Abstract
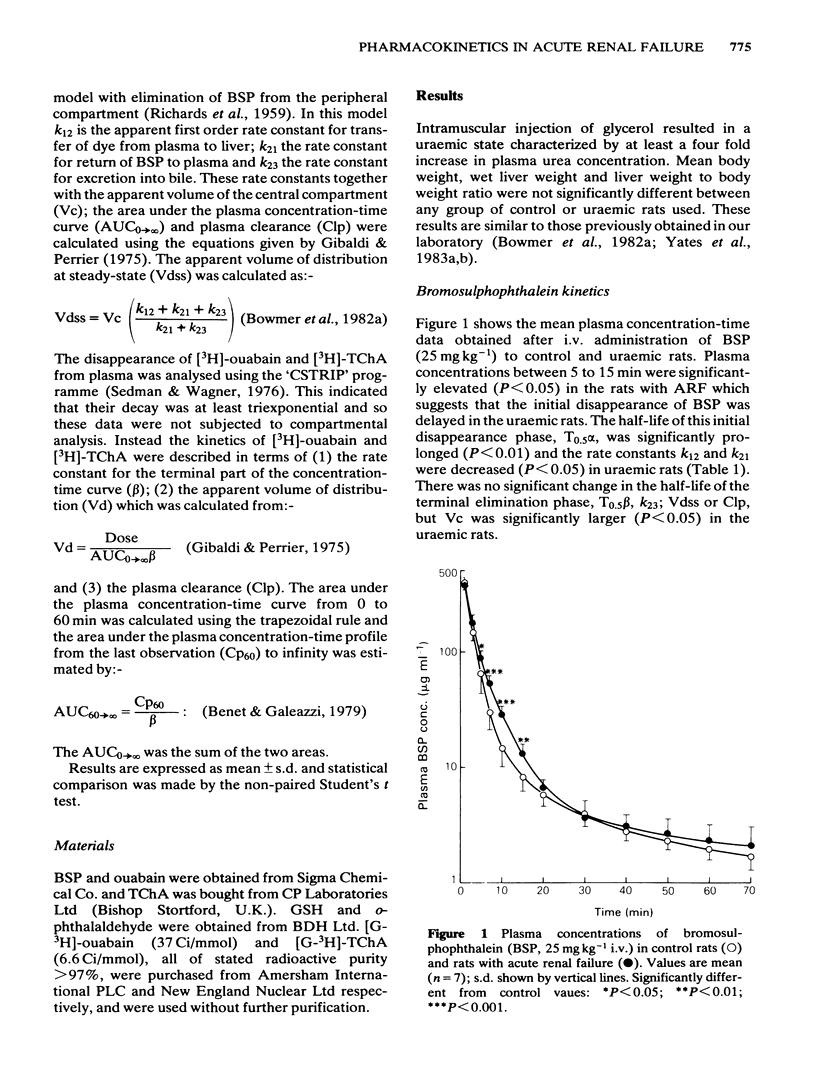
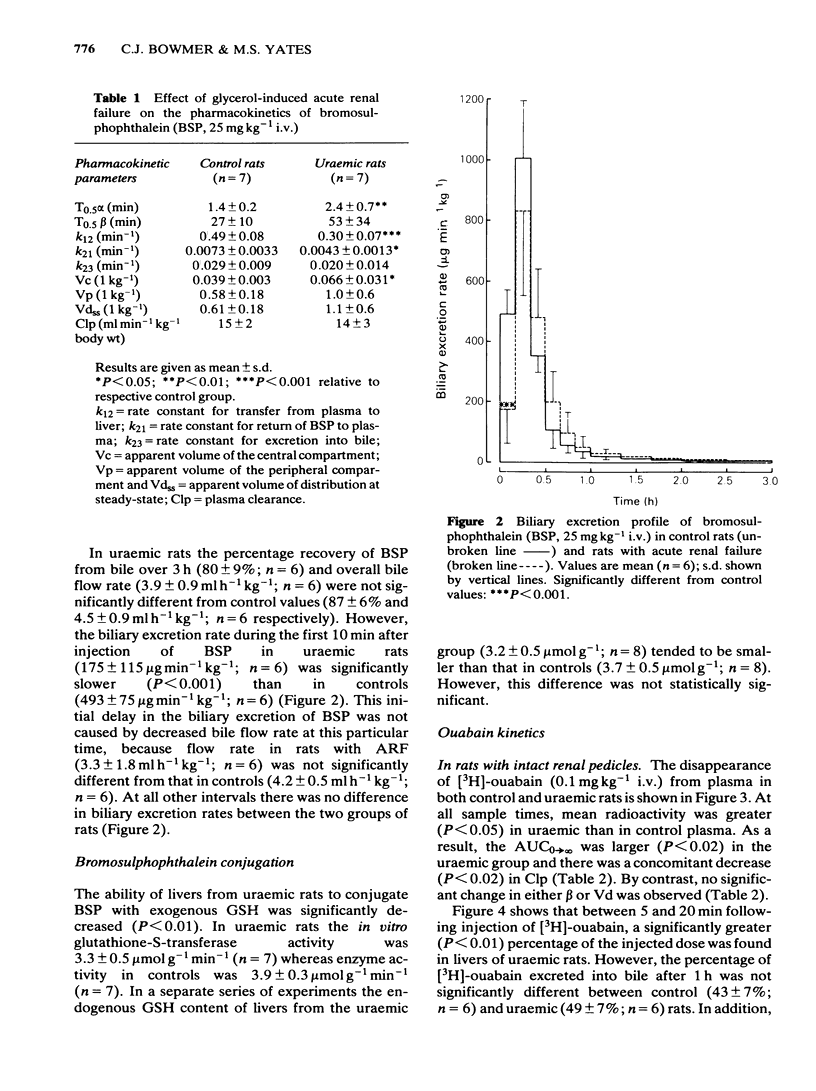
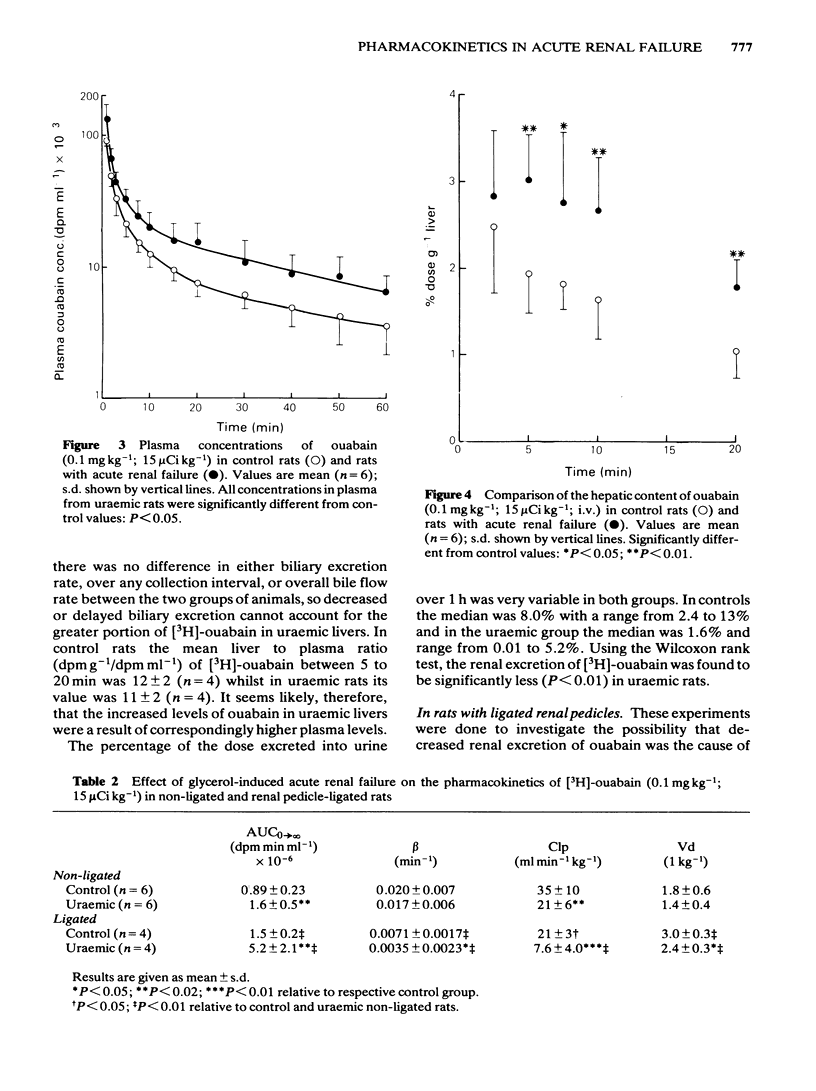
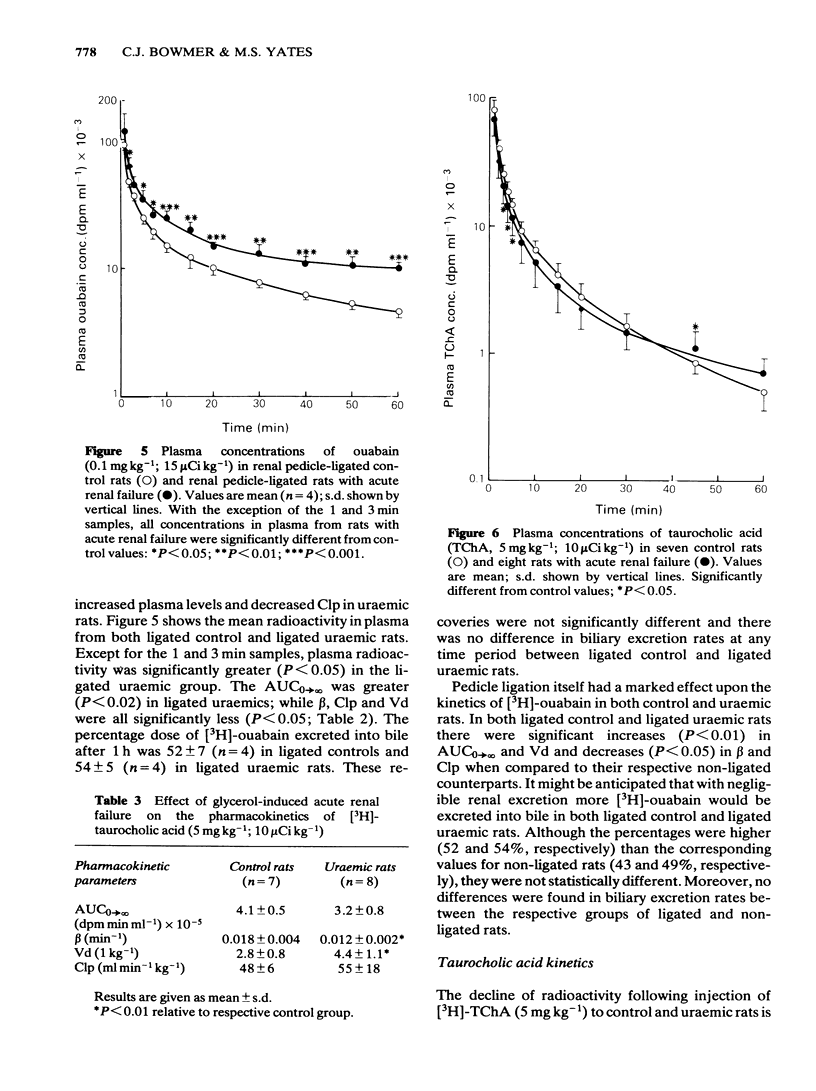
The pharmacokinetics and biliary excretion of bromosulphophthalein (BSP), ouabain and taurocholic acid (TChA) have been studied in rats with glycerol-induced acute renal failure (ARF). In rats with ARF, the hepatic uptake and initial biliary excretion of BSP were decreased. In addition, the rate of BSP conjugation with glutathione by rat liver homogenates was also decreased. This latter change may contribute to the initial decrease in the biliary excretion of BSP. No change was found in the hepatic uptake and biliary excretion of ouabain, but the area under the concentration-time curve was increased and the plasma clearance (Clp) decreased in rats with ARF. This decrease in Clp was not due to reduced renal excretion. The decreased Clp of ouabain in rats with ARF may come from reduced tissue binding and a concomitant decrease in its volume of distribution (Vd). The hepatic handling of TChA appeared unaltered in ARF, but the rate constant for the terminal part of the concentration-time curve (beta) was decreased. This change probably resulted from a large increase in Vd in rats with ARF. It is concluded that the decreased uptake of BSP was not due to a non-specific disturbance of hepatocyte function in ARF because the hepatic handling of ouabain and TChA were unaltered.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alpert S., Mosher M., Shanske A., Arias I. M. Multiplicity of hepatic excretory mechanisms for organic anions. J Gen Physiol. 1969 Feb;53(2):238–247. doi: 10.1085/jgp.53.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benet L. Z., Galeazzi R. L. Noncompartmental determination of the steady-state volume of distribution. J Pharm Sci. 1979 Aug;68(8):1071–1074. doi: 10.1002/jps.2600680845. [DOI] [PubMed] [Google Scholar]
- Bowmer C. J., Emmerson J., Yates M. S. Delayed biliary excretion of indocyanine green in rats with glycerol-induced acute renal failure. Biochem Pharmacol. 1983 May 15;32(10):1641–1642. doi: 10.1016/0006-2952(83)90340-4. [DOI] [PubMed] [Google Scholar]
- Bowmer C. J., Lindup W. E. Decreased binding of drugs and dyes to plasma proteins from rats with acute renal failure: effects of ureter ligation and intramuscular injection of glycerol. Br J Pharmacol. 1979 Jun;66(2):275–281. doi: 10.1111/j.1476-5381.1979.tb13676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COMBES B. THE IMPORTANCE OF CONJUGATION WITH GLUTATHIONE FOR SULFOBROMOPHTHALEIN SODIUM (BSP) TRANSFER FROM BLOOD TO BILE. J Clin Invest. 1965 Jul;44:1214–1224. doi: 10.1172/JCI105227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- COX E., ROXBURGH G., WRIGHT S. E. The metabolism of ouabain in the rat. J Pharm Pharmacol. 1959 Sep;11:535–539. doi: 10.1111/j.2042-7158.1959.tb12592.x. [DOI] [PubMed] [Google Scholar]
- Cohn V. H., Lyle J. A fluorometric assay for glutathione. Anal Biochem. 1966 Mar;14(3):434–440. doi: 10.1016/0003-2697(66)90286-7. [DOI] [PubMed] [Google Scholar]
- Fischer E., Varga F., Gregus Z., Gógl A. Bile flow and biliary excretion rate of some organic anions in phenobarbital-pretreated rats. Digestion. 1978;17(3):211–220. doi: 10.1159/000198112. [DOI] [PubMed] [Google Scholar]
- Goldstein J., Combes B. Spectrophotometric assay of the liver enzyme that catalyzes sulfobromophthalein-glutathione conjugation. J Lab Clin Med. 1966 May;67(5):863–872. [PubMed] [Google Scholar]
- Grausz H., Schmid R. Reciprocal relation between plasma albumin level and hepatic sulfobromophthalein removal. N Engl J Med. 1971 Jun 24;284(25):1403–1406. doi: 10.1056/NEJM197106242842504. [DOI] [PubMed] [Google Scholar]
- Gregus Z., Klaassen C. D. Role of ligandin as a binding protein and as an enzyme in the biliary excretion of sulfobromophthalein. J Pharmacol Exp Ther. 1982 Apr;221(1):242–246. [PubMed] [Google Scholar]
- Habig W. H., Pabst M. J., Fleischner G., Gatmaitan Z., Arias I. M., Jakoby W. B. The identity of glutathione S-transferase B with ligandin, a major binding protein of liver. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3879–3882. doi: 10.1073/pnas.71.10.3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiley C. R., Yates M. S., Roberts P. J., Bloom A. E. Alterations in liver blood flow during glycerol-induced acute renal failure in the rat. Nephron. 1980;26(5):244–248. doi: 10.1159/000181993. [DOI] [PubMed] [Google Scholar]
- Hirom P. C., Millburn P., Smith R. L. Bile and urine as complementary pathways for the excretion of foreign organic compounds. Xenobiotica. 1976 Jan;6(1):55–64. doi: 10.3109/00498257609151612. [DOI] [PubMed] [Google Scholar]
- Hoffman N. E., Iser J. H., Smallwood R. A. Hepatic bile acid transport: effect of conjugation and position of hydroxyl groups. Am J Physiol. 1975 Aug;229(2):298–302. doi: 10.1152/ajplegacy.1975.229.2.298. [DOI] [PubMed] [Google Scholar]
- Inoue M., Okajima K., Nagase S., Morino Y. Plasma clearance of sulfobromophthalein and its interaction with hepatic binding proteins in normal and analbuminemic rats: is plasma albumin essential for vectorial transport of organic anions in the liver? Proc Natl Acad Sci U S A. 1983 Dec;80(24):7654–7658. doi: 10.1073/pnas.80.24.7654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jusko W. J., Weintraub M. Myocardial distribution of digoxin and renal function. Clin Pharmacol Ther. 1974 Sep;16(3):449–454. doi: 10.1002/cpt1974163part1449. [DOI] [PubMed] [Google Scholar]
- Kamisaka K., Listowsky I., Gatmaitan Z., Arias I. M. Interactions of bilirubin and other ligands with ligandin. Biochemistry. 1975 May 20;14(10):2175–2180. doi: 10.1021/bi00681a021. [DOI] [PubMed] [Google Scholar]
- Ketley J. N., Habig W. H., Jakoby W. B. Binding of nonsubstrate ligands to the glutathione S-transferases. J Biol Chem. 1975 Nov 25;250(22):8670–8673. [PubMed] [Google Scholar]
- Ketterer B., Tipping E., Hackney J. F., Beale D. A low-molecular-weight protein from rat liver that resembles ligandin in its binding properties. Biochem J. 1976 Jun 1;155(3):511–521. doi: 10.1042/bj1550511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen C. D. Biliary excretion of drugs: role of ligandin in newborn immaturity and in the action of microsomal enzyme inducers. J Pharmacol Exp Ther. 1975 Nov;195(2):311–319. [PubMed] [Google Scholar]
- Klaassen C. D. Independence of bile acid and ouabain hepatic uptake: studies in the newborn rat. Proc Soc Exp Biol Med. 1978 Jan;157(1):66–69. doi: 10.3181/00379727-157-39992. [DOI] [PubMed] [Google Scholar]
- Kupferberg H. J., Schankl L. S. Biliary secretion of ouabain-3H and its uptake by liver slices in the rat. Am J Physiol. 1968 May;214(5):1048–1053. doi: 10.1152/ajplegacy.1968.214.5.1048. [DOI] [PubMed] [Google Scholar]
- Levi A. J., Gatmaitan Z., Arias I. M. Two hepatic cytoplasmic protein fractions, Y and Z, and their possible role in the hepatic uptake of bilirubin, sulfobromophthalein, and other anions. J Clin Invest. 1969 Nov;48(11):2156–2167. doi: 10.1172/JCI106182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer D. K., Bognacki J., Levine W. G. Effect of nafenopin (SU-13,437) on liver functions. Hepatic uptake and biliary excretion of ouabain in the rat. Drug Metab Dispos. 1975 May-Jun;3(3):220–225. [PubMed] [Google Scholar]
- Meijer D. K., Vonk R. J., Keulemans K., Weitering J. G. Hepatic uptake and biliary excretion of dibromosulphthalein. Albumin dependence, influence of phenobarbital and nafenopin pretreatment and the role of y and z protein. J Pharmacol Exp Ther. 1977 Jul;202(1):8–21. [PubMed] [Google Scholar]
- Meijer D. K., Vonk R. J., Scholtens E. J., Levine W. G. The influence of dehydrocholate on hepatic uptake and biliary excretion of 3H-taurocholate and 3H-ouabain. Drug Metab Dispos. 1976 Jan-Feb;4(1):1–7. [PubMed] [Google Scholar]
- Priestly B. G., Plaa G. L. Effects of benziodarone on the metabolism and biliary excretion of sulfobromophthalein and related dyes. Proc Soc Exp Biol Med. 1969 Dec;132(3):881–885. doi: 10.3181/00379727-132-34328. [DOI] [PubMed] [Google Scholar]
- Reuning R. H., Sams R. A., Notari R. E. Role of pharmacokinetics in drug dosage adjustment. I. Pharmacologic effect kinetics and apparent volume of distribution of digoxin. J Clin Pharmacol New Drugs. 1973 Apr;13(4):127–141. doi: 10.1002/j.1552-4604.1973.tb00074.x. [DOI] [PubMed] [Google Scholar]
- SOLOMON H. M., SCHANKER L. S. Hepatic transport of organic cations: active uptake of a quaternary ammonium compound, procaine amide ethobromide. by rat liver slices. Biochem Pharmacol. 1963 Jul;12:621–626. doi: 10.1016/0006-2952(63)90036-4. [DOI] [PubMed] [Google Scholar]
- Scharschmidt B. F., Waggoner J. G., Berk P. D. Hepatic organic anion uptake in the rat. J Clin Invest. 1975 Nov;56(5):1280–1292. doi: 10.1172/JCI108204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedman A. J., Wagner J. G. CSTRIP, a fortran IV computer program for obtaining initial polyexponential parameter estimates. J Pharm Sci. 1976 Jul;65(7):1006–1010. doi: 10.1002/jps.2600650713. [DOI] [PubMed] [Google Scholar]
- Uptake of bromosulfophthalein by isolated liver cells. Eur J Biochem. 1976 Apr 15;64(1):189–197. [PubMed] [Google Scholar]
- Wernze H., Spech H. J. Untersuchungen über Speicherkapazität und Transportmaximum der Leber für Bromsulphthalein bei chronischer Niereninsuffizienz. Klin Wochenschr. 1971 Dec 15;49(24):1318–1322. doi: 10.1007/BF01495518. [DOI] [PubMed] [Google Scholar]
- Whelan G., Hoch J., Combes B. A direct assessment of the importance of conjugation for biliary transport of sulfobromophthalein sodium. J Lab Clin Med. 1970 Apr;75(4):542–557. [PubMed] [Google Scholar]
- Yates M. S., Bowmer C. J., Emmerson J. Effect of acute renal failure on the clearance and biliary excretion of indocyanine green in perfused rat liver. Biochem Pharmacol. 1984 May 15;33(10):1695–1696. doi: 10.1016/0006-2952(84)90297-1. [DOI] [PubMed] [Google Scholar]
- Yates M. S., Emmerson J., Bowmer C. J. Hepatic clearance of indocyanine green during the course of glycerol-induced acute renal failure in the rat. J Pharm Pharmacol. 1983 May;35(5):335–338. doi: 10.1111/j.2042-7158.1983.tb02950.x. [DOI] [PubMed] [Google Scholar]
- Yates M. S., Emmerson J., Bowmer C. J. Pharmacokinetics of indocyanine green in rats with chronic renal failure. J Pharm Pharmacol. 1983 Sep;35(9):593–594. doi: 10.1111/j.2042-7158.1983.tb04340.x. [DOI] [PubMed] [Google Scholar]


