Abstract
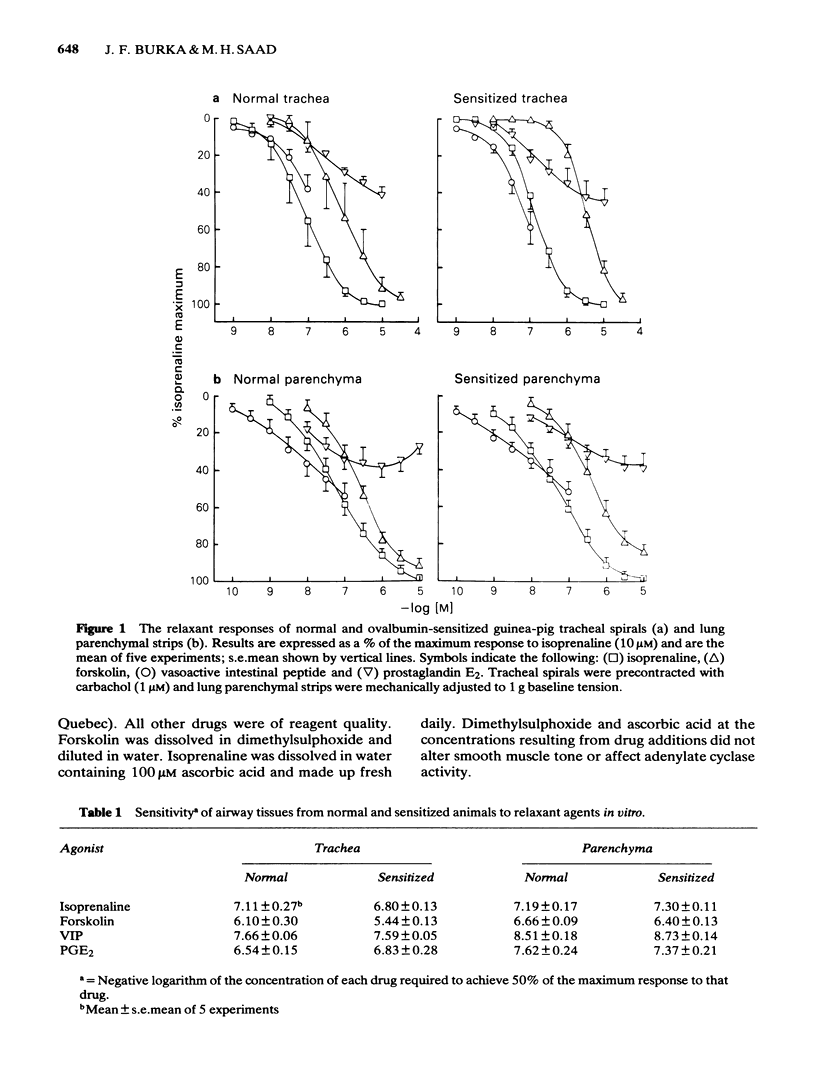
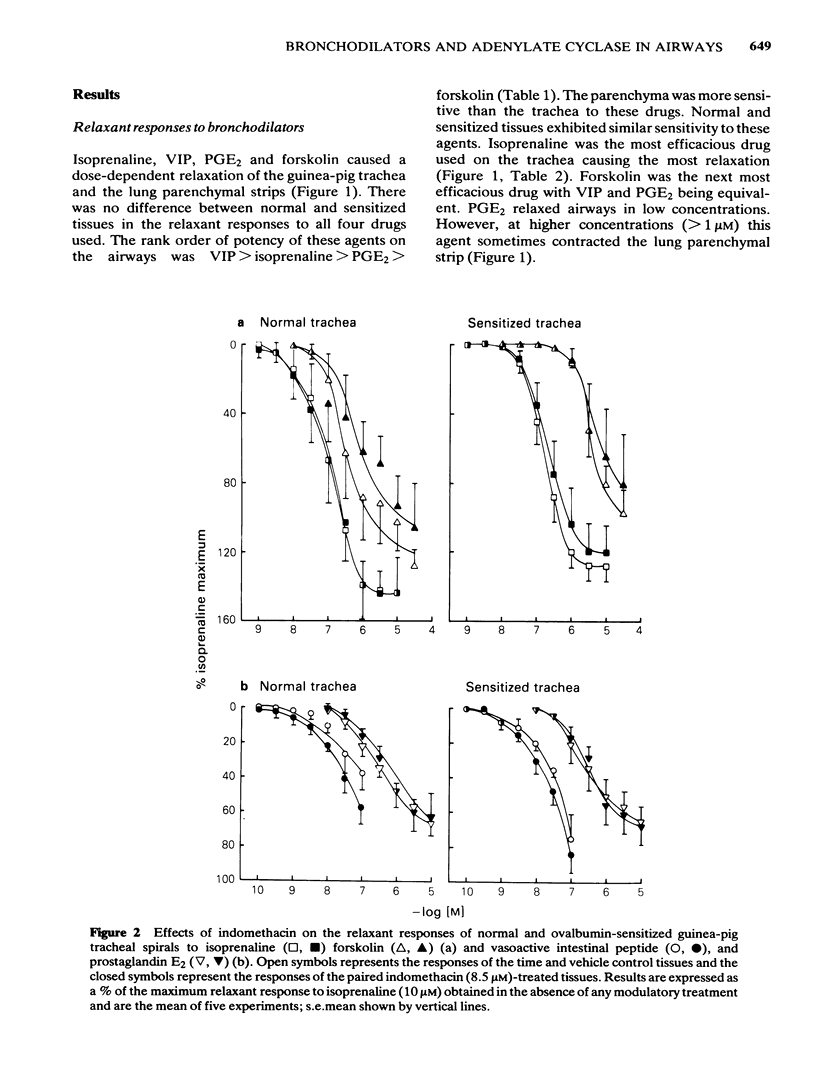
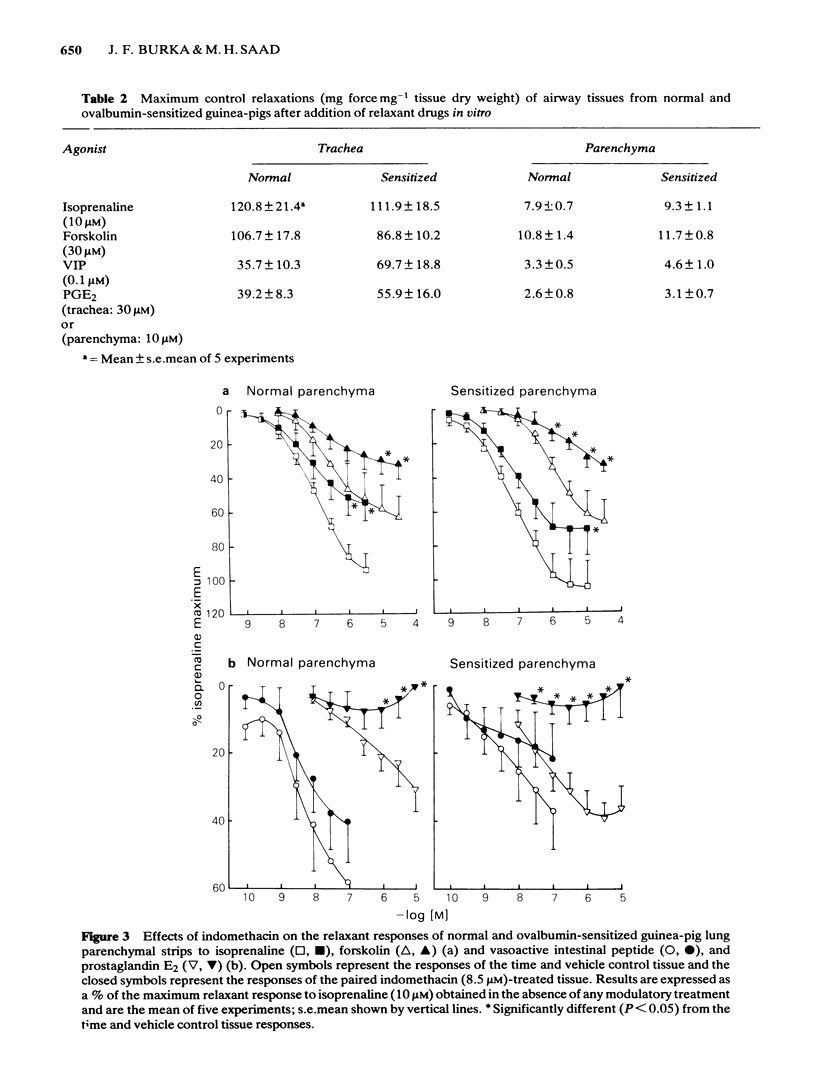
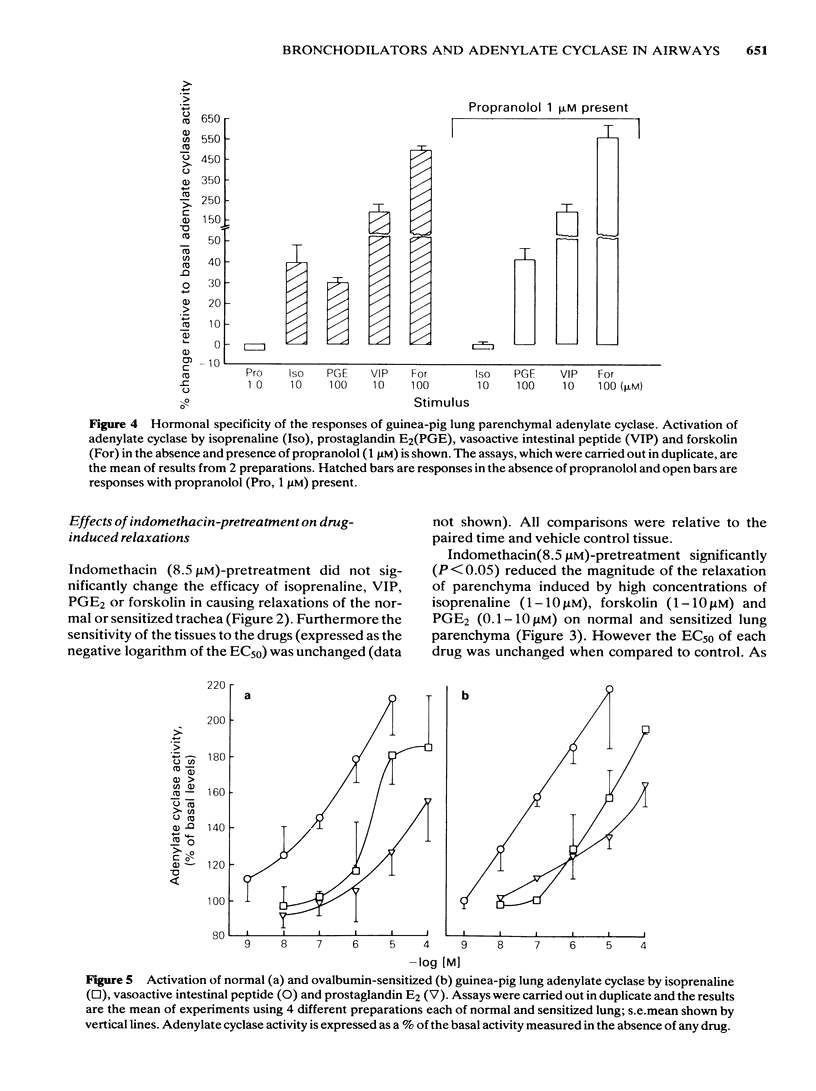
Isoprenaline, vasoactive intestinal peptide (VIP), prostaglandin E2 (PGE2) and forskolin caused a dose-dependent relaxation of normal and ovalbumin-sensitized guinea-pig tracheal spirals and lung parenchymal strips in vitro. There was no difference in magnitude of relaxation or sensitivity to these relaxants between normal and sensitized tissues. The rank order of potency (concentration of each drug at which 50% of the maximum is obtained) for these relaxants on both trachea and parenchyma was VIP greater than isoprenaline greater than PGE2 greater than forskolin, although the parenchyma was more sensitive than the trachea. The rank order of efficacy of the drugs used in relaxing both the trachea and lung parenchyma was isoprenaline (10 microM) greater than forskolin (30 microM) greater than VIP (0.1 microM) greater than PGE2 (10 microM). PGE2 at concentrations greater than 1 microM sometimes contracted the lung strip. Pretreatment with indomethacin (8.5 microM), a cyclo-oxygenase inhibitor, reduced the resting tone of tracheal spirals, but did not significantly affect the tone of lung strips. Indomethacin-pretreatment did not affect drug-induced relaxations of either normal or sensitized tracheal spirals. However, both normal and sensitized indomethacin-pretreated lung strips relaxed significantly less (P less than 0.05) to isoprenaline, PGE2 and forskolin. Indomethacin-pretreatment did not affect sensitivity of normal and sensitized trachea or parenchyma to the relaxant drugs. All the relaxant drugs used stimulated adenylate cyclase activity in normal or sensitized lung parenchyma membrane preparations. The rank order of efficacy (maximal activation) was forskolin greater than isoprenaline = VIP greater than PGE2. There was no difference in response between normal and sensitized lungs. Adenylate cyclase activity of normal lung was stimulated as follows: forskolin (100 microM), 500.0 +/- 50.0%; isoprenaline (100 microM), 186.0 +/- 29.0%; VIP (10 microM), 213.0 +/- 19.0% and PGE2 (100 microM), 155.0 +/- 23.0% of basal activity. Similar values were obtained for sensitized lung parenchyma. Indomethacin-pretreatment did not significantly affect normal or sensitized lung adenylate cyclase stimulation by isoprenaline, VIP, forskolin or PGE2. It was concluded that: Immunological sensitization to ovalbumin does not induce hypoactivity of relaxant drug receptors and/or the adenylate cyclase system of the airway tissues of the guinea-pig.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes P. J., Dollery C. T., MacDermot J. Increased pulmonary alpha-adrenergic and reduced beta-adrenergic receptors in experimental asthma. Nature. 1980 Jun 19;285(5766):569–571. doi: 10.1038/285569a0. [DOI] [PubMed] [Google Scholar]
- Boushey H. A., Holtzman M. J., Sheller J. R., Nadel J. A. Bronchial hyperreactivity. Am Rev Respir Dis. 1980 Feb;121(2):389–413. doi: 10.1164/arrd.1980.121.2.389. [DOI] [PubMed] [Google Scholar]
- Burka J. F., Paterson N. A. Evidence for lipoxygenase pathway involvement in allergic tracheal contraction. Prostaglandins. 1980 Apr;19(4):499–515. doi: 10.1016/s0090-6980(80)80001-3. [DOI] [PubMed] [Google Scholar]
- Burka J. F., Saad M. H. Mediators of arachidonic acid-induced contractions of indomethacin-treated guinea-pig airways: leukotrienes C4 and D4. Br J Pharmacol. 1984 Mar;81(3):465–473. doi: 10.1111/j.1476-5381.1984.tb10099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONSTANTINE J. W. THE SPIRALLY CUT TRACHEAL STRIP PREPARATION. J Pharm Pharmacol. 1965 Jun;17:384–385. doi: 10.1111/j.2042-7158.1965.tb07688.x. [DOI] [PubMed] [Google Scholar]
- Cheng J. B., Townley R. G. Effects of chronic histamine and ovalbumin aerosols on pulmonary beta adrenergic receptors in sensitized guinea pigs. Res Commun Chem Pathol Pharmacol. 1982 Jun;36(3):507–510. [PubMed] [Google Scholar]
- Conolly M. E., Greenacre J. K. The lymphocyte beta-adrenoceptor in normal subjects and patients with bronchial asthma: the effect of different forms of treatment on receptor function. J Clin Invest. 1976 Dec;58(6):1307–1316. doi: 10.1172/JCI108586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundström N., Andersson R. G., Wikberg J. E. Pharmacological characterization of the autonomous innervation of the guinea pig tracheobronchial smooth muscle. Acta Pharmacol Toxicol (Copenh) 1981 Aug;49(2):150–157. doi: 10.1111/j.1600-0773.1981.tb00884.x. [DOI] [PubMed] [Google Scholar]
- Hanna C. J., Bach M. K., Pare P. D., Schellenberg R. R. Slow-reacting substances (leukotrienes) contract human airway and pulmonary vascular smooth muscle in vitro. Nature. 1981 Mar 26;290(5804):343–344. doi: 10.1038/290343a0. [DOI] [PubMed] [Google Scholar]
- Hargreave F. E., Ryan G., Thomson N. C., O'Byrne P. M., Latimer K., Juniper E. F., Dolovich J. Bronchial responsiveness to histamine or methacholine in asthma: measurement and clinical significance. J Allergy Clin Immunol. 1981 Nov;68(5):347–355. doi: 10.1016/0091-6749(81)90132-9. [DOI] [PubMed] [Google Scholar]
- Hyman A. L., Spannhake E. W., Kadowitz P. J. Prostaglandins and the lung. Am Rev Respir Dis. 1978 Jan;117(1):111–136. doi: 10.1164/arrd.1978.117.1.111. [DOI] [PubMed] [Google Scholar]
- Kaliner M., Shelhamer J. H., Davis P. B., Smith L. J., Venter J. C. Autonomic nervous system abnormalities and allergy. Ann Intern Med. 1982 Mar;96(3):349–357. doi: 10.7326/0003-4819-96-3-349. [DOI] [PubMed] [Google Scholar]
- Kitamura S., Ishihara Y., Said S. I. Effect of VIP, phenoxybenzamine and prednisolone on cyclic nucleotide content of isolated guinea-pig lung and trachea. Eur J Pharmacol. 1980 Oct 17;67(2-3):219–223. doi: 10.1016/0014-2999(80)90501-4. [DOI] [PubMed] [Google Scholar]
- Kukovetz W. R., Holzmann S., Wurm A., Pöch G. Evidence for cyclic GMP-mediated relaxant effects of nitro-compounds in coronary smooth muscle. Naunyn Schmiedebergs Arch Pharmacol. 1979 Dec;310(2):129–138. doi: 10.1007/BF00500277. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R. J., Michel T. Plasma membrane receptors. J Clin Invest. 1983 Oct;72(4):1185–1189. doi: 10.1172/JCI111073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewicki J. A., Brandwein H. J., Mittal C. K., Arnold W. P., Murad F. Properties of purified soluble guanylate cyclase activated by nitric oxide and sodium nitroprusside. J Cyclic Nucleotide Res. 1982;8(1):17–25. [PubMed] [Google Scholar]
- Lulich K. M., Papadimitriou J. M., Paterson J. W. The isolated lung strip and single open tracheal ring: a convenient combination for characterizing Schultz Dale anaphylactic contractions in the peripheral and central airways of the guinea-pig. Clin Exp Pharmacol Physiol. 1979 Nov-Dec;6(6):625–629. doi: 10.1111/j.1440-1681.1979.tb00046.x. [DOI] [PubMed] [Google Scholar]
- MacDermot J., Barnes P. J. Activation of guinea pig pulmonary adenylate cyclase by prostacyclin. Eur J Pharmacol. 1980 Oct 31;67(4):419–425. doi: 10.1016/0014-2999(80)90183-1. [DOI] [PubMed] [Google Scholar]
- Meurs H., Koëter G. H., de Vries K., Kauffman H. F. The beta-adrenergic system and allergic bronchial asthma: changes in lymphocyte beta-adrenergic receptor number and adenylate cyclase activity after an allergen-induced asthmatic attack. J Allergy Clin Immunol. 1982 Oct;70(4):272–280. doi: 10.1016/0091-6749(82)90063-x. [DOI] [PubMed] [Google Scholar]
- Muller M. J., Baer H. P. Relaxant effects of forskolin in smooth muscle. Role of cyclic AMP. Naunyn Schmiedebergs Arch Pharmacol. 1983 Feb;322(1):78–82. doi: 10.1007/BF00649356. [DOI] [PubMed] [Google Scholar]
- Orehek J. Asthma without airway hyperreactivity: fact or artifact? Eur J Respir Dis. 1982 Jan;63(1):1–4. [PubMed] [Google Scholar]
- Piper P. J., Tippins J. R., Morris H. R., Taylor G. W. Arachidonic acid metabolism and SRS-A. Agents Actions Suppl. 1979;(4):37–48. [PubMed] [Google Scholar]
- Richardson J. B. Noradrenergic inhibitory innervation of the lung. Lung. 1981;159(6):315–322. doi: 10.1007/BF02713931. [DOI] [PubMed] [Google Scholar]
- Rinard G. A., Rubinfeld A. R., Brunton L. L., Mayer S. E. Depressed cyclic AMP levels in airways smooth muscle from asthmatic dogs. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1472–1476. doi: 10.1073/pnas.76.3.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robberecht P., Chatelain P., De Neef P., Camus J. C., Waelbroeck M., Christophe J. Presence of vasoactive intestinal peptide receptors coupled to adenylate cyclase in rat lung membranes. Biochim Biophys Acta. 1981 Nov 18;678(1):76–82. doi: 10.1016/0304-4165(81)90049-0. [DOI] [PubMed] [Google Scholar]
- Saad M. H., Burka J. F. Effects of immunological sensitization on the responses and sensitivity of guinea pig airways to bronchoconstrictors. Modulation by selective inhibition of arachidonic acid metabolism. Can J Physiol Pharmacol. 1983 Aug;61(8):876–887. doi: 10.1139/y83-133. [DOI] [PubMed] [Google Scholar]
- Said S. I. Vasoactive peptides in the lung, with special reference to vasoactive intestinal peptide. Exp Lung Res. 1982 Nov;3(3-4):343–348. doi: 10.3109/01902148209069662. [DOI] [PubMed] [Google Scholar]
- Sano Y., Watt G., Townley R. G. Decreased mononuclear cell beta-adrenergic receptors in bronchial asthma: parallel studies of lymphocyte and granulocyte desensitization. J Allergy Clin Immunol. 1983 Nov;72(5 Pt 1):495–503. doi: 10.1016/0091-6749(83)90587-0. [DOI] [PubMed] [Google Scholar]
- Scheid C. R., Honeyman T. W., Fay F. S. Mechanism of beta-adrenergic relaxation of smooth muscle. Nature. 1979 Jan 4;277(5691):32–36. doi: 10.1038/277032a0. [DOI] [PubMed] [Google Scholar]
- Schmidt K., Munshi R., Baer H. P. Characterization of forskolin binding sites in rat brain membranes using [14,15-3H]14,15-dihydroforskolin as a ligand. Naunyn Schmiedebergs Arch Pharmacol. 1984 Feb;325(2):153–158. doi: 10.1007/BF00506195. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. Forskolin: a unique diterpene activator of cyclic AMP-generating systems. J Cyclic Nucleotide Res. 1981;7(4):201–224. [PubMed] [Google Scholar]
- Tipton W. R., Nelson H. S., Souhrada J. F., Morris H. G., Jacobson K. W. Dynamics of isoproterenol subsensitivity in guinea pig airway smooth muscle. Comparison of tissue cAMP levels and airway smooth muscle relaxation. Lung. 1981;159(4):199–210. doi: 10.1007/BF02713916. [DOI] [PubMed] [Google Scholar]
- Vane J. R. Inhibition of prostaglandin synthesis as a mechanism of action for aspirin-like drugs. Nat New Biol. 1971 Jun 23;231(25):232–235. doi: 10.1038/newbio231232a0. [DOI] [PubMed] [Google Scholar]
- Walters E. H., Bevan M., Davies B. H. The effects of oral propranolol and indomethacin on the response to inhaled PGE2 in normal human subjects. Prostaglandins Leukot Med. 1982 Feb;8(2):141–150. doi: 10.1016/s0262-1746(82)80006-1. [DOI] [PubMed] [Google Scholar]
- Yamaguchi T., Hitzig B., Coburn R. F. Endogenous prostaglandins and mechanical tension in canine trachealis muscle. Am J Physiol. 1976 Jun;230(6):1737–1743. doi: 10.1152/ajplegacy.1976.230.6.1737. [DOI] [PubMed] [Google Scholar]


