Abstract
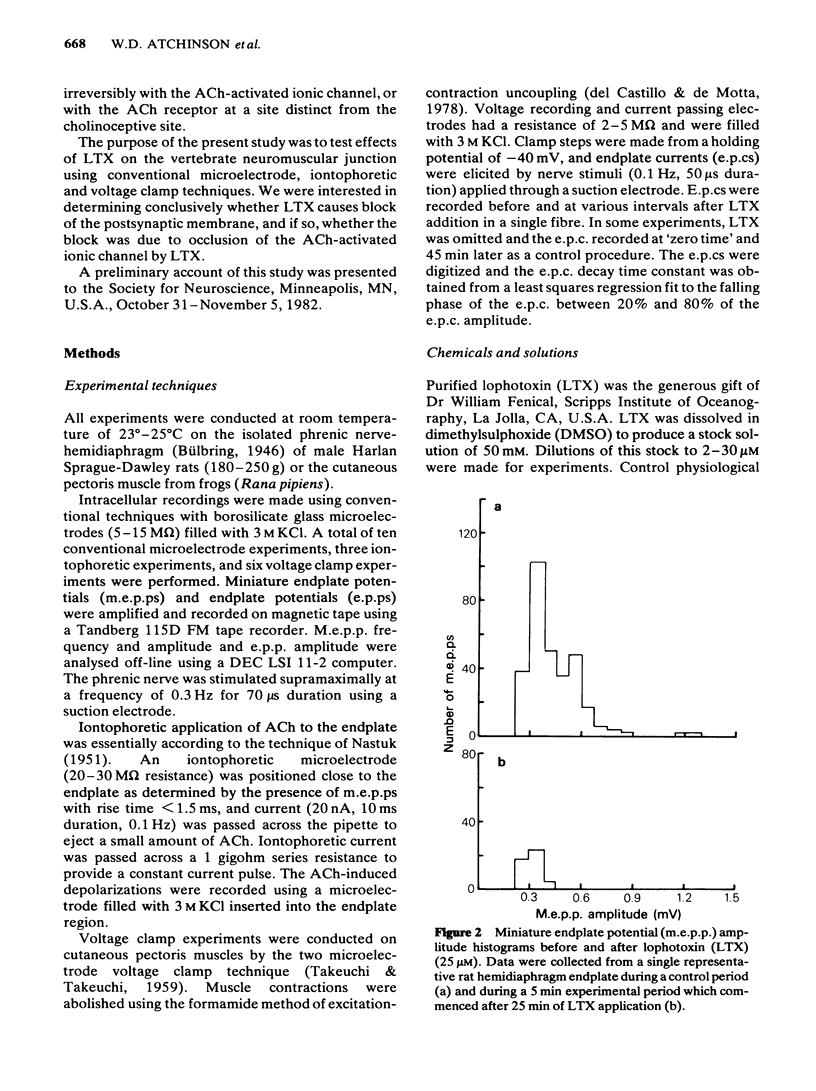
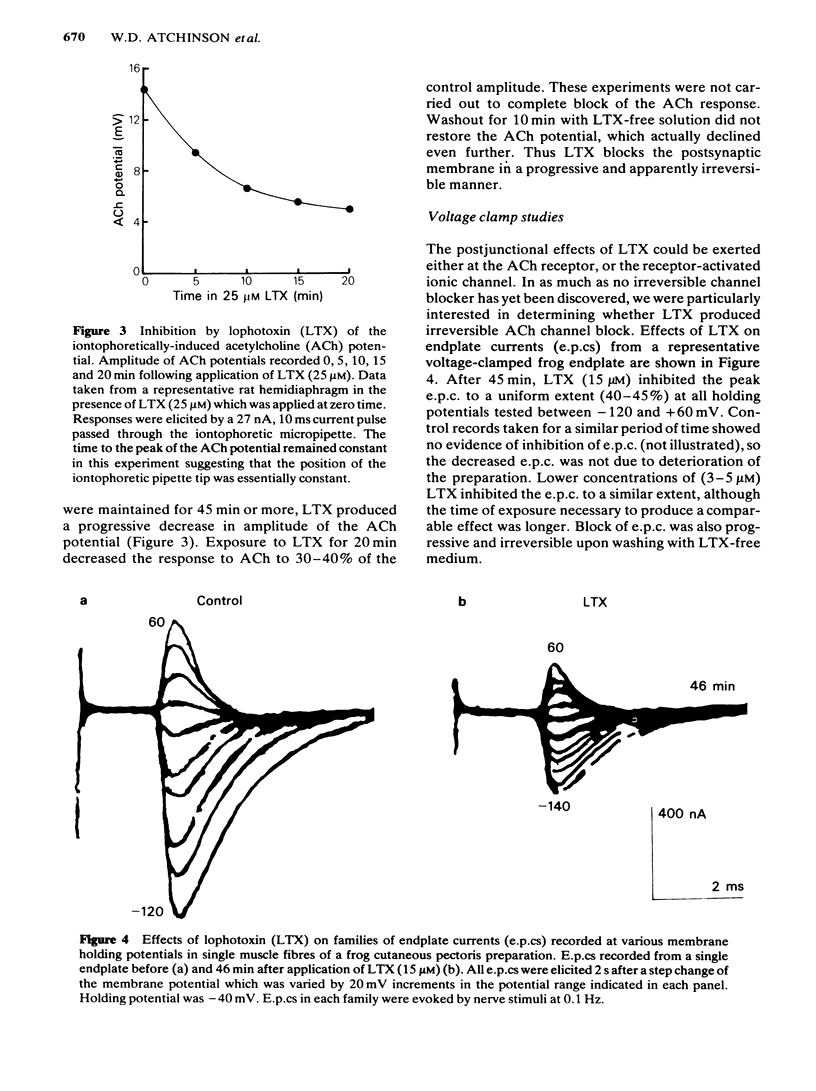
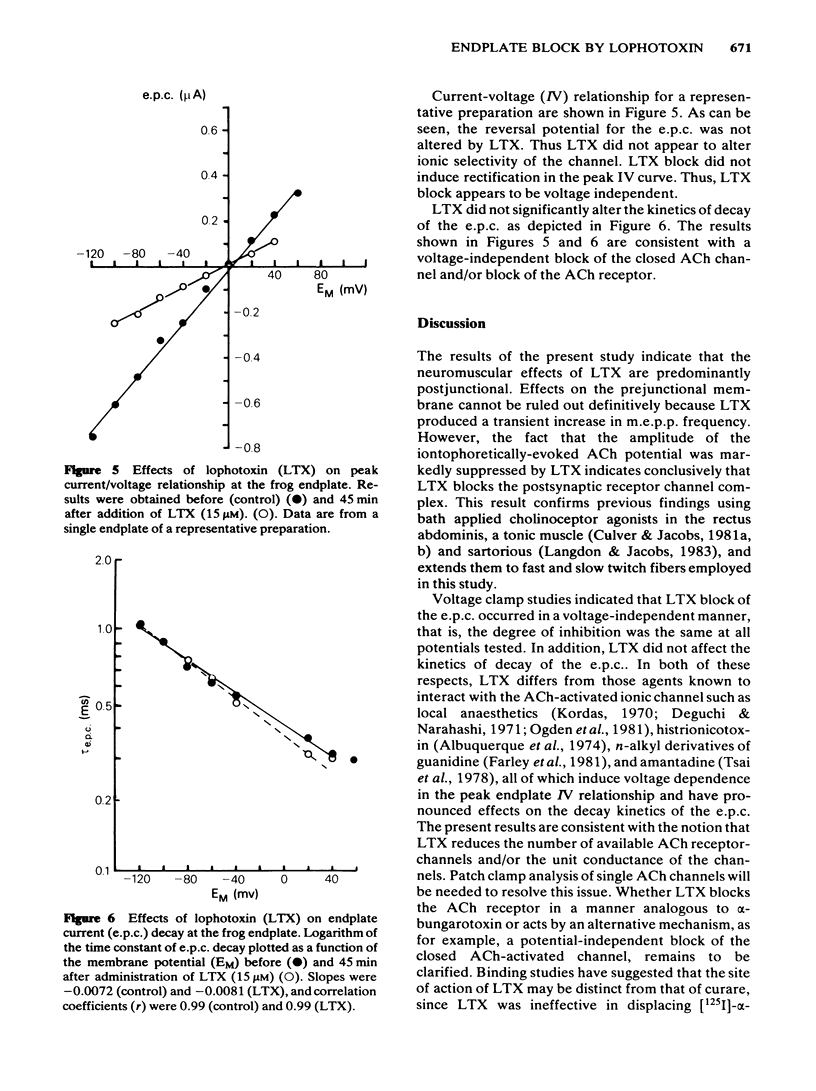
Effects of lophotoxin (LTX), a neurotoxin isolated from Pacific sea whips Lophogorgia rigida and Lophogorgia chelensis, on neuromuscular transmission were assessed in the rat phrenic nerve-hemidiaphragm preparation using conventional microelectrode recording techniques, and in the frog cutaneous pectoris preparation using two microelectrode voltage clamp techniques. LTX (2-25 microM) produced a progressive, irreversible block of miniature endplate potential (m.e.p.p.) and endplate potential (e.p.p.) amplitude. M.e.p.p. amplitude histograms were shifted markedly in the direction of lower amplitude by LTX. These effects occurred following a latency of 25-40 min. The latency to onset of block was decreased with increasing LTX concentrations. In some preparations, LTX produced a transient increase in m.e.p.p. frequency during the first 5 min of application; however, m.e.p.p. frequency then declined to complete block. The depressant effect of LTX on m.e.p.p. and e.p.p. amplitude progressed to complete block irrespective of the LTX concentration. LTX also blocked the endplate depolarization produced by iontophoretic application of acetylcholine (ACh). The resting membrane potential of skeletal muscle fibres was unaffected by LTX. In voltage clamp experiments, LTX (15 microM) depressed the peak amplitude of the endplate current (e.p.c.) nearly uniformly at potentials between -120 and +60 mV. LTX did not affect the e.p.c. reversal potential or the kinetics of e.p.c. decay suggesting that LTX does not block open ACh channels. E.p.c. block by LTX was also progressive and irreversible. The results indicate that LTX blocks neuromuscular transmission by a postjunctional action. The binding site of LTX may be different from that of ACh.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albuquerque E. X., Kuba K., Daly J. Effect of histrionicotoxin on the ionic conductance modulator of the cholinergic receptor: a quantitative analysis of the end-plate current. J Pharmacol Exp Ther. 1974 May;189(2):513–524. [PubMed] [Google Scholar]
- Culver P., Jacobs R. S. Lophotoxin: a neuromuscular acting toxin from the sea whip (Lophogorgia rigida). Toxicon. 1981;19(6):825–830. doi: 10.1016/0041-0101(81)90078-7. [DOI] [PubMed] [Google Scholar]
- Deguchi T., Narahashi T. Effects of procaine on ionic conductances of end-plate membranes. J Pharmacol Exp Ther. 1971 Feb;176(2):423–433. [PubMed] [Google Scholar]
- Farley J. M., Glavinović M. I., Watanabe S., Narahashi T. Stimulation of transmitter release by guanidine derivatives. Neuroscience. 1979;4(10):1511–1519. doi: 10.1016/0306-4522(79)90056-3. [DOI] [PubMed] [Google Scholar]
- Farley J. M., Yeh J. Z., Watanabe S., Narahashi T. Endplate channel block by guanidine derivatives. J Gen Physiol. 1981 Mar;77(3):273–293. doi: 10.1085/jgp.77.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenical W., Okuda R. K., Bandurraga M. M., Culver P., Jacobs R. S. Lophotoxin: a novel neuromuscular toxin from Pacific sea whips of the genus Lophogorgia. Science. 1981 Jun 26;212(4502):1512–1514. doi: 10.1126/science.6112796. [DOI] [PubMed] [Google Scholar]
- Kordas M. The effect of procaine on neuromuscular transmission. J Physiol. 1970 Aug;209(3):689–699. doi: 10.1113/jphysiol.1970.sp009186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langdon R. B., Jacobs R. S. Quantal analysis indicates an alpha-toxin-like block by lophotoxin, a non-ionic marine natural product. Life Sci. 1983 Mar 14;32(11):1223–1228. doi: 10.1016/0024-3205(83)90191-1. [DOI] [PubMed] [Google Scholar]
- NICKERSON M. Mechanism of the prolonged adrenergic blockade produced by haloalkylamines. Arch Int Pharmacodyn Ther. 1962 Nov 1;140:237–250. [PubMed] [Google Scholar]
- NICKERSON M. Nonequilibrium drug antagonism. Pharmacol Rev. 1957 Jun;9(2):246–259. [PubMed] [Google Scholar]
- Ogden D. C., Siegelbaum S. A., Colquhoun D. Block of acetylcholine-activated ion channels by an uncharged local anaesthetic. Nature. 1981 Feb 12;289(5798):596–598. doi: 10.1038/289596a0. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Active phase of frog's end-plate potential. J Neurophysiol. 1959 Jul;22(4):395–411. doi: 10.1152/jn.1959.22.4.395. [DOI] [PubMed] [Google Scholar]
- Tsai M. C., Mansour N. A., Eldefrawi A. T., Eldefrawi M. E., Albuquerque E. X. Mechanism of action of amantadine on neuromuscular transmission. Mol Pharmacol. 1978 Sep;14(5):787–803. [PubMed] [Google Scholar]
- del Castillo J., Escalona de Motta G. A new method for excitation-contraction uncoupling in frog skeletal muscle. J Cell Biol. 1978 Sep;78(3):782–784. doi: 10.1083/jcb.78.3.782. [DOI] [PMC free article] [PubMed] [Google Scholar]


