Abstract
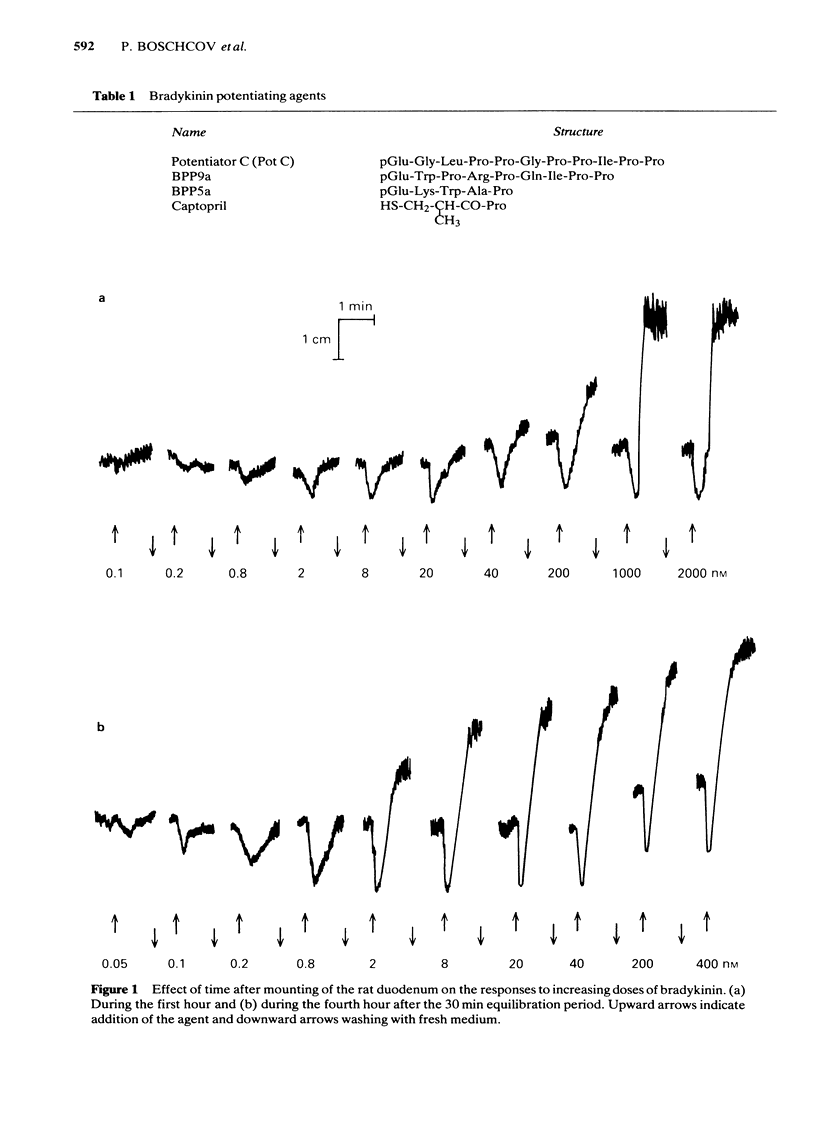
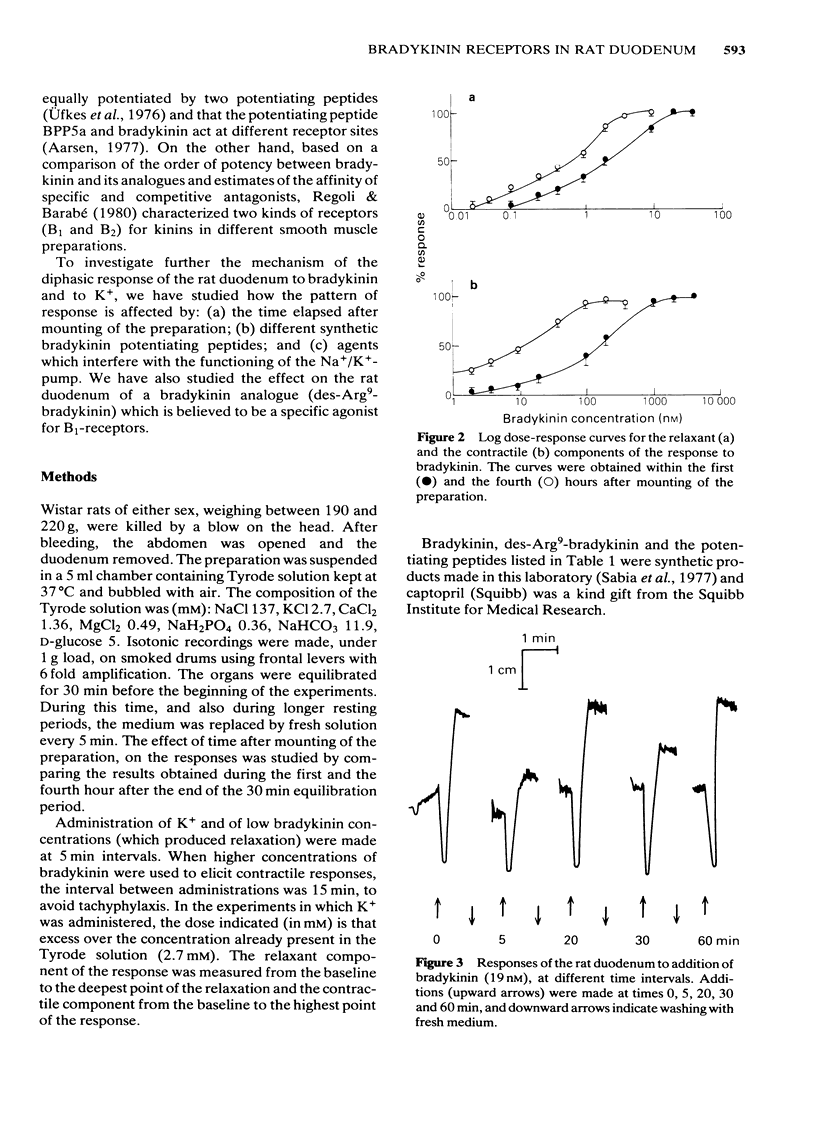
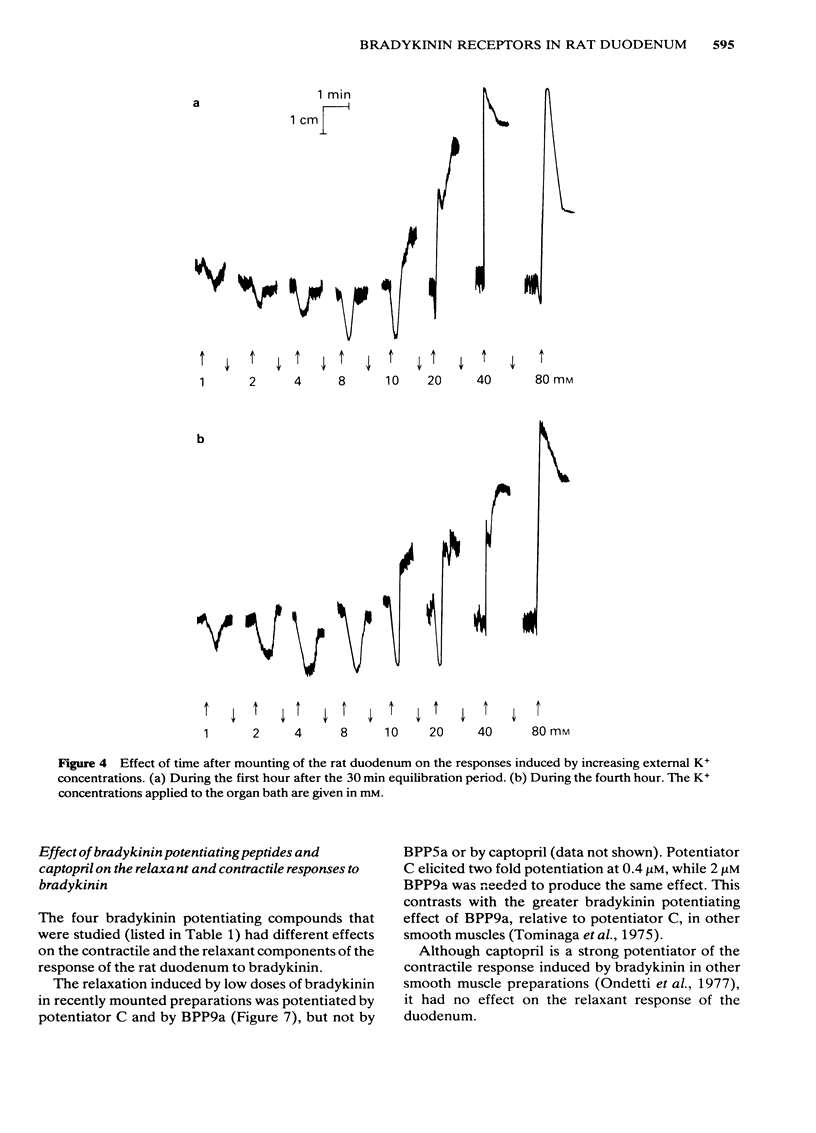
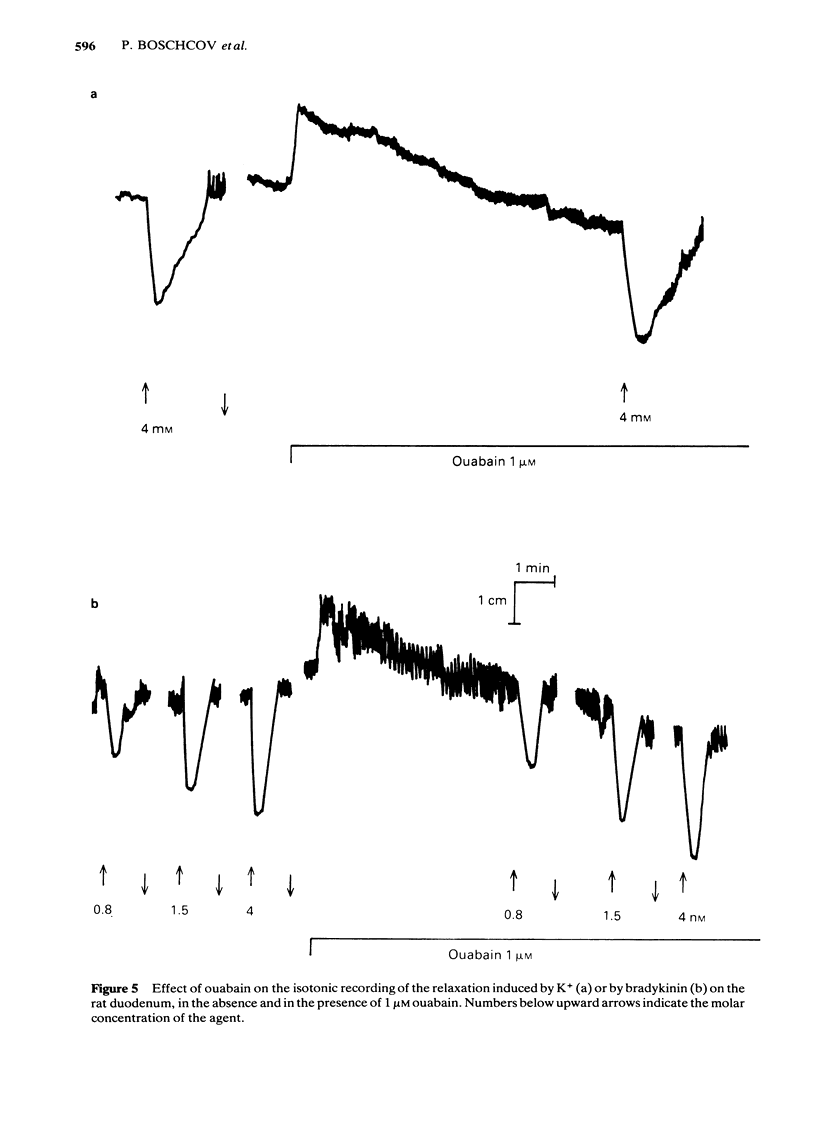
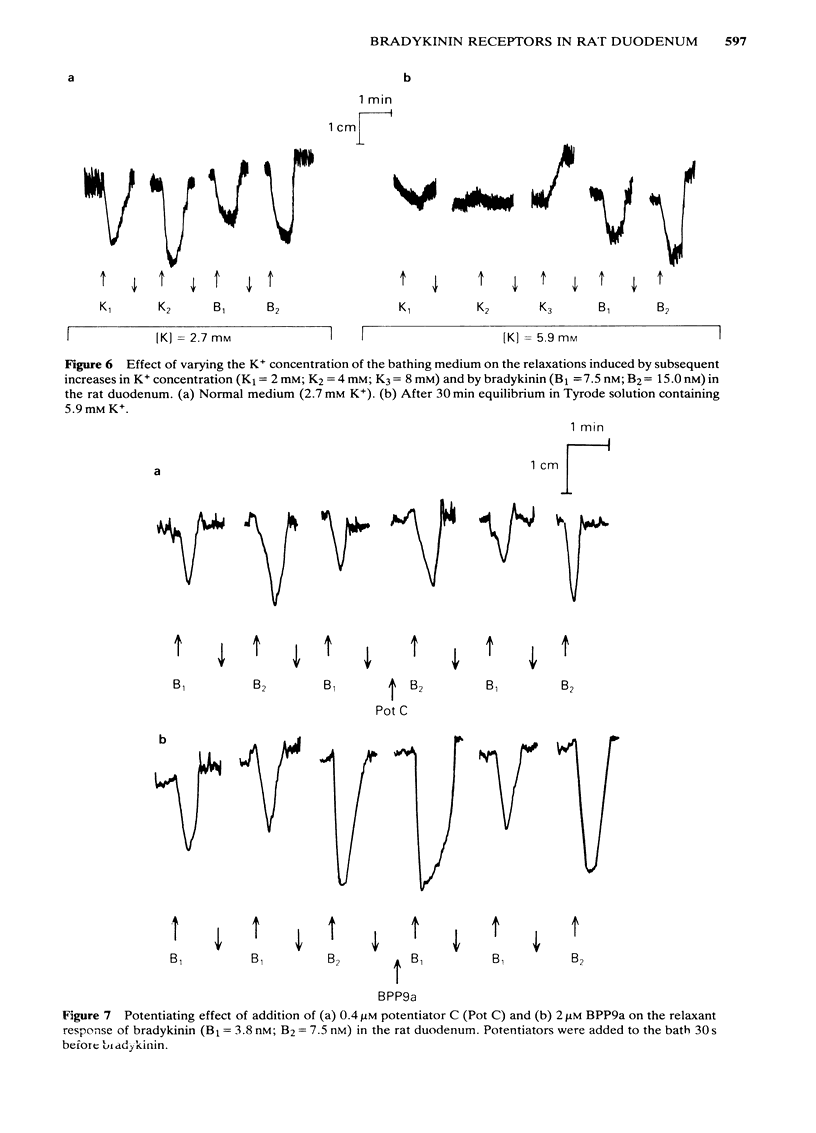
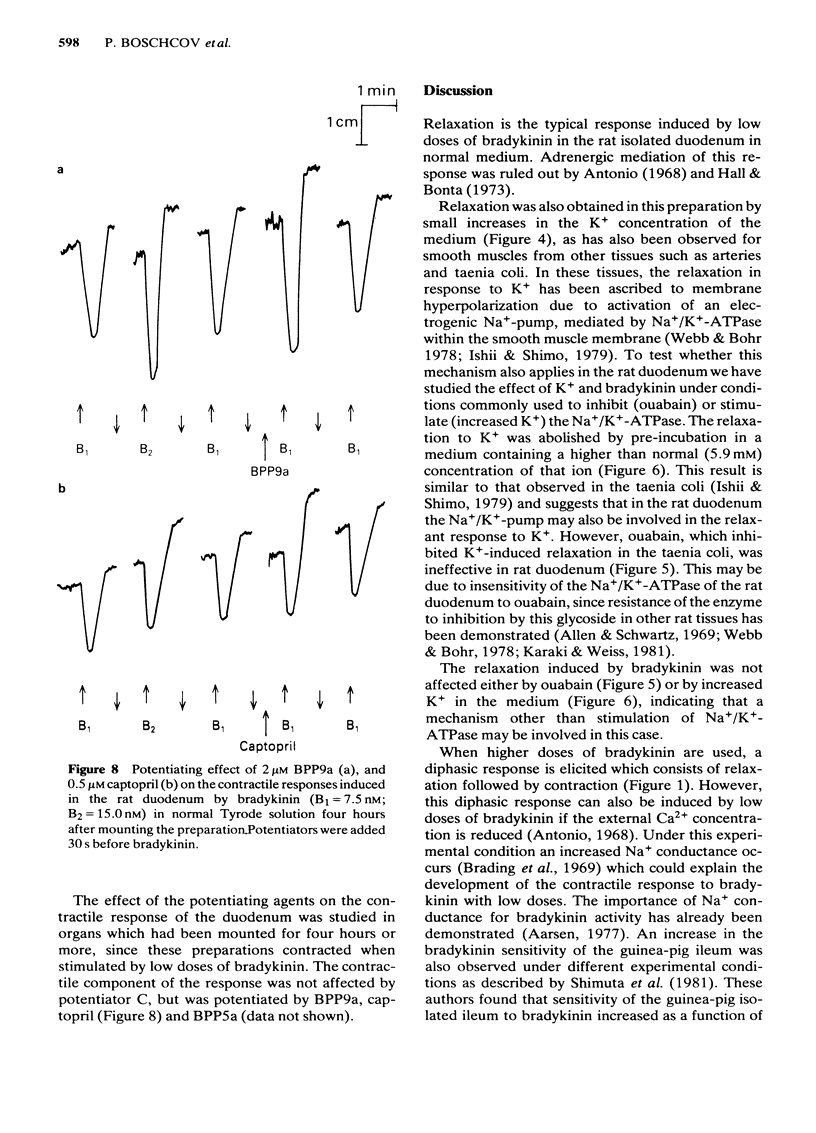
Low doses of bradykinin (below 10 nM), as well as of K+ (below 10 mM) induced relaxation, whereas higher doses caused contraction of the rat duodenum. The relaxant responses induced by bradykinin and K+ were not affected by ouabain (1 microM), but pre-incubation with 5.9 mM K+ abolished the responses to that ion but not those to bradykinin. The contractile and relaxant components of the response to bradykinin (but not those to K+) increased with the time elapsed after mounting of the preparation, and this was due to stretching by the load of the recording system. Specific and reversible desensitization (tachyphylaxis) was observed with the contractile response (but not the relaxation) induced by bradykinin. Des-Arg9-bradykinin, an analogue specific for B1-receptors, was much less active than bradykinin, and elicited only a contractile response. Among four bradykinin potentiating peptides that were tested, potentiator C enhanced the relaxation only, whereas BPP5a and captopril potentiated only the contraction and BPP9a potentiated both types of response to bradykinin. Our results support the hypothesis that the relaxant and contractile components of the rat duodenum's response to bradykinin are due to actions at different receptor sites, which can be distinguished by their properties (desensitization) and their different apparent affinities for agonists and for potentiating peptides.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aarsen P. N. The effects of bradykinin and the bradykinin potentiating peptide BPP5a on the electrical and mechanical responses of the guinea-pig taenia coli. Br J Pharmacol. 1977 Dec;61(4):523–532. doi: 10.1111/j.1476-5381.1977.tb07544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarsen P. N., van Caspel-de Bruyn M. Effect of changes in ionic environment on the action of bradykinin on the guinea-pig taenia coli. Eur J Pharmacol. 1970;12(3):348–358. doi: 10.1016/0014-2999(70)90087-7. [DOI] [PubMed] [Google Scholar]
- Allen J. C., Schwartz A. A possible biochemical explanation for the insensitivity of the rat to cardiac glycosides. J Pharmacol Exp Ther. 1969 Jul;168(1):42–46. [PubMed] [Google Scholar]
- Antonio A. The relaxing effect of bradykinin on intestinal smooth muscle. Br J Pharmacol Chemother. 1968 Jan;32(1):78–86. doi: 10.1111/j.1476-5381.1968.tb00431.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BORN G. V., BULBRING E. The movement of potassium between smooth muscle and the surrounding fluid. J Physiol. 1956 Mar 28;131(3):690–703. doi: 10.1113/jphysiol.1956.sp005494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BULBRING E. Correlation between membrane potential, spike discharge and tension in smooth muscle. J Physiol. 1955 Apr 28;128(1):200–221. doi: 10.1113/jphysiol.1955.sp005299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brading A., Bülbring E., Tomita T. The effect of sodium and calcium on the action potential of the smooth muscle of the guinea-pig taenia coli. J Physiol. 1969 Feb;200(3):637–654. doi: 10.1113/jphysiol.1969.sp008713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FREEMAN-NARROD M., GOODFORD P. J. Sodium and potassium content of the smooth muscle of the guinea-pig taenia coli at different temperatures and tensions. J Physiol. 1962 Oct;163:399–410. doi: 10.1113/jphysiol.1962.sp006985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faber D. B., Van der Meer C. A study of some bradykinin potentiating peptides derived from plasma proteins. Arch Int Pharmacodyn Ther. 1973 Oct;205(2):226–243. [PubMed] [Google Scholar]
- Hall D. W., Bonta I. L. Potentiation of the biphasic bradykinin response of the guinea-pig ileum. Arch Int Pharmacodyn Ther. 1974 Feb;207(2):361–372. [PubMed] [Google Scholar]
- Hall D. W., Bonta I. L. The biphasic response of the isolated guinea-pig ileum by bradykinin. Eur J Pharmacol. 1973 Feb;21(2):147–154. doi: 10.1016/0014-2999(73)90219-7. [DOI] [PubMed] [Google Scholar]
- Ishii T., Shimo Y. Potassium-induced relaxations of the guinea-pig taenia coli. Arch Int Pharmacodyn Ther. 1979 May;239(1):36–44. [PubMed] [Google Scholar]
- Karaki H., Weiss G. B. Effect of transmembrane pH gradient changes on potassium-induced relaxation in vascular smooth muscle. Blood Vessels. 1981;18(1-2):36–44. doi: 10.1159/000158336. [DOI] [PubMed] [Google Scholar]
- Ondetti M. A., Rubin B., Cushman D. W. Design of specific inhibitors of angiotensin-converting enzyme: new class of orally active antihypertensive agents. Science. 1977 Apr 22;196(4288):441–444. doi: 10.1126/science.191908. [DOI] [PubMed] [Google Scholar]
- Regoli D., Barabé J. Pharmacology of bradykinin and related kinins. Pharmacol Rev. 1980 Mar;32(1):1–46. [PubMed] [Google Scholar]
- Sabia E. B., Tominaga M., Paiva A. C., Paiva T. B. Bradykinin potentiating and sensitizing activities of new synthetic analogues of snake venom peptides. J Med Chem. 1977 Dec;20(12):1679–1681. doi: 10.1021/jm00222a030. [DOI] [PubMed] [Google Scholar]
- Shimuta S. I., Sabia E. B., Paiva A. C., Paiva T. B. Effect of stretching on the sensitivity of the guinea pig ileum to bradykinin and on its modification by bradykinin potentiating peptides. Eur J Pharmacol. 1981 Apr 9;70(4):551–558. doi: 10.1016/0014-2999(81)90366-6. [DOI] [PubMed] [Google Scholar]
- Tominaga M., Stewart J. M. Synthesis and properties of new bradykinin potentiating peptides. J Med Chem. 1975 Feb;18(2):130–133. doi: 10.1021/jm00236a003. [DOI] [PubMed] [Google Scholar]
- Ufkes J. G., Aarsen P. N., Van Der Meer C. The bradykinin potentiating activity of two pentapeptides on various isolated smooth muscle preparations. Eur J Pharmacol. 1976 Nov;40(1):137–144. doi: 10.1016/0014-2999(76)90363-0. [DOI] [PubMed] [Google Scholar]
- Webb R. C., Bohr D. F. Potassium-induced relaxation as an indicator of Na+-K+ ATPase activity in vascular smooth muscle. Blood Vessels. 1978;15(1-3):198–207. doi: 10.1159/000158166. [DOI] [PubMed] [Google Scholar]


