Abstract
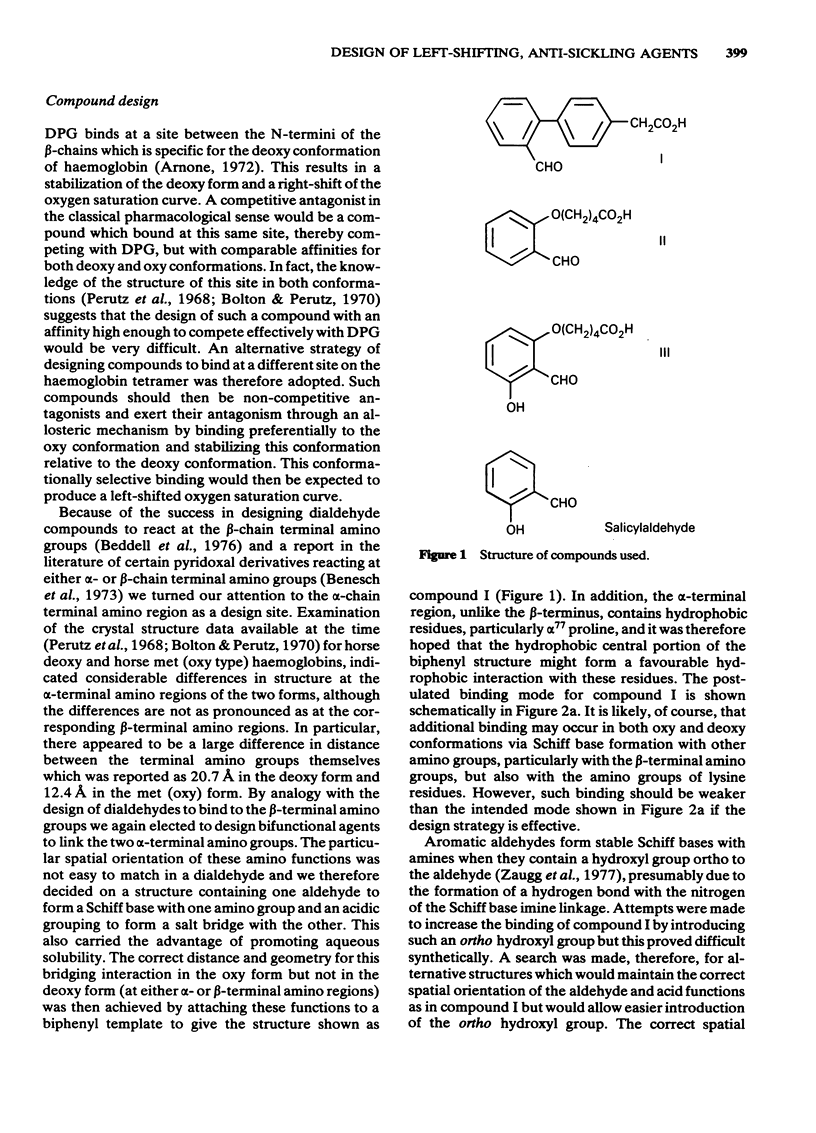
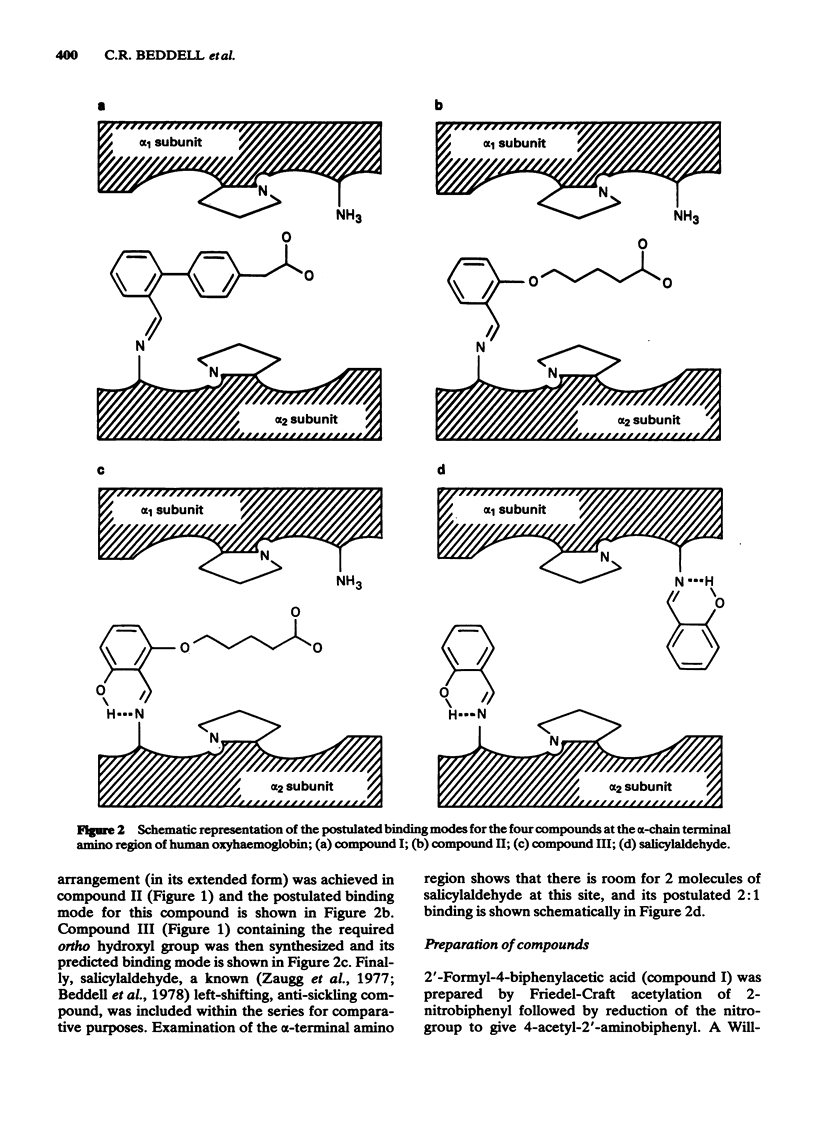
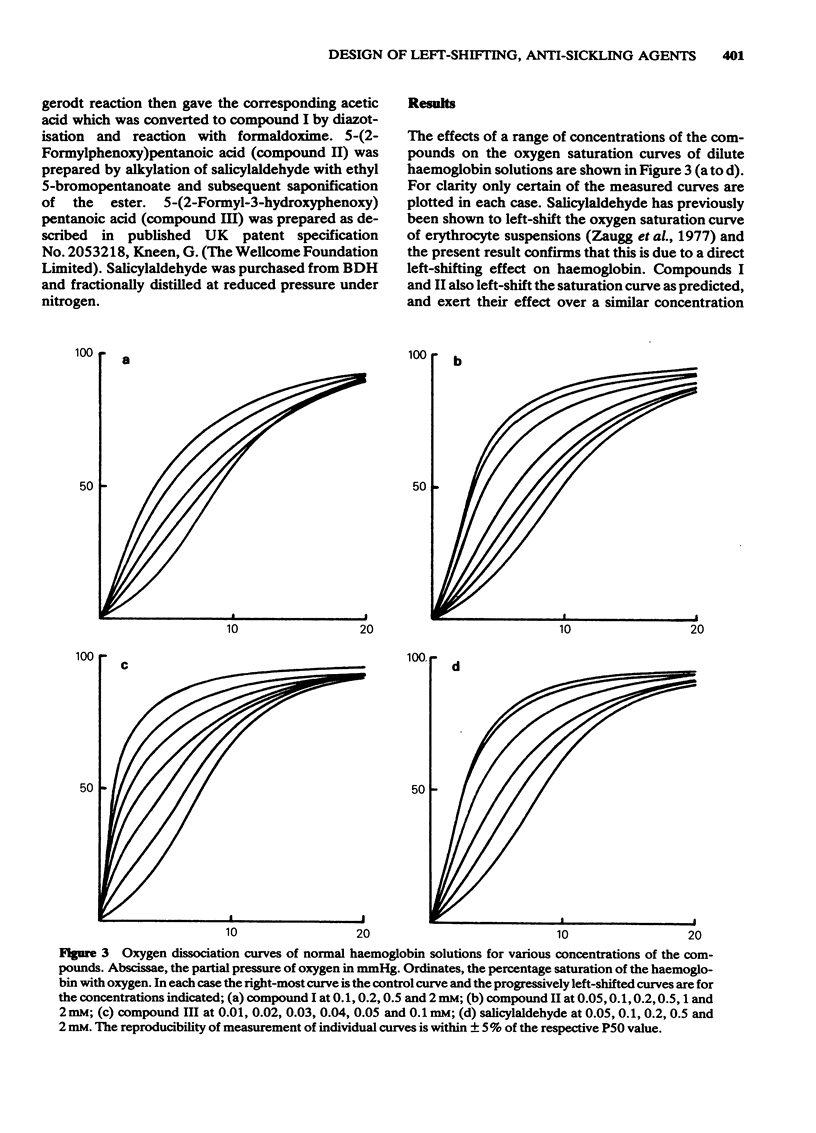
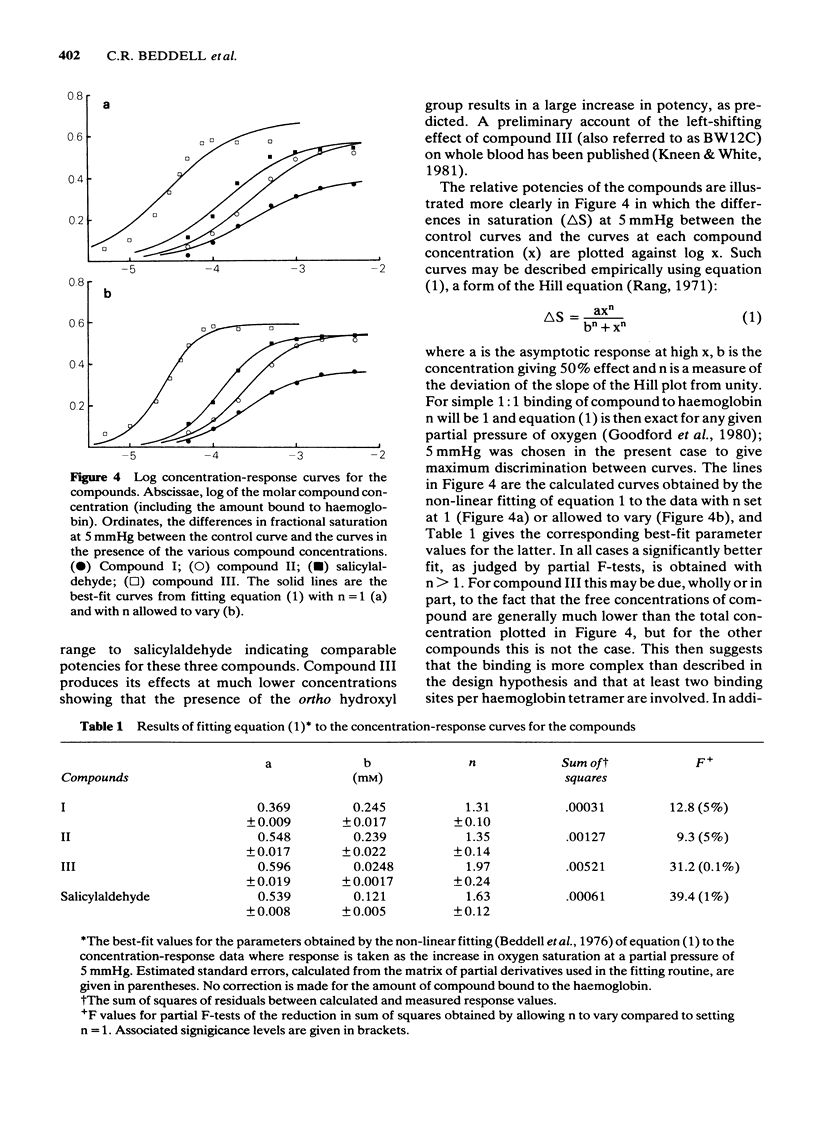
Substituted benzaldehydes have been designed to bind preferentially to the oxy conformation of human haemoglobin at a site between the amino terminal residues of the alpha-subunits. Such compounds should stabilize the oxygenated form of haemoglobin and thereby increase its oxygen affinity. The compounds produce the expected effect, left-shifting the oxygen saturation curve of dilute haemoglobin solutions and of whole blood, although the binding pattern to haemoglobin is more complex than envisaged by the design hypothesis. The predicted best compound is also a potent inhibitor, at low oxygen pressure, of the sickling of erythrocytes from patients homozygous for sickle cell disease, and may prove to be a clinically useful anti-sickling agent.
Full text
PDF

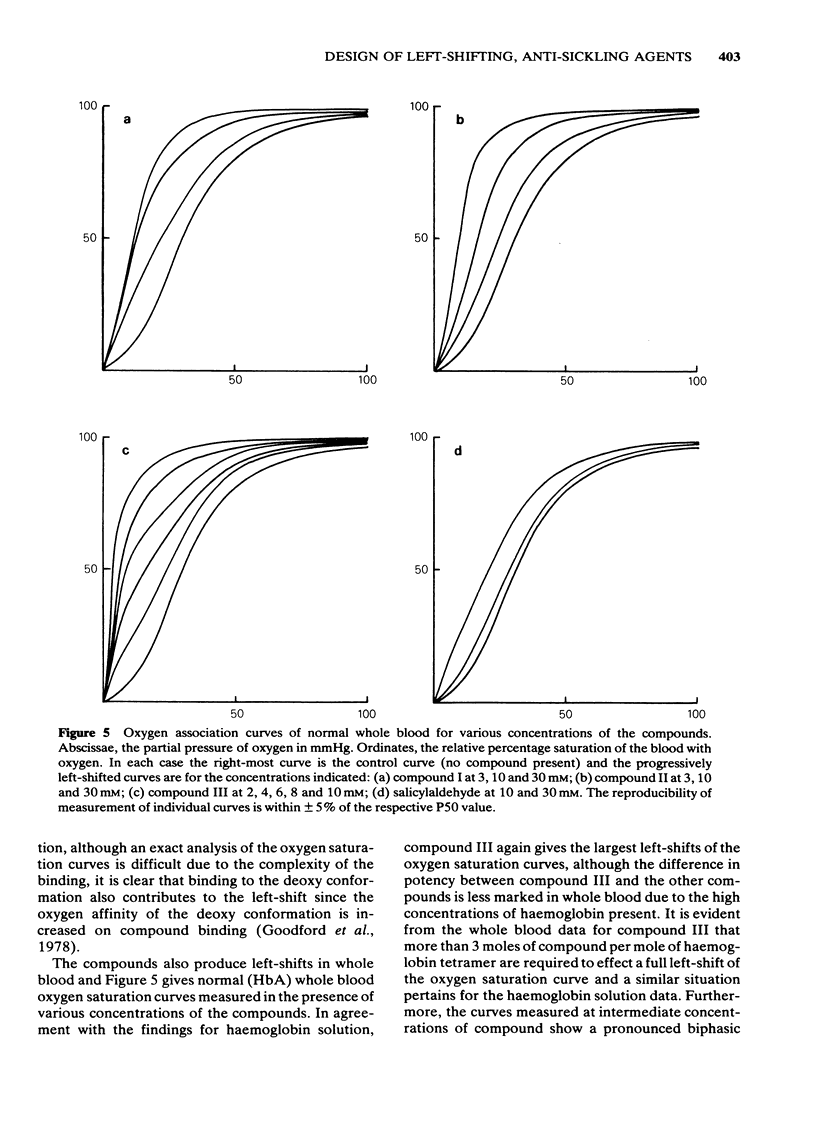
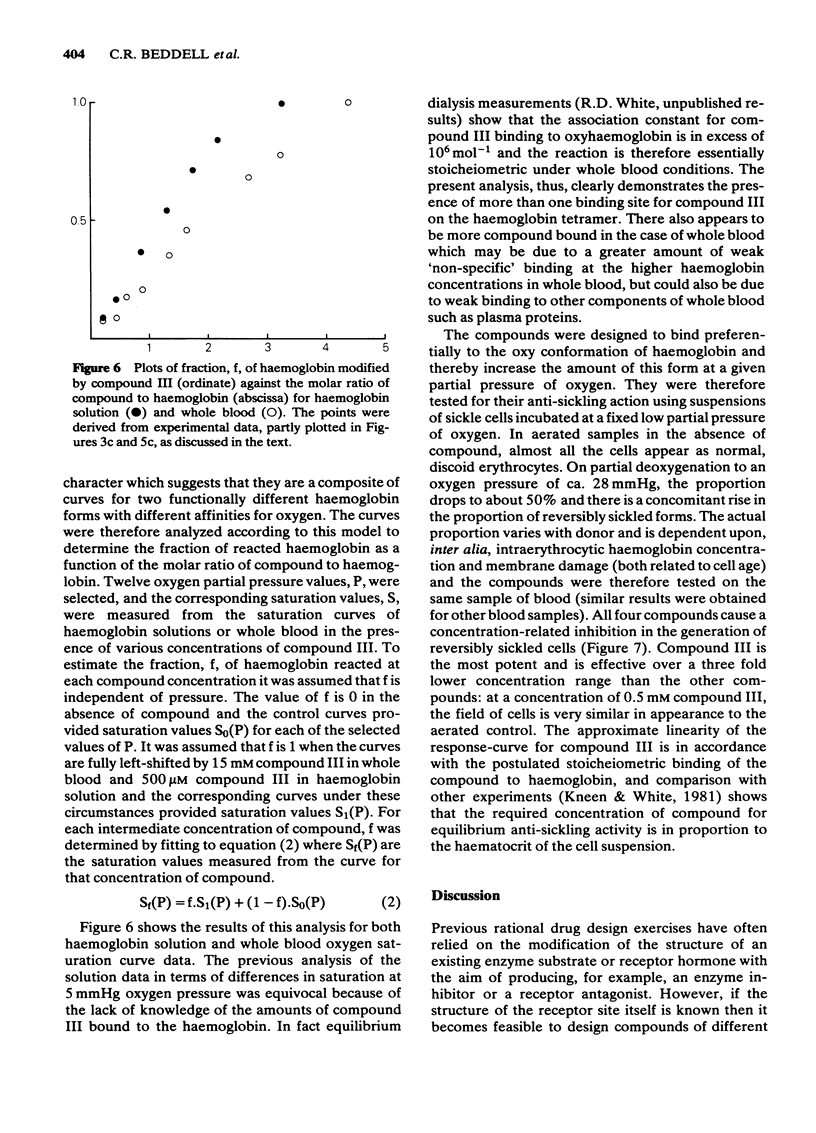
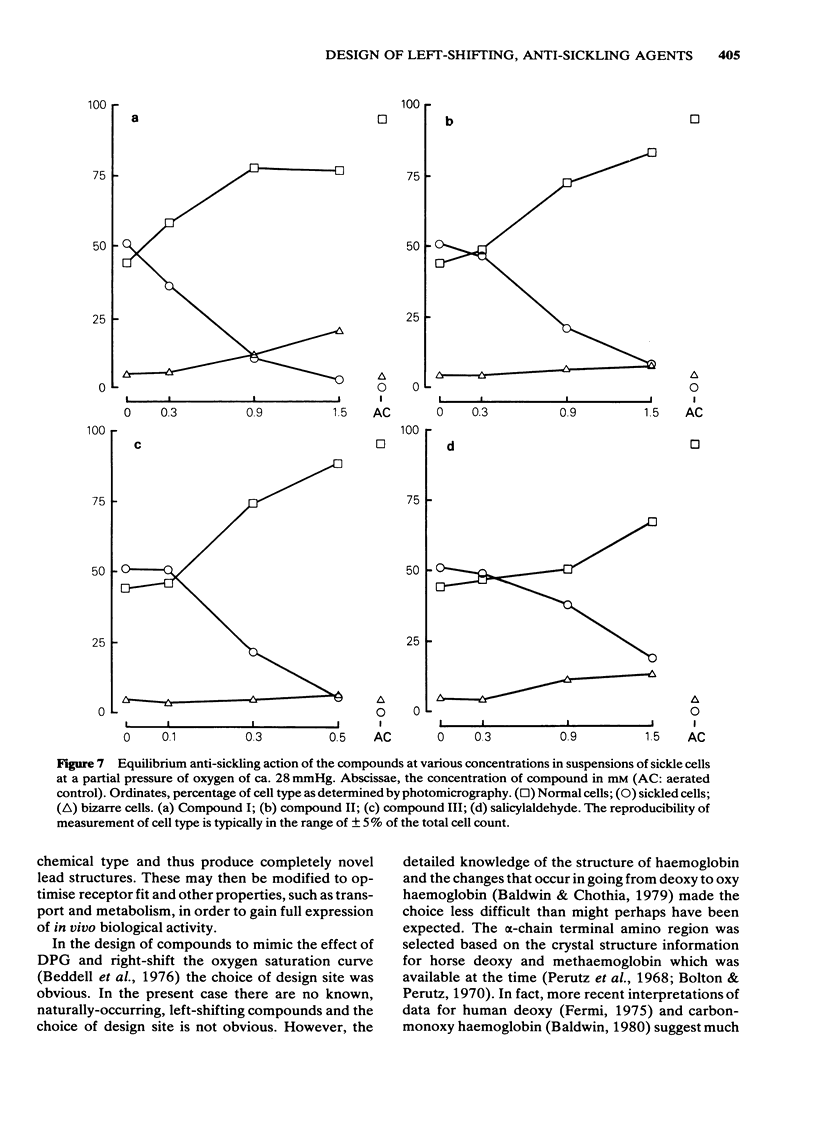


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnone A. X-ray diffraction study of binding of 2,3-diphosphoglycerate to human deoxyhaemoglobin. Nature. 1972 May 19;237(5351):146–149. doi: 10.1038/237146a0. [DOI] [PubMed] [Google Scholar]
- Baldwin J. M. The structure of human carbonmonoxy haemoglobin at 2.7 A resolution. J Mol Biol. 1980 Jan 15;136(2):103–128. doi: 10.1016/0022-2836(80)90308-3. [DOI] [PubMed] [Google Scholar]
- Baldwin J., Chothia C. Haemoglobin: the structural changes related to ligand binding and its allosteric mechanism. J Mol Biol. 1979 Apr 5;129(2):175–220. doi: 10.1016/0022-2836(79)90277-8. [DOI] [PubMed] [Google Scholar]
- Beddell C. R., Goodford P. J., Norrington F. E., Wilkinson S., Wootton R. Compounds designed to fit a site of known structure in human haemoglobin. Br J Pharmacol. 1976 Jun;57(2):201–209. doi: 10.1111/j.1476-5381.1976.tb07468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beddell C. R., Goodford P. J., Stammers D. K., Wootton R. Species differences in the binding of compounds designed to fit a site of known structure in adult human haemoglobin. Br J Pharmacol. 1979 Mar;65(3):535–543. doi: 10.1111/j.1476-5381.1979.tb07862.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beddell C. R., Kneen G., White R. D. The anti-sickling activity of a series of aromatic aldehydes [proceedings]. Br J Pharmacol. 1979 May;66(1):70P–70P. [PMC free article] [PubMed] [Google Scholar]
- Benesch R., Benesch R. E. The effect of organic phosphates from the human erythrocyte on the allosteric properties of hemoglobin. Biochem Biophys Res Commun. 1967 Jan 23;26(2):162–167. doi: 10.1016/0006-291x(67)90228-8. [DOI] [PubMed] [Google Scholar]
- Blaney J. M., Jorgensen E. C., Connolly M. L., Ferrin T. E., Langridge R., Oatley S. J., Burridge J. M., Blake C. C. Computer graphics in drug design: molecular modeling of thyroid hormone-prealbumin interactions. J Med Chem. 1982 Jul;25(7):785–790. doi: 10.1021/jm00349a004. [DOI] [PubMed] [Google Scholar]
- Bolton W., Perutz M. F. Three dimensional fourier synthesis of horse deoxyhaemoglobin at 2.8 Angstrom units resolution. Nature. 1970 Nov 7;228(5271):551–552. doi: 10.1038/228551a0. [DOI] [PubMed] [Google Scholar]
- Chanutin A., Curnish R. R. Effect of organic and inorganic phosphates on the oxygen equilibrium of human erythrocytes. Arch Biochem Biophys. 1967 Jul;121(1):96–102. doi: 10.1016/0003-9861(67)90013-6. [DOI] [PubMed] [Google Scholar]
- Dean J., Schechter A. N. Sickle-cell anemia: molecular and cellular bases of therapeutic approaches (second of three parts). N Engl J Med. 1978 Oct 12;299(15):804–811. doi: 10.1056/NEJM197810122991504. [DOI] [PubMed] [Google Scholar]
- Fermi G. Three-dimensional fourier synthesis of human deoxyhaemoglobin at 2-5 A resolution: refinement of the atomic model. J Mol Biol. 1975 Sep 15;97(2):237–256. doi: 10.1016/s0022-2836(75)80037-4. [DOI] [PubMed] [Google Scholar]
- Goodford P. J., St-Louis J., Wootton R. A quantitative analysis of the effects of 2,3-diphosphoglycerate, adenosine triphosphate and inositol hexaphosphate on the oxygen dissociation curve of human haemoglobin. J Physiol. 1978 Oct;283:397–407. doi: 10.1113/jphysiol.1978.sp012508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodford P. J., St-Louis J., Wootton R. The interaction of human haemoglobin with allosteric effectors as a model for drug-receptor interactions. Br J Pharmacol. 1980 Apr;68(4):741–748. doi: 10.1111/j.1476-5381.1980.tb10867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- INGRAM V. M. A specific chemical difference between the globins of normal human and sickle-cell anaemia haemoglobin. Nature. 1956 Oct 13;178(4537):792–794. doi: 10.1038/178792a0. [DOI] [PubMed] [Google Scholar]
- Rang H. P. Drug receptors and their function. Nature. 1971 May 14;231(5298):91–96. doi: 10.1038/231091a0. [DOI] [PubMed] [Google Scholar]
- Zaugg R. H., Walder J. A., Klotz I. M. Schiff base adducts of hemoglobin. Modifications that inhibit erythrocyte sickling. J Biol Chem. 1977 Dec 10;252(23):8542–8548. [PubMed] [Google Scholar]


