Abstract
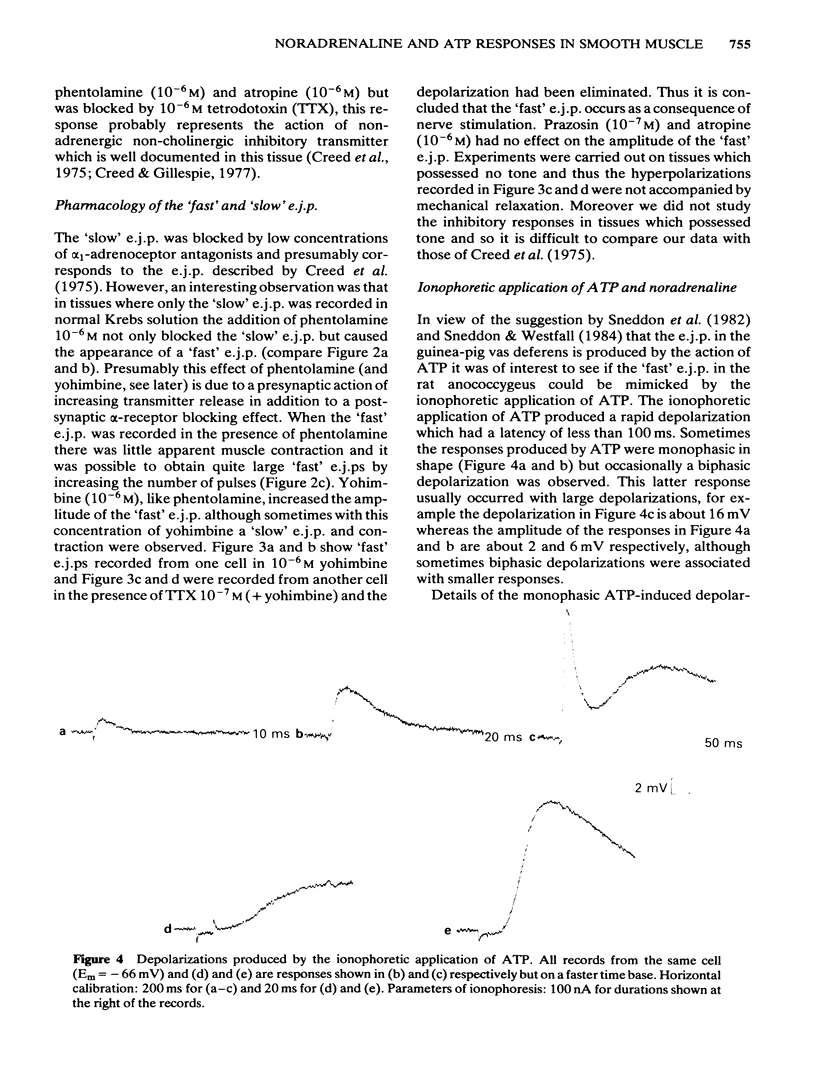
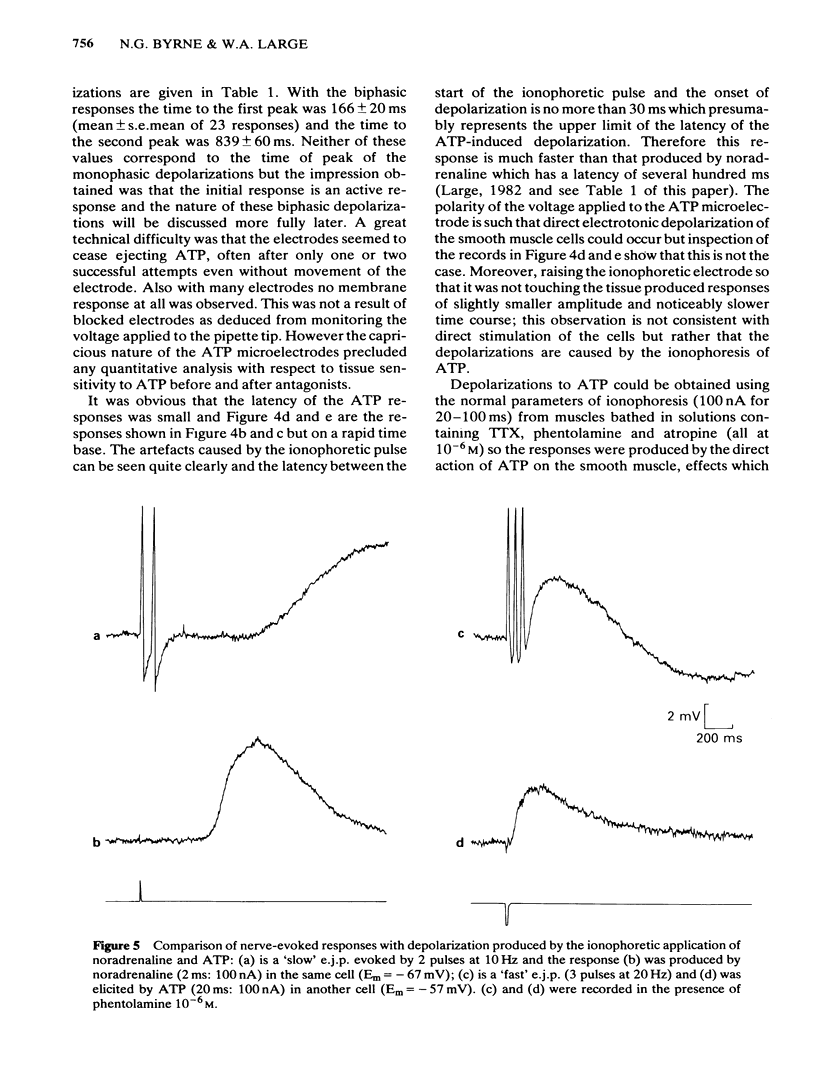
The effects of field stimulation and ionophoretic application of adenosine triphosphate (ATP) and noradrenaline were studied in the rat anococcygeus by means of an intracellular microelectrode. Field stimulation at room temperature produced three types of electrical membrane response: (a) a 'fast' excitatory junction potential (e.j.p.) which had a latency of less than 100 ms and a time to peak of 300 ms; (b) a 'slow' e.j.p. which had a latency of several hundred ms and a time to peak of 1-2 s, and (c) an inhibitory junction potential (i.j.p.) which had a time to peak of about 1.5 s. All three responses were blocked by tetrodotoxin. The ionophoretic application of ATP produced both monophasic and biphasic depolarizations; these responses had a latency of less than 30 ms and a time to peak of 150-300 ms. In contrast, ionophoretically-applied noradrenaline produced a depolarization which had a mean latency of 471 ms and a time to peak of 861 ms. The 'slow' e.j.p. and the noradrenaline-induced depolarization were blocked by prazosin whereas the 'fast' e.j.p. and the ATP responses were resistant to this antagonist and also to atropine. These results are further evidence that the 'fast' e.j.p. in some smooth muscle tissues is mediated by ATP.
Full text
PDF
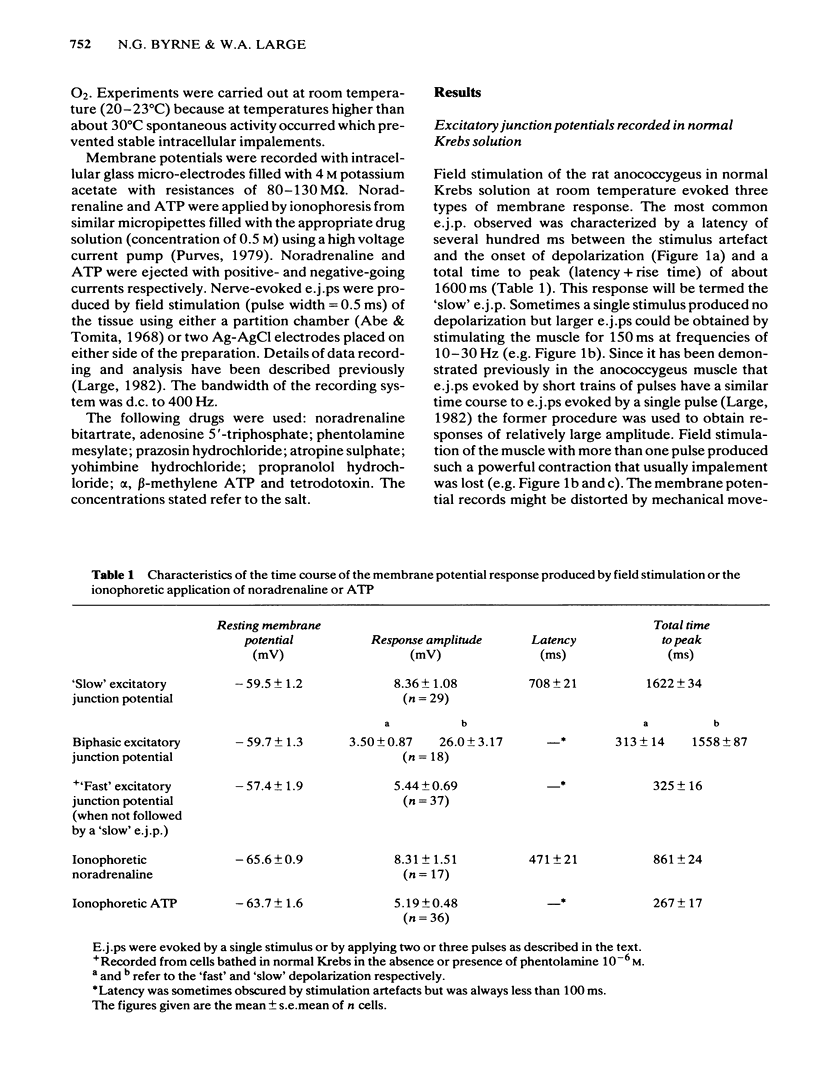
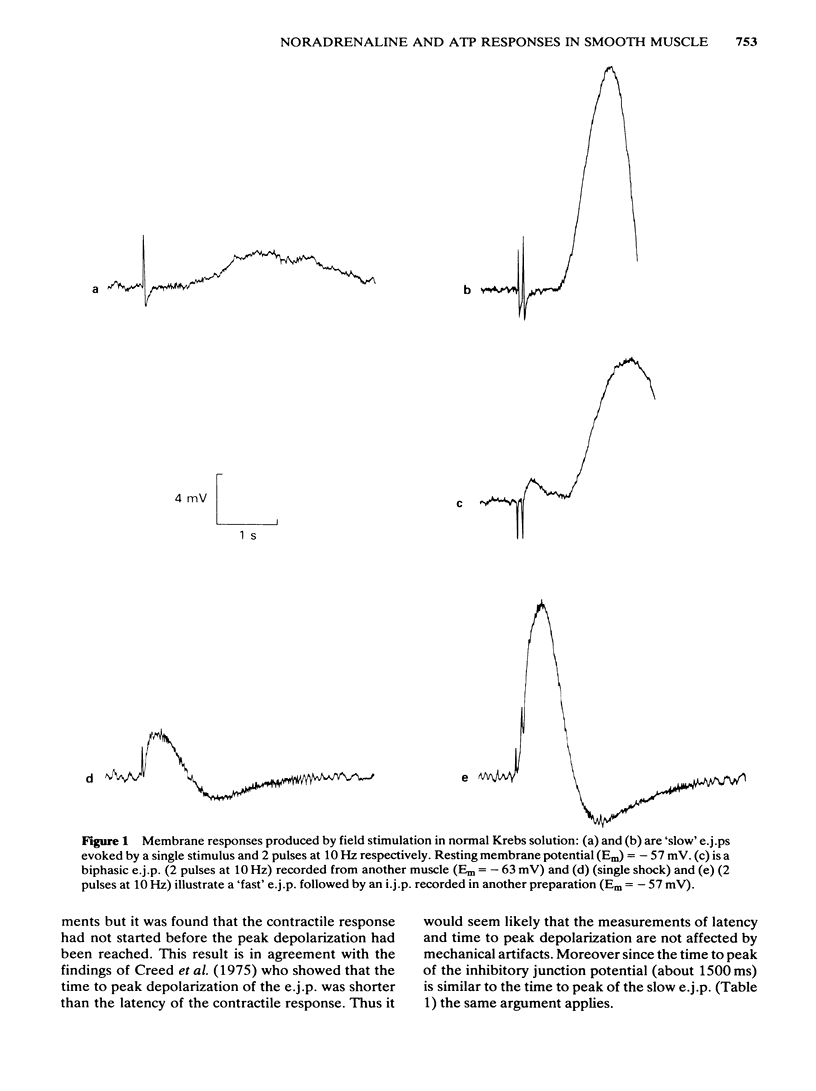
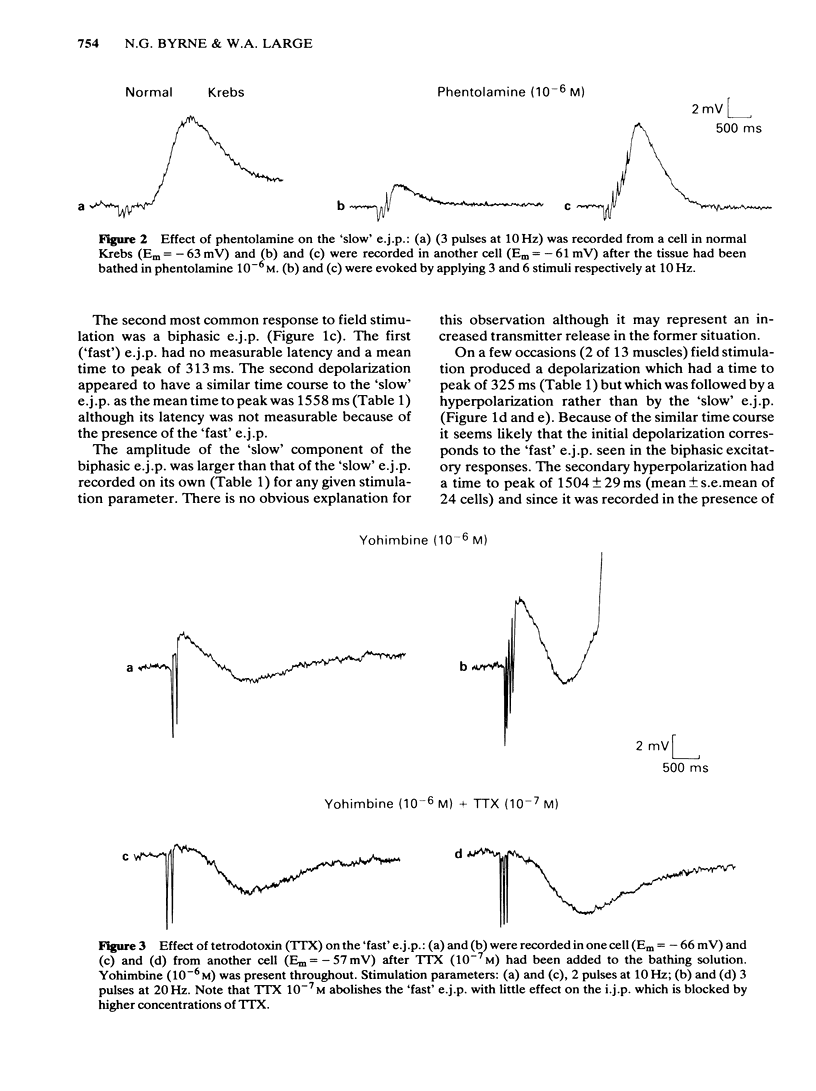


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abe Y., Tomita T. Cable properties of smooth muscle. J Physiol. 1968 May;196(1):87–100. doi: 10.1113/jphysiol.1968.sp008496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURNSTOCK G., HOLMAN M. E. AN ELECTROPHYSIOLOGICAL INVESTIGATION OF THE ACTIONS OF SOME AUTONOMIC BLOCKING DRUGS ON TRANSMISSION IN THE GUINEA-PIG VAS DEFERENS. Br J Pharmacol Chemother. 1964 Dec;23:600–612. doi: 10.1111/j.1476-5381.1964.tb01613.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G., Cocks T., Crowe R. Evidence for purinergic innervation of the anococcygeus muscle. Br J Pharmacol. 1978 Sep;64(1):13–20. doi: 10.1111/j.1476-5381.1978.tb08635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed K. E., Gillespie J. S., Muir T. C. The electrical basis of excitation and inhibition in the rat anoccygeus muscle. J Physiol. 1975 Feb;245(1):33–47. doi: 10.1113/jphysiol.1975.sp010833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed K. E., Gillespie J. S. Some electrical properties of the rabbit anococcygeus muscle and a comparison of the effects of inhibitory nerve stimulation in the rat and rabbit. J Physiol. 1977 Dec;273(1):137–153. doi: 10.1113/jphysiol.1977.sp012086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creed K. E. Membrane properties of the smooth muscle cells of the rat anococcygeus muscle. J Physiol. 1975 Feb;245(1):49–62. doi: 10.1113/jphysiol.1975.sp010834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. Evidence for two populations of excitatory receptors for noradrenaline on arteriolar smooth muscle. Nature. 1980 Feb 21;283(5749):767–768. doi: 10.1038/283767a0. [DOI] [PubMed] [Google Scholar]
- Holman M. E., Surprenant A. An electrophysiological analysis of the effects of noradrenaline and alpha-receptor antagonists on neuromuscular transmission in mammalian muscular arteries. Br J Pharmacol. 1980;71(2):651–661. doi: 10.1111/j.1476-5381.1980.tb10986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KURIYAMA H. ELECTROPHYSIOLOGICAL OBSERVATIONS ON THE MOTOR INNERVATION OF THE SMOOTH MUSCLE CELLS IN THE GUINEA-PIG VAS DEFERENS. J Physiol. 1963 Nov;169:213–228. doi: 10.1113/jphysiol.1963.sp007251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large W. A. Membrane potential responses of the mouse anococcygeus muscle to ionophoretically applied noradrenaline. J Physiol. 1982 May;326:385–400. doi: 10.1113/jphysiol.1982.sp014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Large W. A. The effect of chloride removal on the responses of the isolated rat anococcygeus muscle to alpha 1-adrenoceptor stimulation. J Physiol. 1984 Jul;352:17–29. doi: 10.1113/jphysiol.1984.sp015275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meldrum L. A., Burnstock G. Evidence that ATP acts as a co-transmitter with noradrenaline in sympathetic nerves supplying the guinea-pig vas deferens. Eur J Pharmacol. 1983 Aug 19;92(1-2):161–163. doi: 10.1016/0014-2999(83)90126-7. [DOI] [PubMed] [Google Scholar]
- Purves R. D. The physics of iontophoretic pipettes. J Neurosci Methods. 1979 Aug;1(2):165–178. doi: 10.1016/0165-0270(79)90014-1. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Westfall D. P., Fedan J. S. Cotransmitters in the motor nerves of the guinea pig vas deferens: electrophysiological evidence. Science. 1982 Nov 12;218(4573):693–695. doi: 10.1126/science.6291151. [DOI] [PubMed] [Google Scholar]
- Sneddon P., Westfall D. P. Pharmacological evidence that adenosine triphosphate and noradrenaline are co-transmitters in the guinea-pig vas deferens. J Physiol. 1984 Feb;347:561–580. doi: 10.1113/jphysiol.1984.sp015083. [DOI] [PMC free article] [PubMed] [Google Scholar]


