Abstract
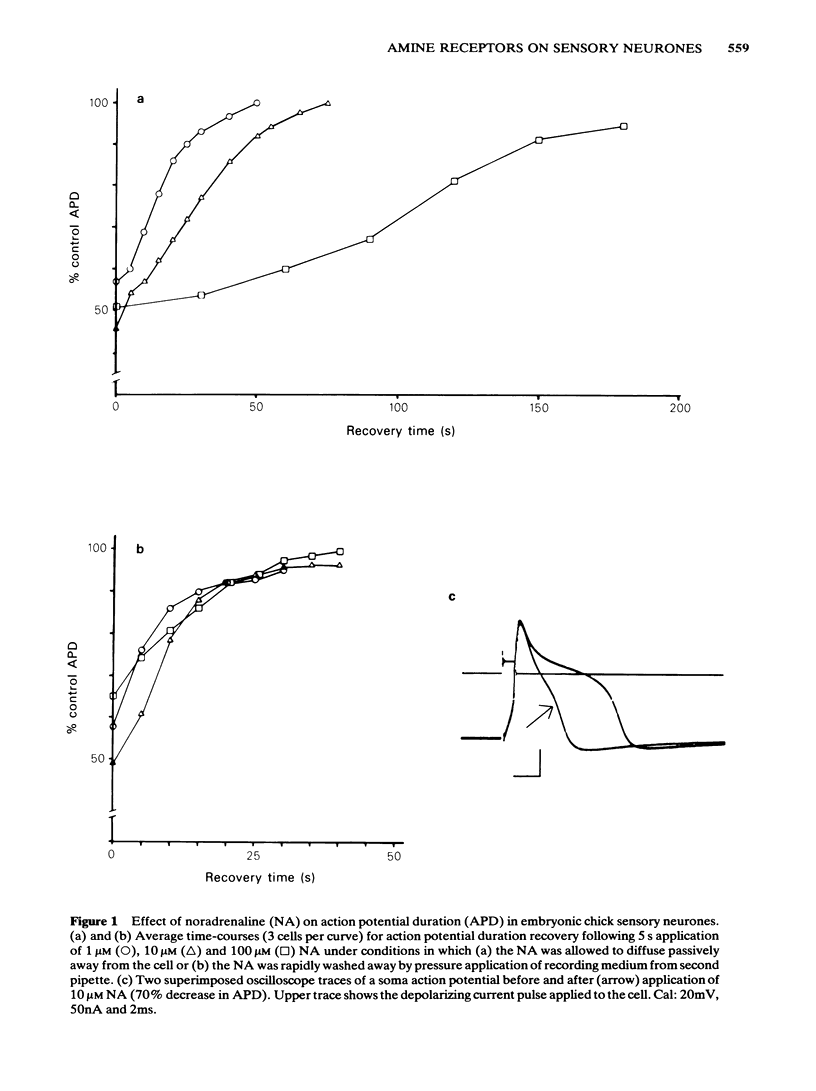
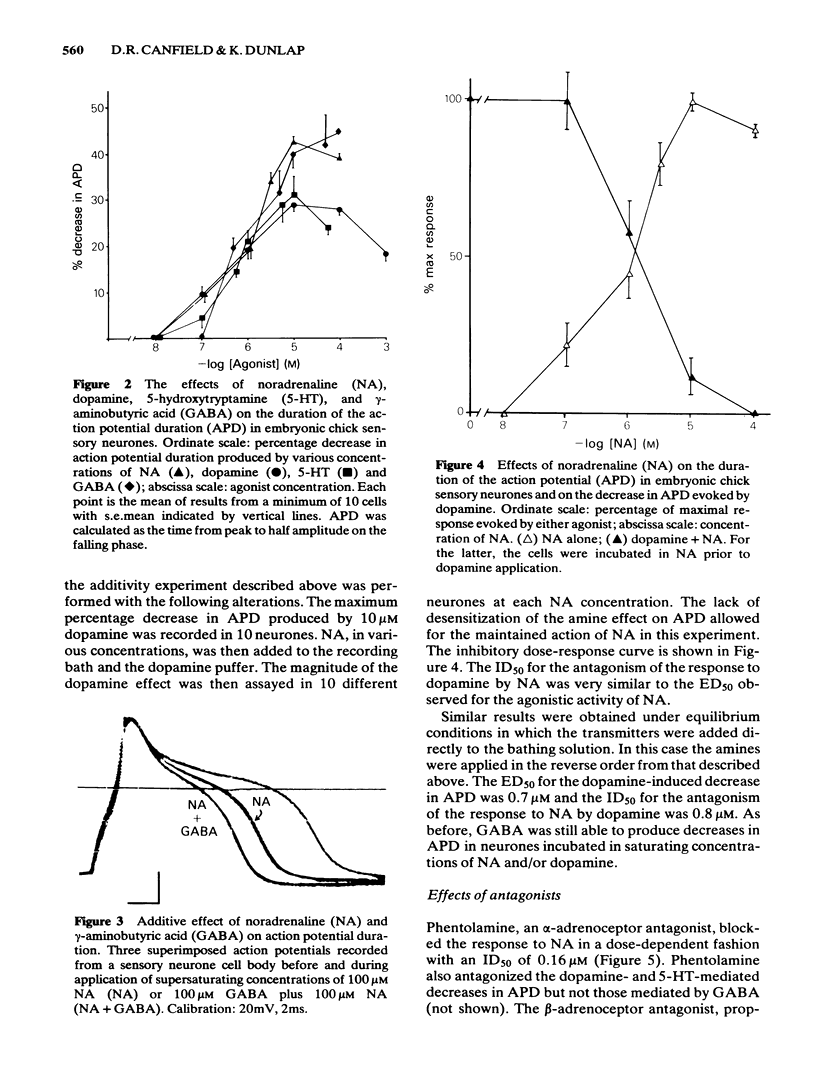
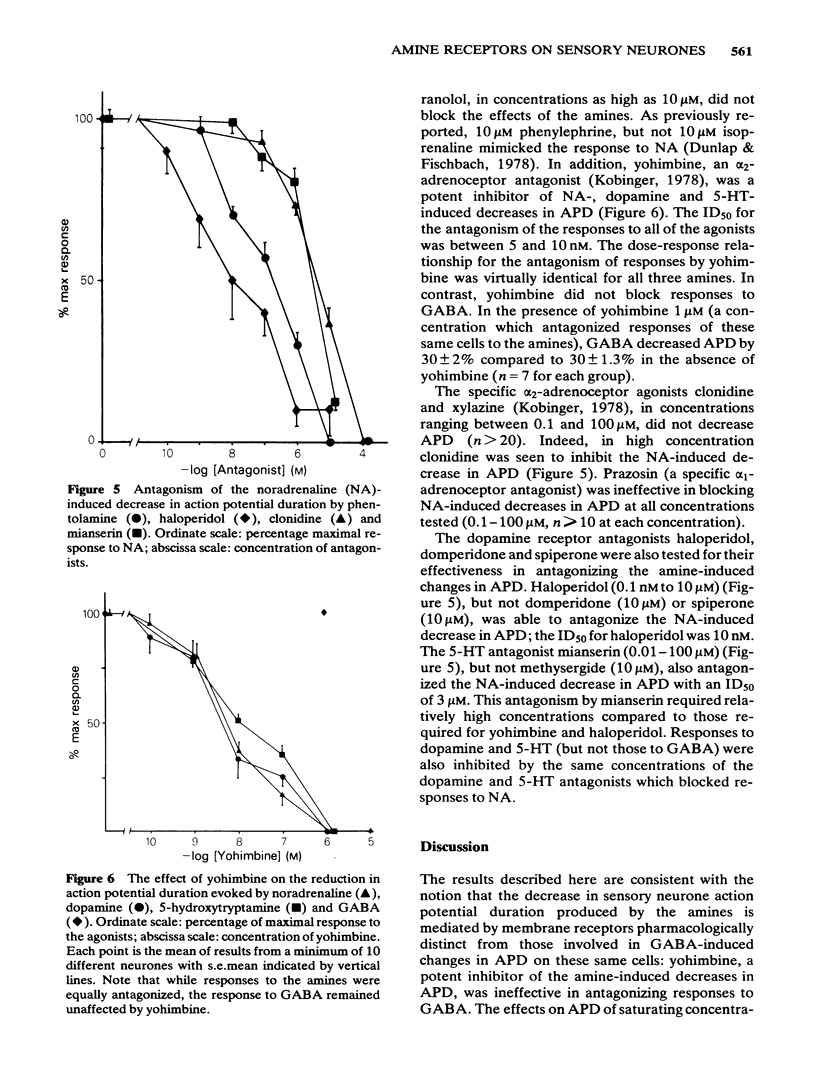
The effects of noradrenaline, dopamine and 5-hydroxytryptamine were investigated on the duration of the action potential of embryonic chick sensory neurones in vitro. All three amines, like gamma-aminobutyric acid, decreased the duration of the action potential evoked by current injection. The onset of the noradrenaline-induced decrease in action potential duration was fast (less than 1s) and the recovery phase was dependent upon the dose of noradrenaline applied. Rapid washout of the noradrenaline revealed a minimum 30s recovery time which was independent of the initial noradrenaline concentration. Dopamine and 5-hydroxytryptamine could mimic the effects of noradrenaline on action potential duration. The ED50 for all three amines was approximately 1 microM. At a saturating concentration of 10 microM, noradrenaline was more potent than dopamine and 5-hydroxytryptamine. Saturating doses of noradrenaline and dopamine or 5-hydroxytryptamine were not additive. Responses to all three amines were affected similarly by antagonists: they were antagonized by yohimbine, phentolamine, haloperidol and mianserin but not by propranolol, prazosin, domperidone, spiperone or methysergide. Clonidine and xylazine (alpha 2-adrenoceptor agonists) were also without effect. In contrast to the amines, saturating concentrations of gamma-aminobutyric acid were additive with those of noradrenaline. Responses to GABA were not antagonized by the amine receptor antagonists. The evidence described here suggests that the amines and gamma-aminobutyric acid acid decrease sensory neurone action potential duration via pharmacologically-distinct membrane receptors. In addition, it is likely that the amines are acting via a single class of receptor whose pharmacology is different from classical adrenoceptors, dopamine receptors and 5-hydroxytryptamine receptors.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Choi D. W., Fischbach G. D. GABA conductance of chick spinal cord and dorsal root ganglion neurons in cell culture. J Neurophysiol. 1981 Apr;45(4):605–620. doi: 10.1152/jn.1981.45.4.605. [DOI] [PubMed] [Google Scholar]
- Dunlap K., Fischbach G. D. Neurotransmitters decrease the calcium conductance activated by depolarization of embryonic chick sensory neurones. J Physiol. 1981 Aug;317:519–535. doi: 10.1113/jphysiol.1981.sp013841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K., Fischbach G. D. Neurotransmitters decrease the calcium ocmponent of sensory neurone action potentials. Nature. 1978 Dec 21;276(5690):837–839. doi: 10.1038/276837a0. [DOI] [PubMed] [Google Scholar]
- Dunlap K. Two types of gamma-aminobutyric acid receptor on embryonic sensory neurones. Br J Pharmacol. 1981 Nov;74(3):579–585. doi: 10.1111/j.1476-5381.1981.tb10467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvan M., Adams P. R. Control of calcium current in rat sympathetic neurons by norepinephrine. Brain Res. 1982 Jul 22;244(1):135–144. doi: 10.1016/0006-8993(82)90911-8. [DOI] [PubMed] [Google Scholar]
- Horn J. P., McAfee D. A. Alpha-drenergic inhibition of calcium-dependent potentials in rat sympathetic neurones. J Physiol. 1980 Apr;301:191–204. doi: 10.1113/jphysiol.1980.sp013198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn J. P., McAfee D. A. Norepinephrine inhibits calcium-dependent potentials in rat sympathetic neurons. Science. 1979 Jun 15;204(4398):1233–1235. doi: 10.1126/science.221979. [DOI] [PubMed] [Google Scholar]
- Kobinger Central alpha-adrenergic systems as targets for hypotensive drugs. Rev Physiol Biochem Pharmacol. 1978;81:39–100. doi: 10.1007/BFb0034091. [DOI] [PubMed] [Google Scholar]
- Starke K. Regulation of noradrenaline release by presynaptic receptor systems. Rev Physiol Biochem Pharmacol. 1977;77:1–124. doi: 10.1007/BFb0050157. [DOI] [PubMed] [Google Scholar]
- Young W. S., 3rd, Kuhar M. J. Noradrenergic alpha 1 and alpha 2 receptors: autoradiographic visualization. Eur J Pharmacol. 1979 Nov 16;59(3-4):317–319. doi: 10.1016/0014-2999(79)90299-1. [DOI] [PubMed] [Google Scholar]



