Abstract
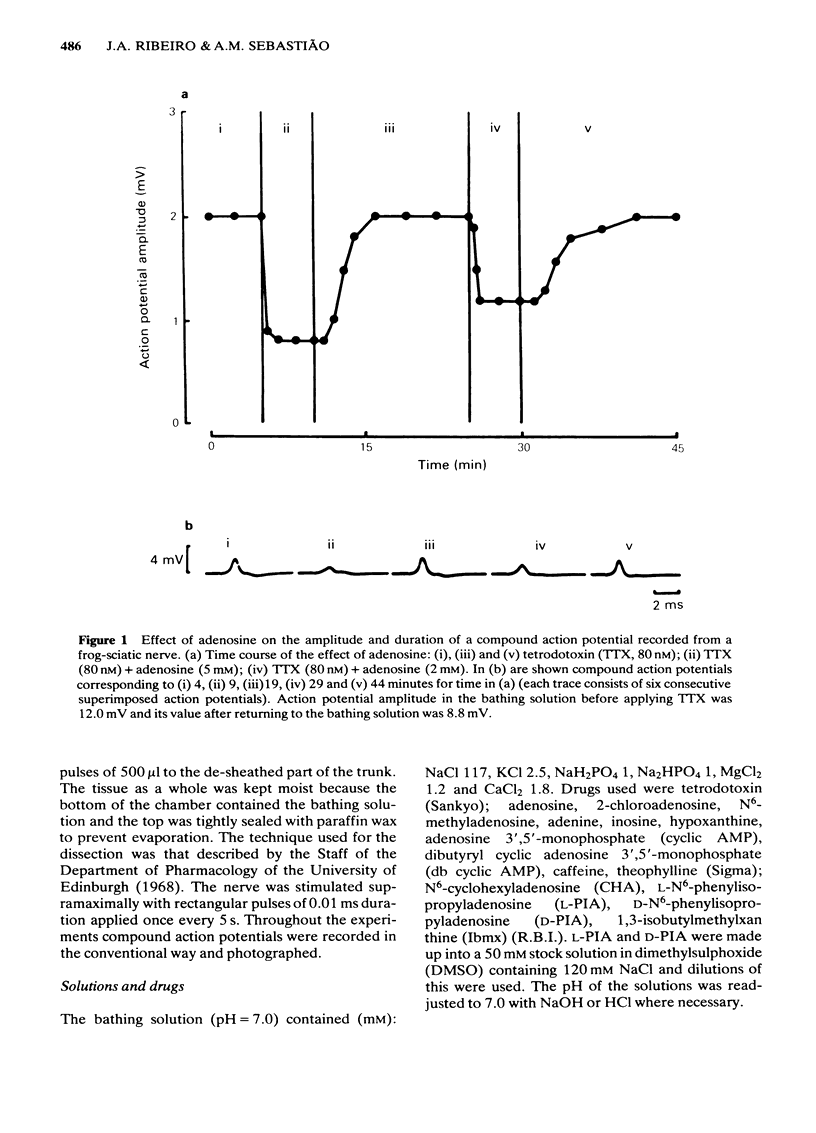
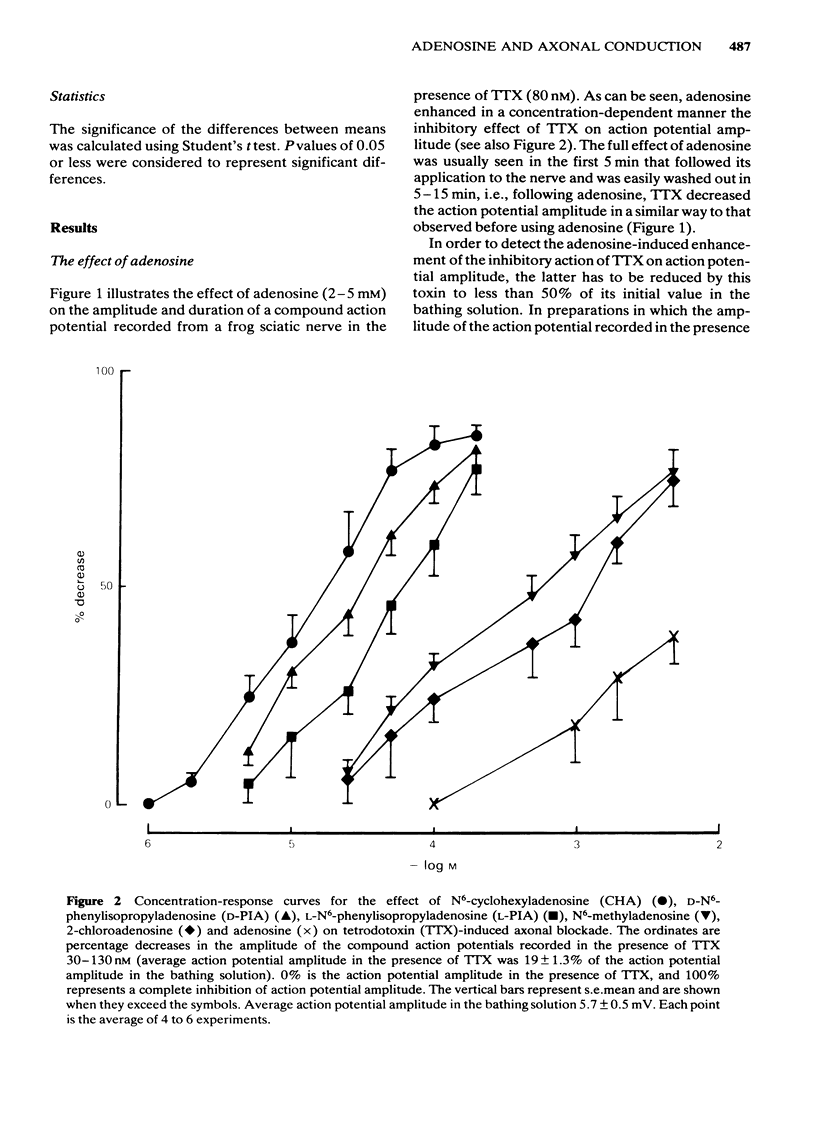
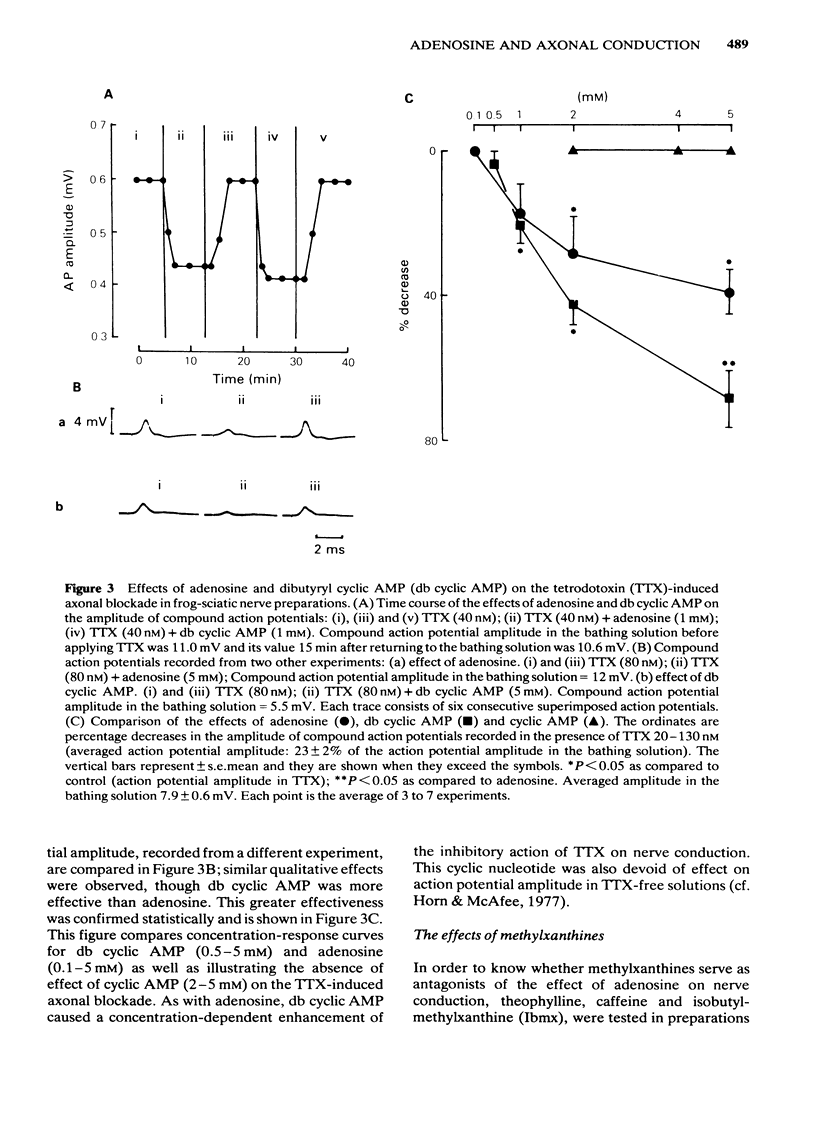
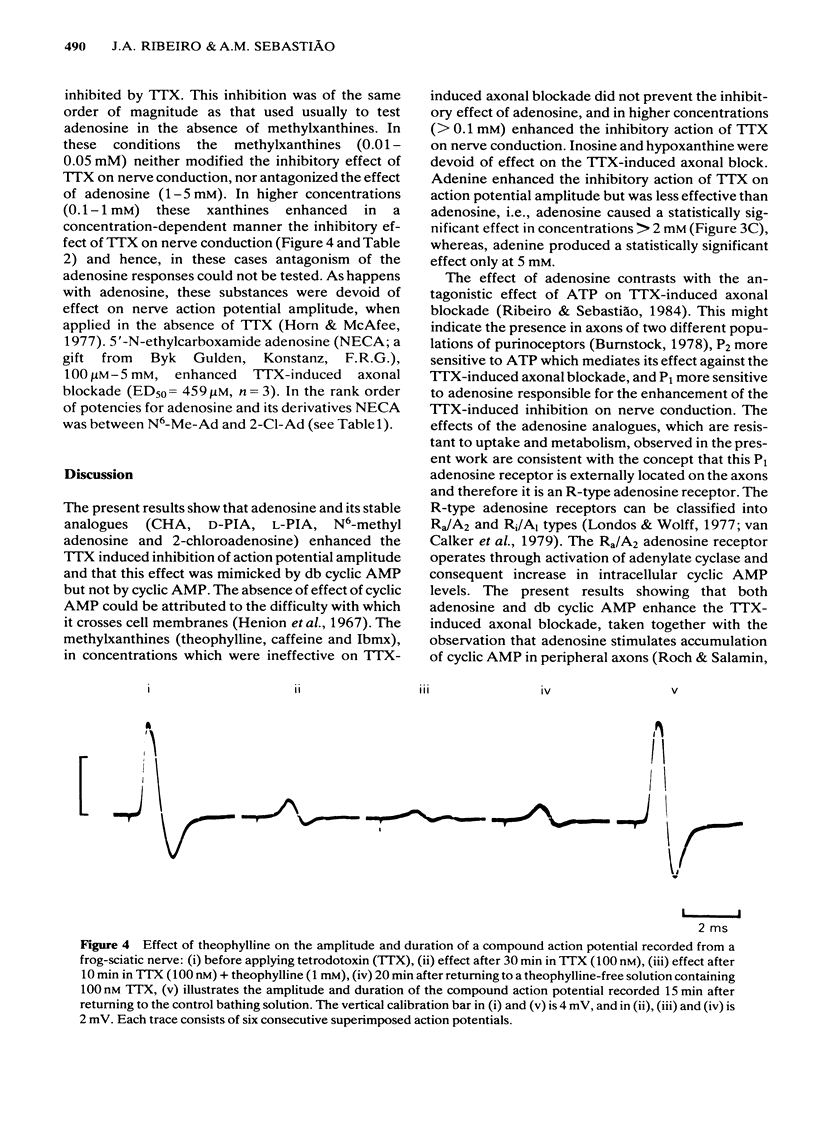
The effects of adenosine, adenosine analogues (N6-cyclohexyladenosine (CHA), L-N6-phenylisopropyladenosine (L-PIA), D-N6-phenylisopropyladenosine (D-PIA), N6-methyladenosine and 2-chloroadenosine), adenine, inosine, hypoxanthine, cyclic AMP and its analogue the dibutyryl cyclic AMP (db cyclic AMP), and methylxanthines (theophylline, caffeine and isobutylmethylxanthine (Ibmx) on compound action potentials were investigated in de-sheathed sciatic nerve preparations of the frog. Adenosine and its analogues enhanced, in a concentration-dependent manner, the inhibitory action of tetrodotoxin (TTX) on nerve conduction. The order of potencies was: CHA greater than D-PIA greater than L-PIA greater than N6-methyladenosine greater than 2-chloroadenosine greater than adenosine. The adenosine metabolites, inosine and hypoxanthine, were inactive on TTX-induced axonal blockade. Adenine enhanced the inhibitory action of TTX on nerve conduction, but was less effective than adenosine. db Cyclic AMP, but not cyclic AMP, mimicked the inhibitory effect of adenosine on nerve conduction. Methylxanthines did not antagonize the effect of adenosine on TTX-induced axonal block and in high concentrations also mimicked the effect of adenosine on nerve conduction. The possibility of adenosine acting on TTX-induced axonal block through an adenosine receptor positively coupled to adenylate cyclase is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Collis M. G. Evidence for an A1-adenosine receptor in the guinea-pig atrium. Br J Pharmacol. 1983 Jan;78(1):207–212. doi: 10.1111/j.1476-5381.1983.tb09381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa M. R., Casnellie J. E., Catterall W. A. Selective phosphorylation of the alpha subunit of the sodium channel by cAMP-dependent protein kinase. J Biol Chem. 1982 Jul 25;257(14):7918–7921. [PubMed] [Google Scholar]
- Henion W. F., Sutherland E. W., Posternak T. Effects of derivatives of adenosine 3',5'-phosphate on liver slices and intact animals. Biochim Biophys Acta. 1967 Oct 9;148(1):106–113. doi: 10.1016/0304-4165(67)90284-x. [DOI] [PubMed] [Google Scholar]
- Horn J. P., McAfee D. A. Modulation of cyclic nucleotide levels in peripheral nerve without effect on resting or compound action potentials. J Physiol. 1977 Aug;269(3):753–766. doi: 10.1113/jphysiol.1977.sp011927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londos C., Wolff J. Two distinct adenosine-sensitive sites on adenylate cyclase. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5482–5486. doi: 10.1073/pnas.74.12.5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maire J. C., Medilanski J., Straub R. W. Uptake of adenosine and release of adenine derivatives in mammalian non-myelinated nerve fibres at rest and during activity. J Physiol. 1982 Feb;323:589–602. doi: 10.1113/jphysiol.1982.sp014093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- OKAMOTO M., ASKARI A., KUPERMAN A. S. THE STABILIZING ACTIONS OF ADENOSINE TRIPHOSPHATE AND RELATED NUCLEOTIDES ON CALCIUM-DEFICIENT NERVE. J Pharmacol Exp Ther. 1964 May;144:229–235. [PubMed] [Google Scholar]
- Ribeiro J. A., Dominguez M. L. Mechanisms of depression of neuromuscular transmission by ATP and adenosine. J Physiol (Paris) 1978;74(5):491–496. [PubMed] [Google Scholar]
- Ribeiro J. A., Sebastião A. M. Antagonism of tetrodotoxin- and procaine-induced axonal blockade by adenine nucleotides in the frog sciatic nerve. Br J Pharmacol. 1984 Feb;81(2):277–282. doi: 10.1111/j.1476-5381.1984.tb10075.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roch P., Salamin A. Adenosine promoted accumulation of adenosine 3',5'-monophosphate in rabbit vagus nerve. Experientia. 1976 Nov 15;32(11):1419–1421. doi: 10.1007/BF01937411. [DOI] [PubMed] [Google Scholar]
- Silinsky E. M. On the mechanism by which adenosine receptor activation inhibits the release of acetylcholine from motor nerve endings. J Physiol. 1984 Jan;346:243–256. doi: 10.1113/jphysiol.1984.sp015019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smellie F. W., Daly J. W., Dunwiddie T. V., Hoffer B. J. The dextro and levorotatory isomers of N-phenylisopropyladenosine: stereospecific effects on cyclic AMP-formation and evoked synaptic responses in brain slices. Life Sci. 1979 Nov 12;25(20):1739–1748. doi: 10.1016/0024-3205(79)90477-6. [DOI] [PubMed] [Google Scholar]
- Wu P. H., Phillis J. W., Nye M. J. Alkylxanthines as adenosine receptor antagonists and membrane phosphodiesterase inhibitors in central nervous tissue: evaluation of structure-activity relationships. Life Sci. 1982 Dec 20;31(25):2857–2867. doi: 10.1016/0024-3205(82)90676-2. [DOI] [PubMed] [Google Scholar]
- van Calker D., Müller M., Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979 Nov;33(5):999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]


