Abstract
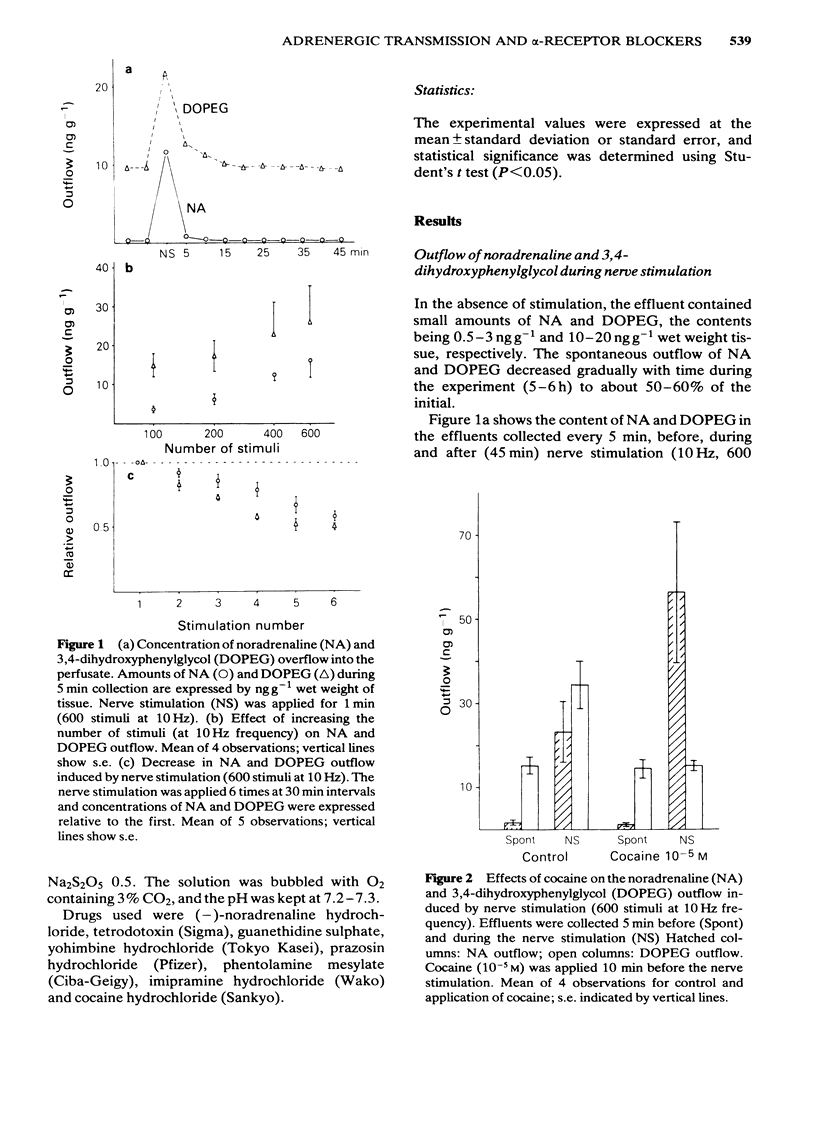
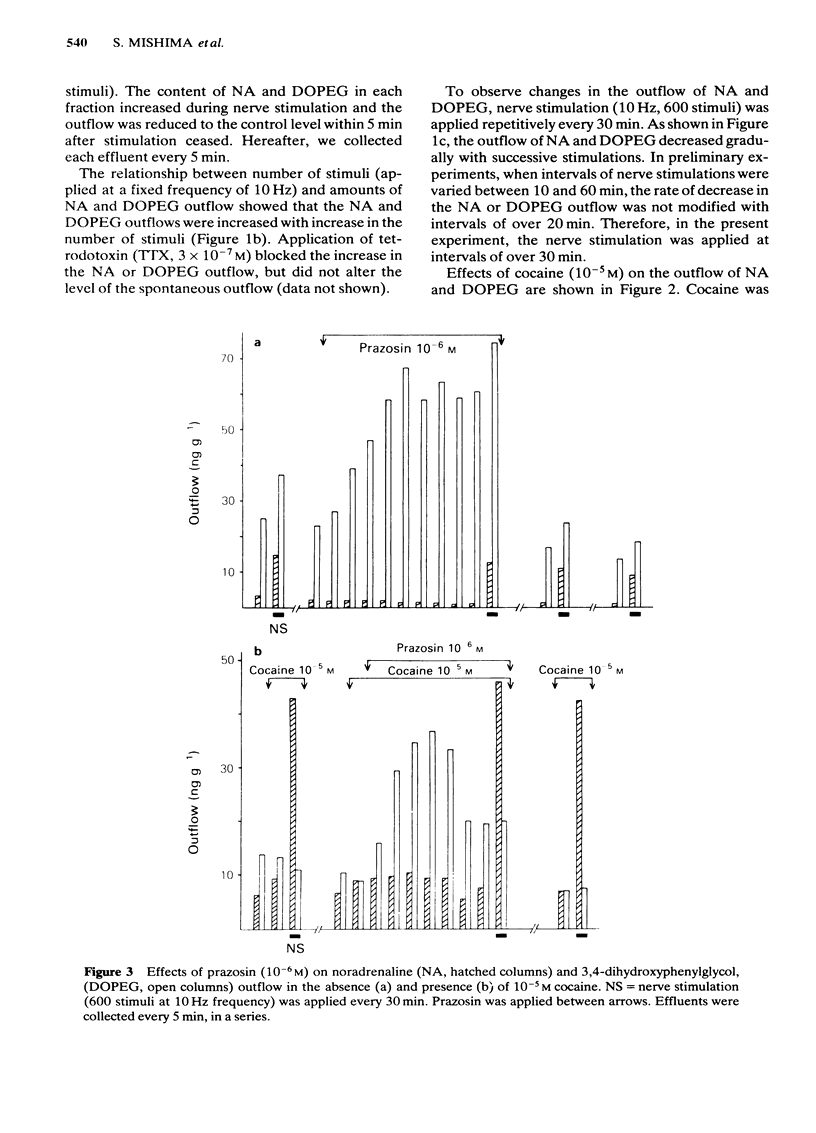
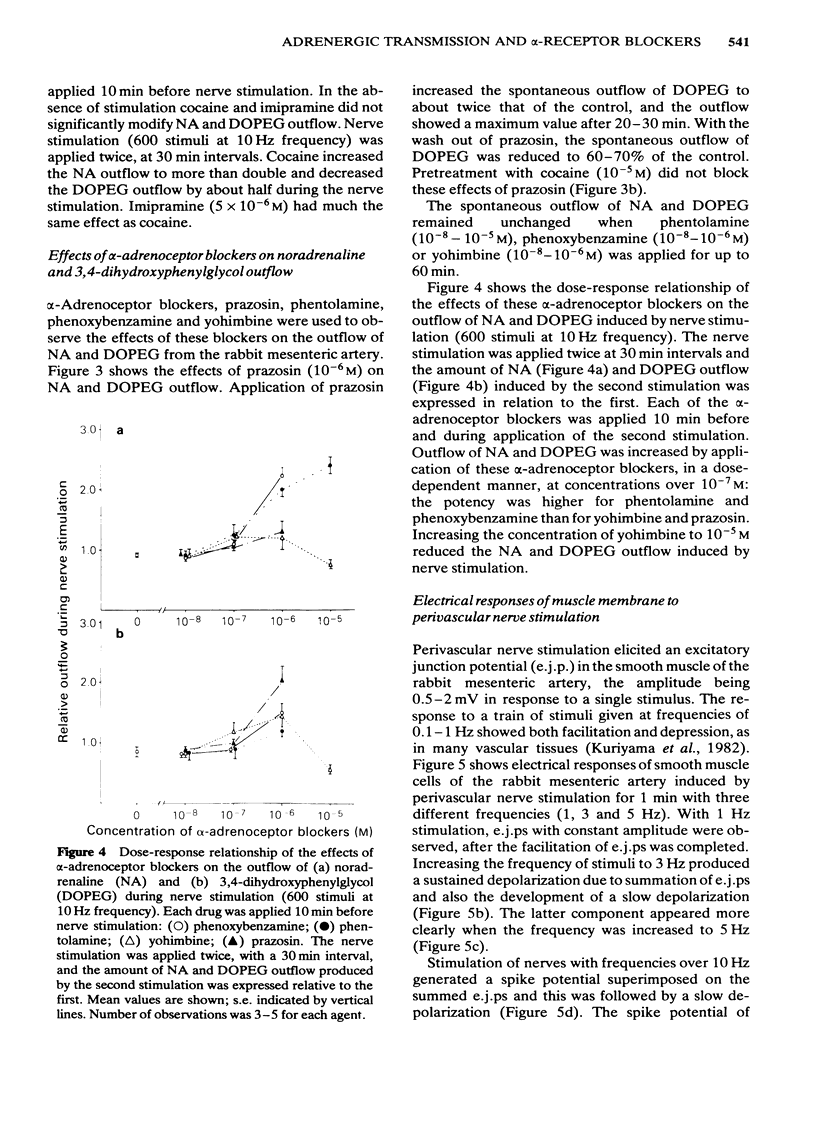
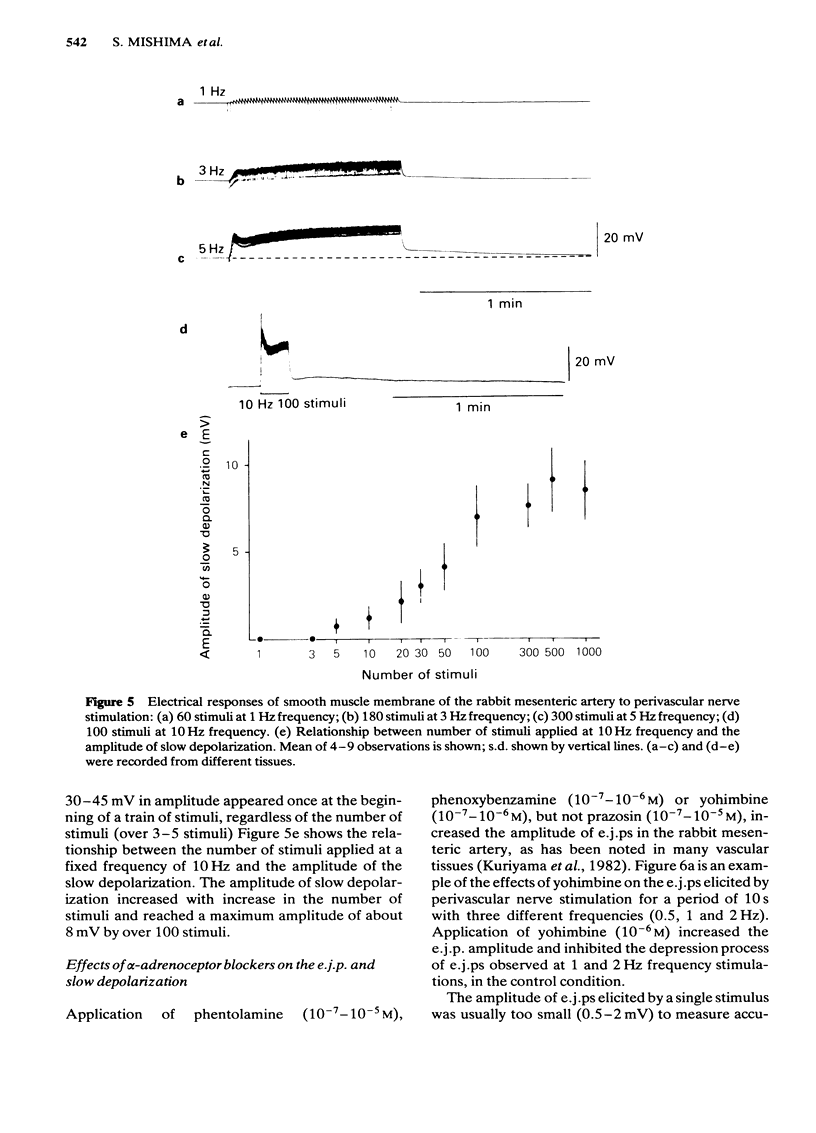
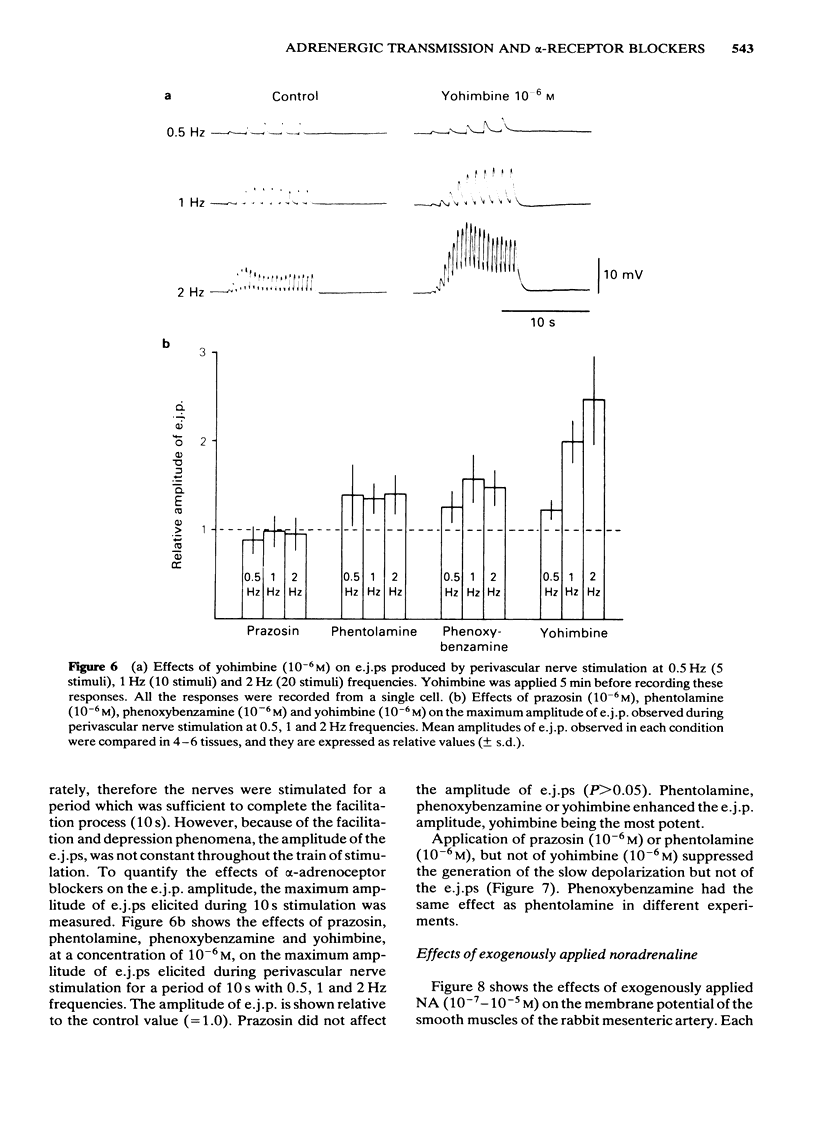
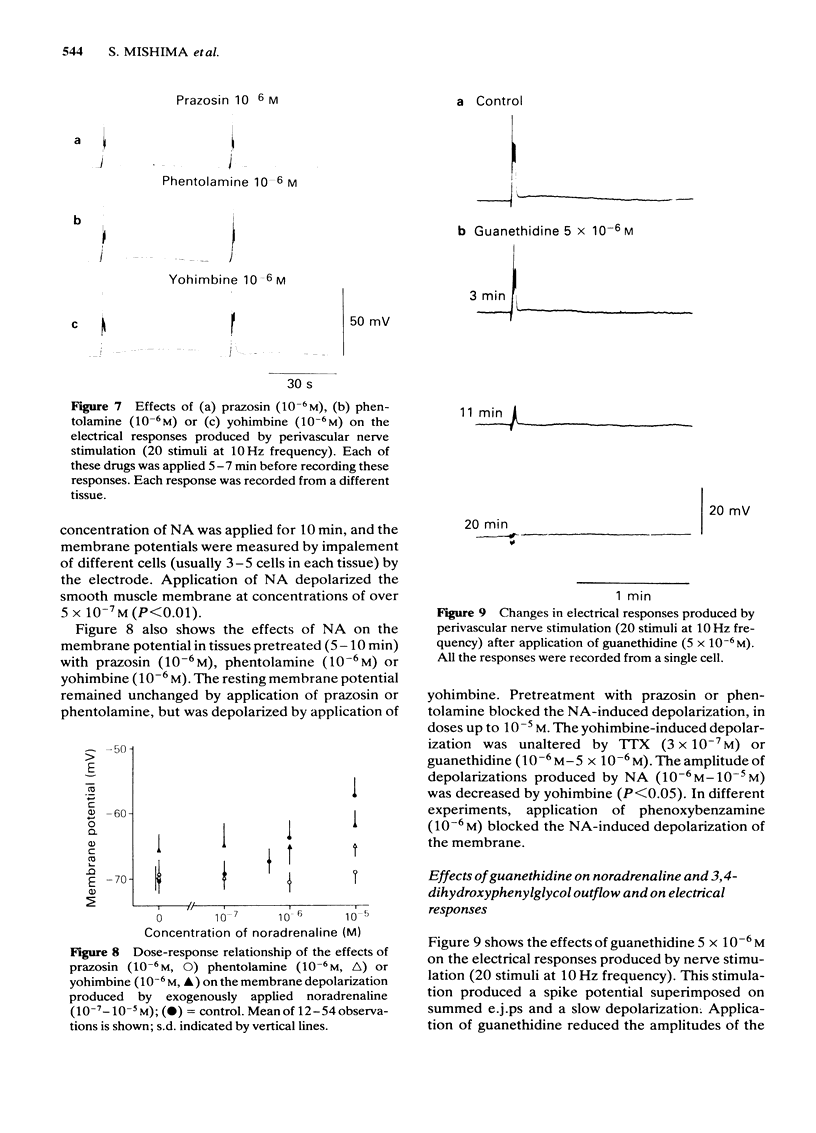
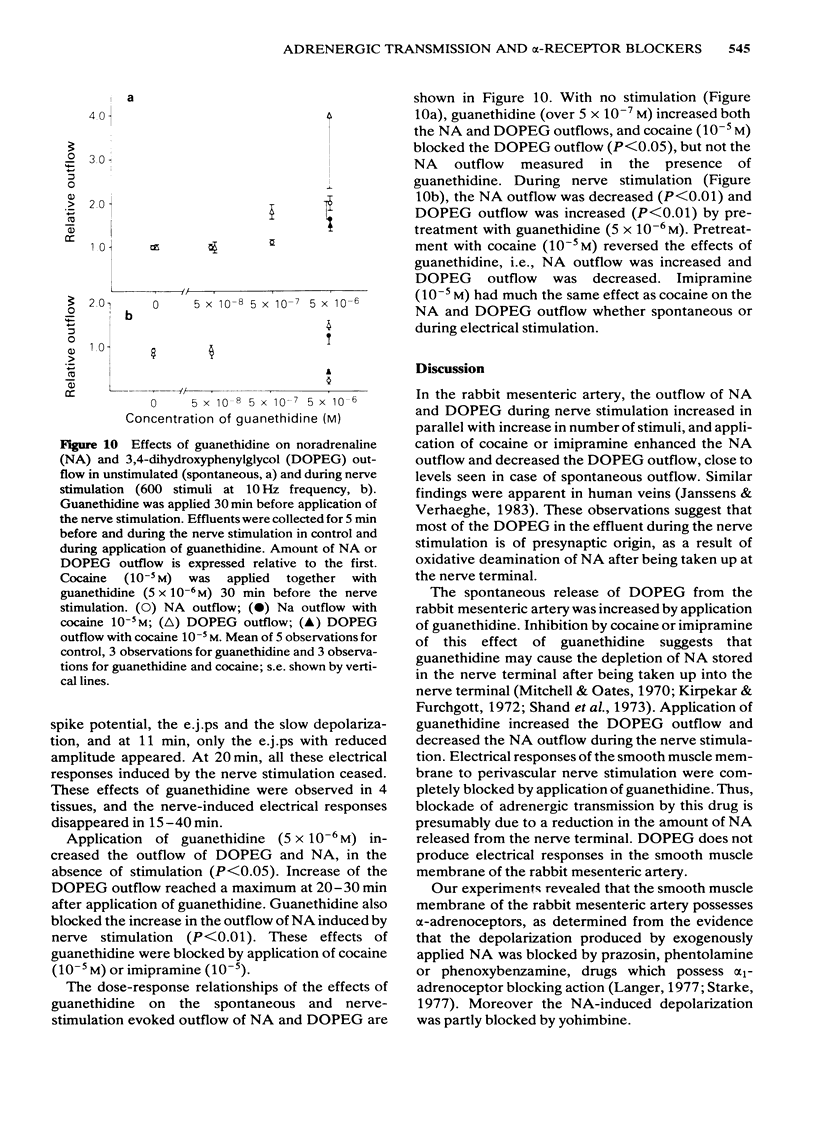
Effects of alpha-adrenoceptor blockers (prazosin, yohimbine, phentolamine and phenoxybenzamine) on the outflow of noradrenaline (NA) and 3,4-dihydroxyphenylglycol (DOPEG) during perivascular nerve stimulation were observed in relation to electrical events in the rabbit mesenteric artery. Cocaine or imipramine increased the NA outflow and reduced the DOPEG outflow induced by nerve stimulation. In the absence of stimulation, cocaine and imipramine did not significantly modify the NA and DOPEG outflows. The alpha-adrenoceptor blockers we used enhanced the NA and DOPEG outflow during nerve stimulation, in a dose-dependent manner; the potency of the enhancement was higher for phentolamine and phenoxybenzamine than for prazosin and yohimbine. Higher concentrations (10(-5) M) of yohimbine reduced the NA and DOPEG outflows induced by nerve stimulation. Prazosin increased the DOPEG outflow in the absence of stimulation, and this effect was not inhibited by pretreatment with cocaine. Guanethidine increased the NA and DOPEG outflow in the absence of stimulation, and the NA outflow was reduced during nerve stimulation. These effects of guanethidine were prevented by pretreatment with cocaine or imipramine. Perivascular nerve stimulation evoked excitatory junction potentials (e.j.ps) and with high frequency stimulation, slow depolarization and spike potentials. Application of phentolamine, phenoxybenzamine or yohimbine enhanced, and of prazosin had no effect, on the amplitude of the e.j.p. Spike potentials were not affected by these alpha-adrenoceptor blockers. Slow depolarization ceased in the presence of prazosin, phentolamine or phenoxybenzamine, and was slightly inhibited by yohimbine. Guanethidine blocked all of these electrical responses induced by perivascular nerve stimulation. Application of prazosin, phentolamine or phenoxybenzamine did not alter the resting membrane potential of the smooth muscle cells. Depolarizations of smooth muscle membrane produced by exogenously applied NA were inhibited by prazosin, phentolamine or phenoxybenzamine. Yohimbine itself depolarized the membrane and the inhibitory effects on the NA-induced depolarization were weaker. We conclude that the smooth muscle membrane of the rabbit mesenteric artery possesses alpha 1-adrenoceptors. Increase in NA outflow by alpha-adrenoceptor antagonists during nerve stimulation was not always consistent with increase in e.j.p. amplitude, presumably due to involvement of actions other than alpha-adrenoceptor blockade with each of these antagonists.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angus J. A., Bobik A., Jackman G. P., Kopin I. J., Korner P. I. Role of auto-inhibitory feed-back in cardiac sympathetic transmission assessed by simultaneous measurements of changes in 3H-efflux and atrial rate in guinea-pig atrium. Br J Pharmacol. 1984 Jan;81(1):201–214. doi: 10.1111/j.1476-5381.1984.tb10762.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angus J. A., Korner P. I. Evidence against presynaptic alpha-adrenoreceptor modulation of cardiac sympathetic transmission. Nature. 1980 Jul 17;286(5770):288–291. doi: 10.1038/286288a0. [DOI] [PubMed] [Google Scholar]
- Cheung D. W. Two components in the cellular response of rat tail arteries to nerve stimulation. J Physiol. 1982 Jul;328:461–468. doi: 10.1113/jphysiol.1982.sp014277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara S., Itoh T., Suzuki H. Membrane properties and excitatory neuromuscular transmission in the smooth muscle of dog cerebral arteries. Br J Pharmacol. 1982 Oct;77(2):197–208. doi: 10.1111/j.1476-5381.1982.tb09286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graefe K. H., Stefano F. J., Langer S. Z. Stereoselectivity in the metabolism of 3H-noradrenaline during uptake into and efflux from the isolated rat vas deferens. Naunyn Schmiedebergs Arch Pharmacol. 1977 Oct;299(3):225–238. doi: 10.1007/BF00500315. [DOI] [PubMed] [Google Scholar]
- Henseling M., Graefe K. H., Trendelenburg U. The rate constants for the efflux of the metabolites of noradrenaline from rabbit aortic strips. Naunyn Schmiedebergs Arch Pharmacol. 1978 Apr;302(2):207–215. doi: 10.1007/BF00517987. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. Evidence for two populations of excitatory receptors for noradrenaline on arteriolar smooth muscle. Nature. 1980 Feb 21;283(5749):767–768. doi: 10.1038/283767a0. [DOI] [PubMed] [Google Scholar]
- Hirst G. D., Neild T. O. Localization of specialized noradrenaline receptors at neuromuscular junctions on arterioles of the guinea-pig. J Physiol. 1981;313:343–350. doi: 10.1113/jphysiol.1981.sp013669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holman M. E., Surprenant A. An electrophysiological analysis of the effects of noradrenaline and alpha-receptor antagonists on neuromuscular transmission in mammalian muscular arteries. Br J Pharmacol. 1980;71(2):651–661. doi: 10.1111/j.1476-5381.1980.tb10986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssens W., Verhaeghe R. Modulation of the concentration of noradrenaline at the neuro-effector junction in human saphenous vein. Br J Pharmacol. 1983 Jun;79(2):577–585. doi: 10.1111/j.1476-5381.1983.tb11032.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpekar S. M., Furchgott R. F. Interaction of tyramine and guanethidine in the spleen of the cat. J Pharmacol Exp Ther. 1972 Jan;180(1):38–46. [PubMed] [Google Scholar]
- Kou K., Kuriyama H., Suzuki H. Effects of 3,4-dihydro-8-(2-hydroxy-3-isopropylaminopropoxy)-3-nitroxy-2H-1-benzopyran (K-351) on smooth muscle cells and neuromuscular transmission in the canine mesenteric artery. Br J Pharmacol. 1982 Dec;77(4):679–689. doi: 10.1111/j.1476-5381.1982.tb09346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuriyama H., Ito Y., Suzuki H., Kitamura K., Itoh T. Factors modifying contraction-relaxation cycle in vascular smooth muscles. Am J Physiol. 1982 Nov;243(5):H641–H662. doi: 10.1152/ajpheart.1982.243.5.H641. [DOI] [PubMed] [Google Scholar]
- Kuriyama H., Makita Y. Modulation of noradrenergic transmission in the guinea-pig mesenteric artery: an electrophysiological study. J Physiol. 1983 Feb;335:609–627. doi: 10.1113/jphysiol.1983.sp014554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer S. Z. Sixth gaddum memorial lecture, National Institute for Medical Research, Mill Hill, January 1977. Presynaptic receptors and their role in the regulation of transmitter release. Br J Pharmacol. 1977 Aug;60(4):481–497. doi: 10.1111/j.1476-5381.1977.tb07526.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin J. A. The uptake and metabolism of 3H-l-and 3H-dl-norepinephrine by intact rabbit aorta and by isolated adventitia and media. J Pharmacol Exp Ther. 1974 Aug;190(2):210–226. [PubMed] [Google Scholar]
- Mitchell J. R., Oates J. A. Guanethidine and related agents. I. Mechanism of the selective blockade of adrenergic neurons and its antagonism by drugs. J Pharmacol Exp Ther. 1970 Mar;172(1):100–107. [PubMed] [Google Scholar]
- Oishi R., Mishima S., Kuriyama H. Determination of norepinephrine and its metabolites released from rat vas deferens using high-performance liquid chromatography with electrochemical detection. Life Sci. 1983 Feb 28;32(9):933–940. doi: 10.1016/0024-3205(83)90922-0. [DOI] [PubMed] [Google Scholar]
- Shand D. G., Morgan D. H., Oates J. A. The release of guanethidine and bethanidine by splenic nerve stimulation: a quantitative evaluation showing dissociation from adrenergic blockade. J Pharmacol Exp Ther. 1973 Jan;184(1):73–80. [PubMed] [Google Scholar]
- Starke K., Borowski E., Endo T. Preferential blockade of presynaptic alpha-adrenoceptors by yohimbine. Eur J Pharmacol. 1975 Dec;34(2):385–388. doi: 10.1016/0014-2999(75)90268-x. [DOI] [PubMed] [Google Scholar]
- Starke K. Regulation of noradrenaline release by presynaptic receptor systems. Rev Physiol Biochem Pharmacol. 1977;77:1–124. doi: 10.1007/BFb0050157. [DOI] [PubMed] [Google Scholar]
- Story D. F., McCulloch M. W., Rand M. J., Standford-Starr C. A. Conditions required for the inhibitory feedback loop in noradrenergic transmission. Nature. 1981 Sep 3;293(5827):62–65. doi: 10.1038/293062a0. [DOI] [PubMed] [Google Scholar]
- Suzuki H. Adrenergic transmission in the dog mesenteric vein and its modulation by alpha-adrenoceptor antagonists. Br J Pharmacol. 1984 Mar;81(3):479–489. doi: 10.1111/j.1476-5381.1984.tb10101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki H., Fujiwara S. Neurogenic electrical responses of single smooth muscle cells of the dog middle cerebral artery. Circ Res. 1982 Dec;51(6):751–759. doi: 10.1161/01.res.51.6.751. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Kou K. Electrical components contributing to the nerve-mediated contractions in the smooth muscles of the rabbit ear artery. Jpn J Physiol. 1983;33(5):743–756. doi: 10.2170/jjphysiol.33.743. [DOI] [PubMed] [Google Scholar]
- Vanhoutte P. M., Verbeuren T. J., Webb R. C. Local modulation of adrenergic neuroeffector interaction in the blood vessel well. Physiol Rev. 1981 Jan;61(1):151–247. doi: 10.1152/physrev.1981.61.1.151. [DOI] [PubMed] [Google Scholar]



