysiol.1895.sp000587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. P., Kuo J. F., Greengard P. Role of muscarinic cholinergic receptors in regulation of guanosine 3':5'-cyclic monophosphate content in mammalian brain, heart muscle, and intestinal smooth muscle. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3287–3291. doi: 10.1073/pnas.69.11.3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal C. K., Arnold W. P., Murad F. Characterization of protein inhibitors of guanylate cyclase activation from rat heart and bovine lung. J Biol Chem. 1978 Feb 25;253(4):1266–1271. [PubMed] [Google Scholar]
- Mittal C. K., Murad F. Properties and oxidative regulation of guanylate cyclase. J Cyclic Nucleotide Res. 1977 Dec;3(6):381–391. [PubMed] [Google Scholar]
- Murad F., Kimura H. Cyclic nucleotide levels in incubations of guinea pig trachea. Biochim Biophys Acta. 1974 Apr 22;343(2):275–286. doi: 10.1016/0304-4165(74)90092-0. [DOI] [PubMed] [Google Scholar]
- Osaka K. Prolonged vasospasm produced by the breakdown products of erythrocytes. J Neurosurg. 1977 Sep;47(3):403–411. doi: 10.3171/jns.1977.47.3.0403. [DOI] [PubMed] [Google Scholar]
- Rapoport R. M., Murad F. Agonist-induced endothelium-dependent relaxation in rat thoracic aorta may be mediated through cGMP. Circ Res. 1983 Mar;52(3):352–357. doi: 10.1161/01.res.52.3.352. [DOI] [PubMed] [Google Scholar]
- Schultz K., Schultz K., Schultz G. Sodium nitroprusside and other smooth muscle-relaxants increase cyclic GMP levels in rat ductus deferens. Nature. 1977 Feb 24;265(5596):750–751. doi: 10.1038/265750a0. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Padgett W., Daly J. W. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner A. L., Parker C. W., Kipnis D. M. Radioimmunoassay for cyclic nucleotides. I. Preparation of antibodies and iodinated cyclic nucleotides. J Biol Chem. 1972 Feb 25;247(4):1106–1113. [PubMed] [Google Scholar]
- Tanishima T. Cerebral vasospasm: contractile activity of hemoglobin in isolated canine basilar arteries. J Neurosurg. 1980 Dec;53(6):787–793. doi: 10.3171/jns.1980.53.6.0787. [DOI] [PubMed] [Google Scholar]
- Thompson W. J., Appleman M. M. Multiple cyclic nucleotide phosphodiesterase activities from rat brain. Biochemistry. 1971 Jan 19;10(2):311–316. [PubMed] [Google Scholar]


