Abstract
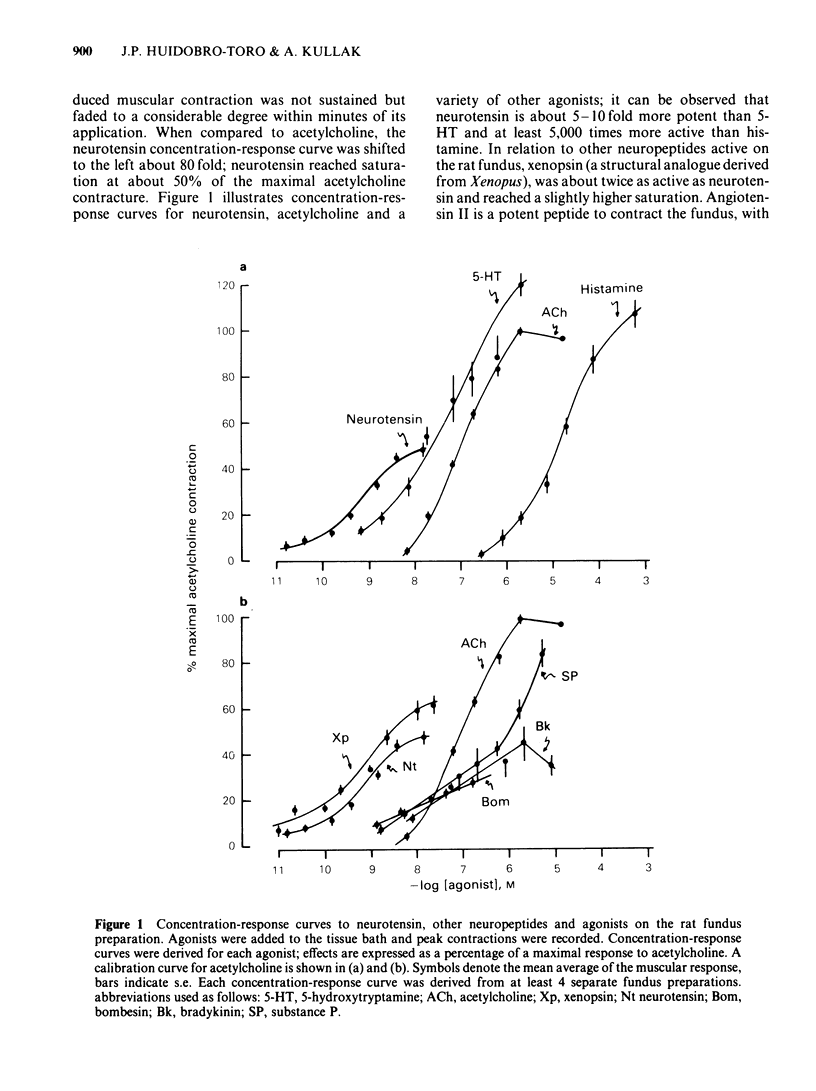
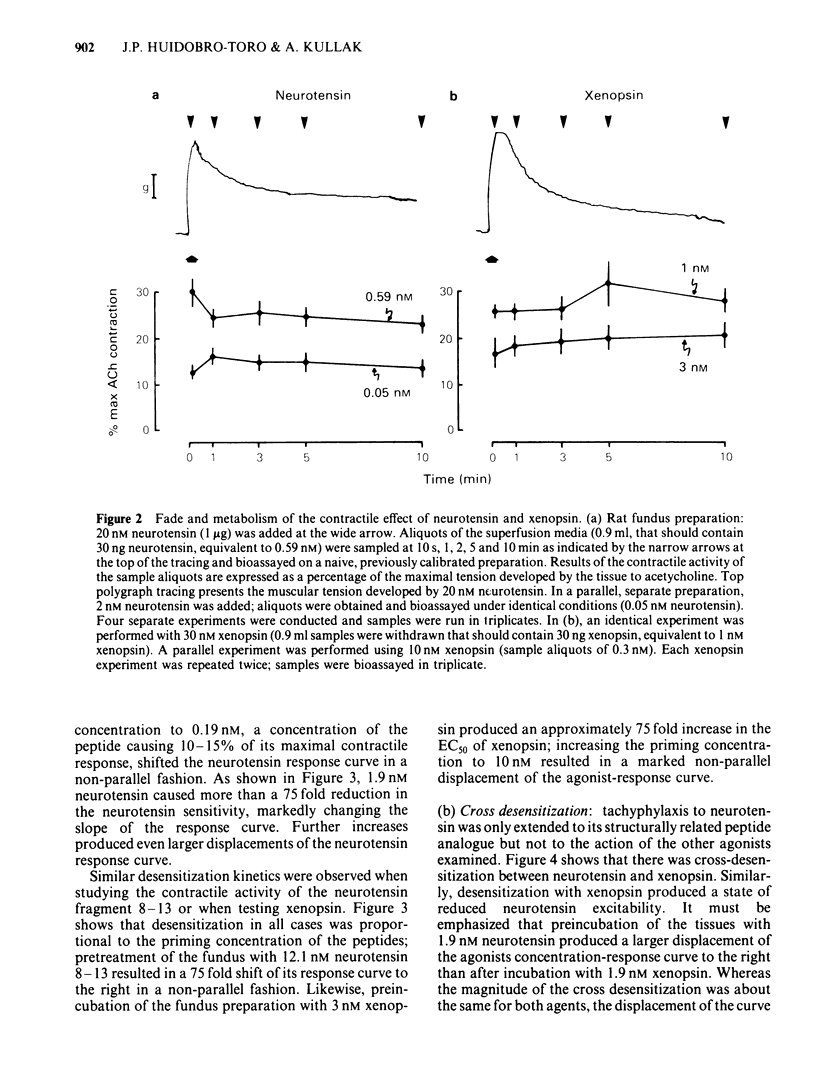
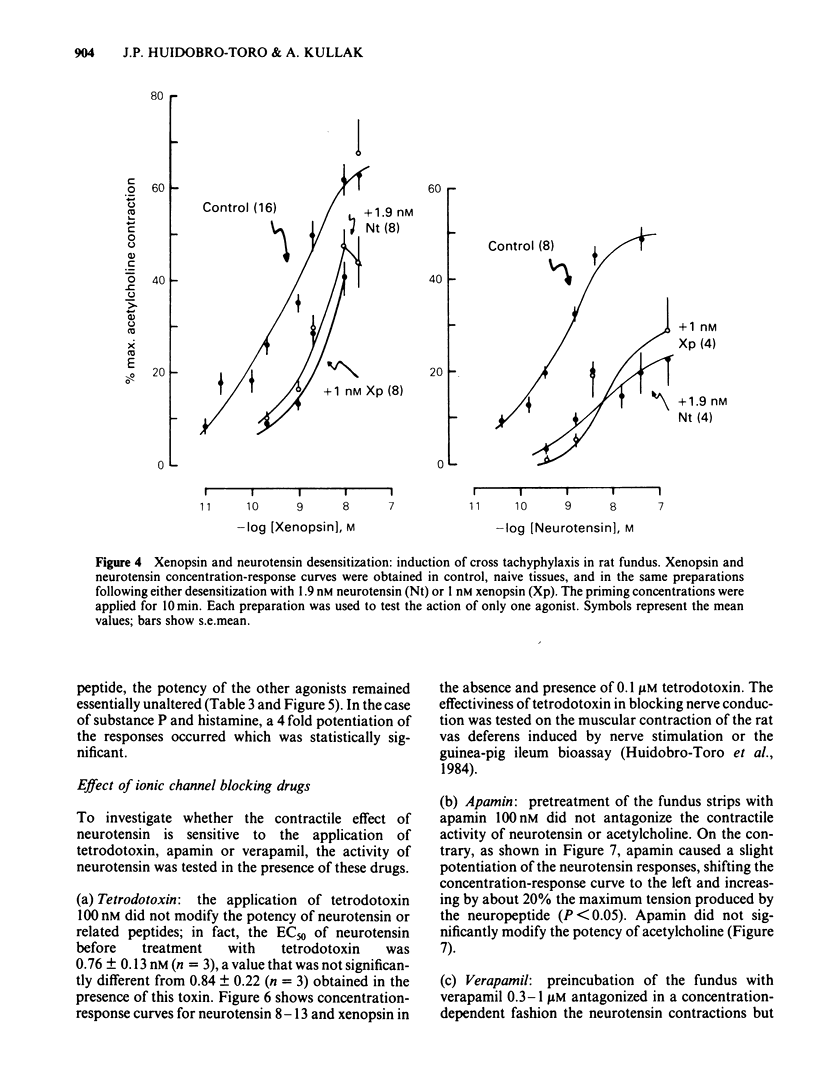
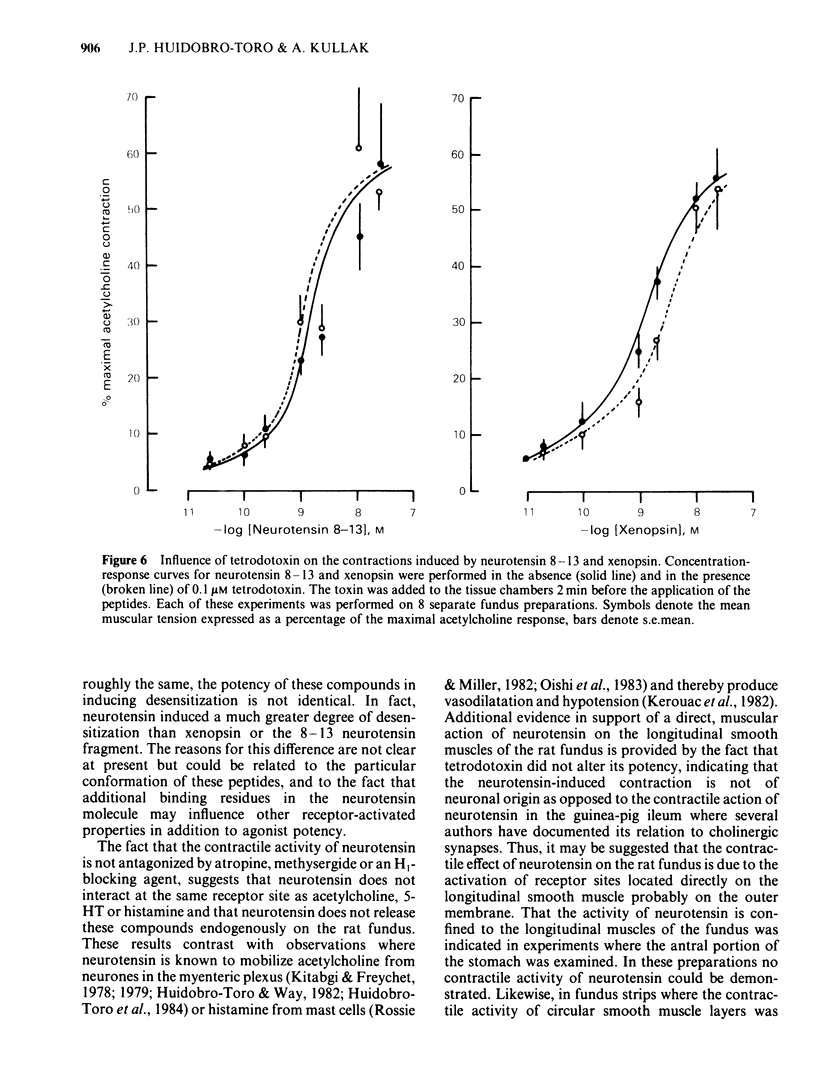
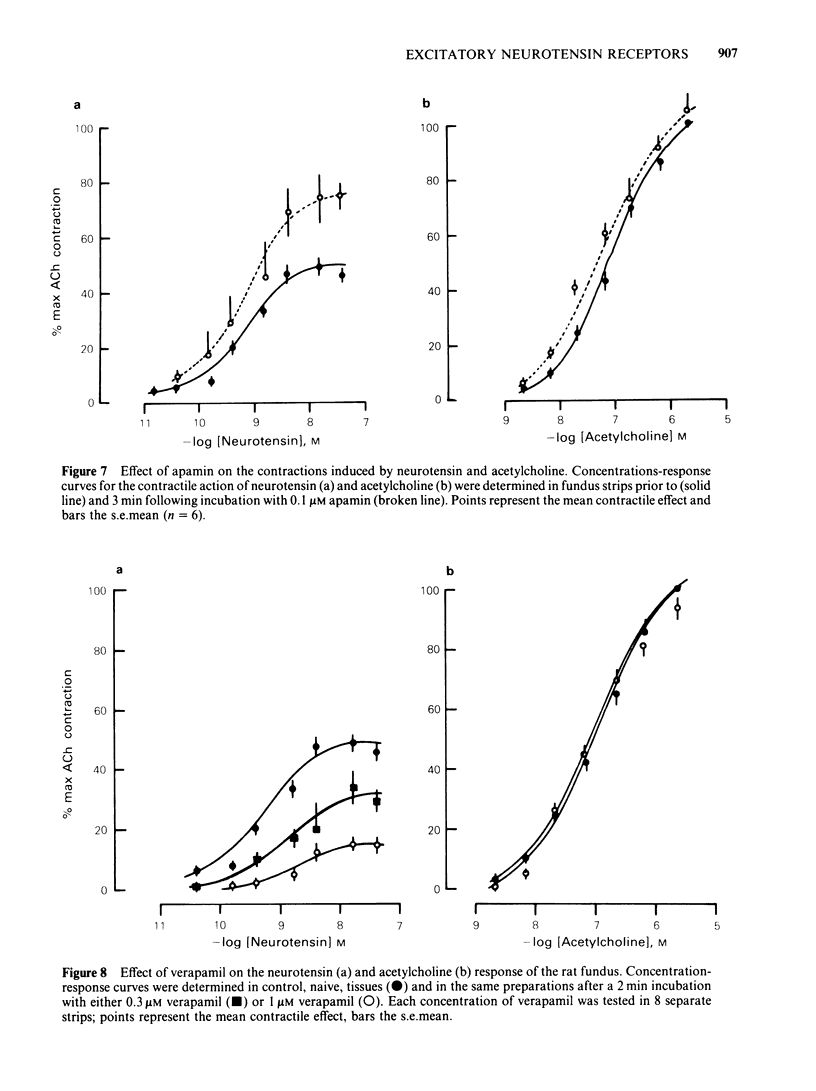
Picomolar concentrations of neurotensin caused concentration-dependent contractions of the longitudinal musculature of the fundus of the rat stomach. The EC50 of neurotensin was approximately 1.5 nM. On a molar basis neurotensin was about 5-10 times more potent than 5-hydroxytryptamine (5-HT) and approximately 80 times as active as acetylcholine in producing similar contractions. Studies with structurally related peptides indicated that whereas the carboxy terminal portion of neurotensin was essential for biological activity, a substantial part of its amino terminus end could be removed without affecting its potency. The EC50 for the neurotensin fragment 8-13 was identical to that of neurotensin, however its 1-8 or 1-11 fragments were completely inactive. Tetrodotoxin did not modify the potency of neurotensin or structurally related analogues suggesting that the neurotensin receptor is probably located on the smooth muscle membrane. In addition, the potency of neurotensin in contracting the fundus was not modified by pretreatment with atropine, methysergide or diphenhydramine. Fade to the contractile response of neurotensin was followed by the development of tachyphylaxis; desensitization was concentration-dependent and characterized by a shift in the agonist concentration-response curve to the right and downwards. Desensitization with a priming concentration of neurotensin (approx. EC50) caused a substantial blockade of its excitability. There was cross-desensitization between neurotensin and the contractile activity of neurotensin 8-13 or xenopsin, but not with angiotensin II, bradykinin, substance P, acetylcholine, 5-HT or histamine. Pretreatment of the fundus strip with verapamil 0.3-1 microM antagonized in a concentration-dependent fashion the neurotensin-induced contractions but not the muscular contractions caused by acetylcholine. It is concluded that neurotensin activates a specific excitatory receptor probably located on the cell membrane of the smooth muscles of the rat fundus. In addition, we suggest that this receptor is somehow related to a voltage-dependent calcium channel, sensitive to verapamil.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Araki K., Tachibana S., Uchiyama M., Nakajima T., Yasuhara T. Isolation and structure of a new active peptide "Xenopsin" on the smooth muscle, especially on a strip of fundus from a rat stomach, from the skin of Xenopus laevis. Chem Pharm Bull (Tokyo) 1973 Dec;21(12):2801–2804. doi: 10.1248/cpb.21.2801. [DOI] [PubMed] [Google Scholar]
- Banks B. E., Brown C., Burgess G. M., Burnstock G., Claret M., Cocks T. M., Jenkinson D. H. Apamin blocks certain neurotransmitter-induced increases in potassium permeability. Nature. 1979 Nov 22;282(5737):415–417. doi: 10.1038/282415a0. [DOI] [PubMed] [Google Scholar]
- Emson P. C., Goedert M., Benton H., St-Pierre S., Rioux F. The regional distribution and chromatographic characterization of neurotensin-like immunoreactivity in the rat. Adv Biochem Psychopharmacol. 1982;33:477–485. [PubMed] [Google Scholar]
- Hammer R. A., Carraway R. E., Leeman S. E. Elevation of plasma neurotensinlike immunoreactivity after a meal. Characterization of the elevated components. J Clin Invest. 1982 Jul;70(1):74–81. doi: 10.1172/JCI110605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstaedtar V., Mühlmann G., Feurle G. E., Forssmann W. G. Immunohistochemical identification of gastrointestinal neurotensin cells in human embryos. Cell Tissue Res. 1977 Nov 7;184(3):315–320. doi: 10.1007/BF00219893. [DOI] [PubMed] [Google Scholar]
- Huidobro-Toro J. P. Non-neuronal excitatory neurotensin receptors on the taenia coli of the guinea pig: lack of influence of tetrodotoxin and dynorphin. Neurosci Lett. 1983 Aug 8;38(3):309–314. doi: 10.1016/0304-3940(83)90387-7. [DOI] [PubMed] [Google Scholar]
- Huidobro-Toro J. P., Way E. L. Possible modulatory role of dynorphin on the excitation by neurotensin on the guinea pig myenteric plexus. Neurosci Lett. 1982 Jun 30;30(3):309–314. doi: 10.1016/0304-3940(82)90418-9. [DOI] [PubMed] [Google Scholar]
- Huidobro-Toro J. P., Yoshimura K. Pharmacological characterization of the inhibitory effects of neurotensin on the rabbit ileum myenteric plexus preparation. Br J Pharmacol. 1983 Dec;80(4):645–653. doi: 10.1111/j.1476-5381.1983.tb10054.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huidobro-Toro J. P., Zhu Y. X., Lee N. M., Loh H. H., Way E. L. Dynorphin inhibition of the neurotensin contractile activity on the myenteric plexus. J Pharmacol Exp Ther. 1984 Feb;228(2):293–303. [PubMed] [Google Scholar]
- Huidobro-Toro J. P., Zhu Y. X. Neurotensin receptors on the ileum of the guinea-pig: evidence for the coexistence of inhibitory and excitatory receptors. Eur J Pharmacol. 1984 Jul 13;102(2):237–250. doi: 10.1016/0014-2999(84)90255-3. [DOI] [PubMed] [Google Scholar]
- Kitabgi P. Effects of neurotensin on intestinal smooth muscle: application to the study of structure-activity relationships. Ann N Y Acad Sci. 1982;400:37–55. doi: 10.1111/j.1749-6632.1982.tb31559.x. [DOI] [PubMed] [Google Scholar]
- Kitabgi P., Freychet P. Effects of neurotensin on isolated intestinal smooth muscles. Eur J Pharmacol. 1978 Aug 15;50(4):349–357. doi: 10.1016/0014-2999(78)90140-1. [DOI] [PubMed] [Google Scholar]
- Kitabgi P., Freychet P. Neurotensin contracts the guinea-pig longitudinal ileal smooth muscle by inducing acetylcholine release. Eur J Pharmacol. 1979 Jul 1;56(4):403–406. doi: 10.1016/0014-2999(79)90272-3. [DOI] [PubMed] [Google Scholar]
- Kitabgi P., Hamon G., Worcel M. Electrophysiological study of the action of neurotensin on the smooth muscle of the guinea-pig taenia coli. Eur J Pharmacol. 1979 Jun;56(1-2):87–93. doi: 10.1016/0014-2999(79)90437-0. [DOI] [PubMed] [Google Scholar]
- Kitabgi P., Poustis C., Granier C., Van Rietschoten J., Rivier J., Morgat J. L., Freychet P. Neurotensin binding to extraneural and neural receptors: comparison with biological activity and structure--activity relationships. Mol Pharmacol. 1980 Jul;18(1):11–19. [PubMed] [Google Scholar]
- Kitabgi P., Vincent J. P. Neurotensin is a potent inhibitor of guinea-pig colon contractile activity. Eur J Pharmacol. 1981 Sep 24;74(4):311–318. doi: 10.1016/0014-2999(81)90050-9. [DOI] [PubMed] [Google Scholar]
- Kérouac R., St-Pierre S., Rioux F. Influence of various drugs on the variations of blood pressure, hematocrit and plasma histamine caused by neurotensin and compound 48/80 in rats. Neuropeptides. 1982 Dec;3(2):145–157. doi: 10.1016/0143-4179(82)90010-5. [DOI] [PubMed] [Google Scholar]
- Leeman S. E., Carraway R. E. Neurotensin: discovery, isolation, characterization, synthesis and possible physiological roles. Ann N Y Acad Sci. 1982;400:1–16. doi: 10.1111/j.1749-6632.1982.tb31557.x. [DOI] [PubMed] [Google Scholar]
- Mashford M. L., Nilsson G., Rökaeus A., Rosell S. The effect of food ingestion on circulating neurotensin-like immunoreactivity (NTLI) in the human. Acta Physiol Scand. 1978 Oct;104(2):244–246. doi: 10.1111/j.1748-1716.1978.tb06275.x. [DOI] [PubMed] [Google Scholar]
- Oishi M., Ishiko J., Inagaki C., Takaori S. Release of histamine and adrenaline in vivo following intravenous administration of neurotensin. Life Sci. 1983 May 9;32(19):2231–2239. doi: 10.1016/0024-3205(83)90421-6. [DOI] [PubMed] [Google Scholar]
- Polak J. M., Sullivan S. N., Bloom S. R., Buchan A. M., Facer P., Brown M. R., Pearse A. G. Specific localisation of neurotensin to the N cell in human intestine by radioimmunoassay and immunocytochemistry. Nature. 1977 Nov 10;270(5633):183–184. doi: 10.1038/270183a0. [DOI] [PubMed] [Google Scholar]
- Quirion R., Regoli D., Rioux F., St-Pierre S. The stimulatory effects of neurotensin and related peptides in rat stomach strips and guinea-pig atria. Br J Pharmacol. 1980 Jan;68(1):83–91. doi: 10.1111/j.1476-5381.1980.tb10702.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosell S., Rökaeus A. The effect of ingestion of amino acids, glucose and fat on circulating neurotensin-like immunoreactivity (NTLI) in man. Acta Physiol Scand. 1979 Nov;107(3):263–267. doi: 10.1111/j.1748-1716.1979.tb06472.x. [DOI] [PubMed] [Google Scholar]
- Rosell S. The role of neurotensin in the uptake and distribution of fat. Ann N Y Acad Sci. 1982;400:183–197. doi: 10.1111/j.1749-6632.1982.tb31569.x. [DOI] [PubMed] [Google Scholar]
- Rossie S. S., Miller R. J. Regulation of mast cell histamine release by neurotensin. Life Sci. 1982 Aug 9;31(6):509–516. doi: 10.1016/0024-3205(82)90478-7. [DOI] [PubMed] [Google Scholar]
- Schultzberg M., Hökfelt T., Nilsson G., Terenius L., Rehfeld J. F., Brown M., Elde R., Goldstein M., Said S. Distribution of peptide- and catecholamine-containing neurons in the gastro-intestinal tract of rat and guinea-pig: immunohistochemical studies with antisera to substance P, vasoactive intestinal polypeptide, enkephalins, somatostatin, gastrin/cholecystokinin, neurotensin and dopamine beta-hydroxylase. Neuroscience. 1980;5(4):689–744. doi: 10.1016/0306-4522(80)90166-9. [DOI] [PubMed] [Google Scholar]
- VANE J. R. A sensitive method for the assay of 5-hydroxytryptamine. Br J Pharmacol Chemother. 1957 Sep;12(3):344–349. doi: 10.1111/j.1476-5381.1957.tb00146.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. T., Katayama Y., North R. A. The action of neurotensin on single myenteric neurones. Eur J Pharmacol. 1979 Nov 16;59(3-4):181–186. doi: 10.1016/0014-2999(79)90280-2. [DOI] [PubMed] [Google Scholar]
- Zetler G. Antagonism of the gut-contracting effects of bombesin and neurotensin by opioid peptides, morphine, atropine or tetrodotoxin. Pharmacology. 1980;21(5):348–354. doi: 10.1159/000137451. [DOI] [PubMed] [Google Scholar]


