Abstract
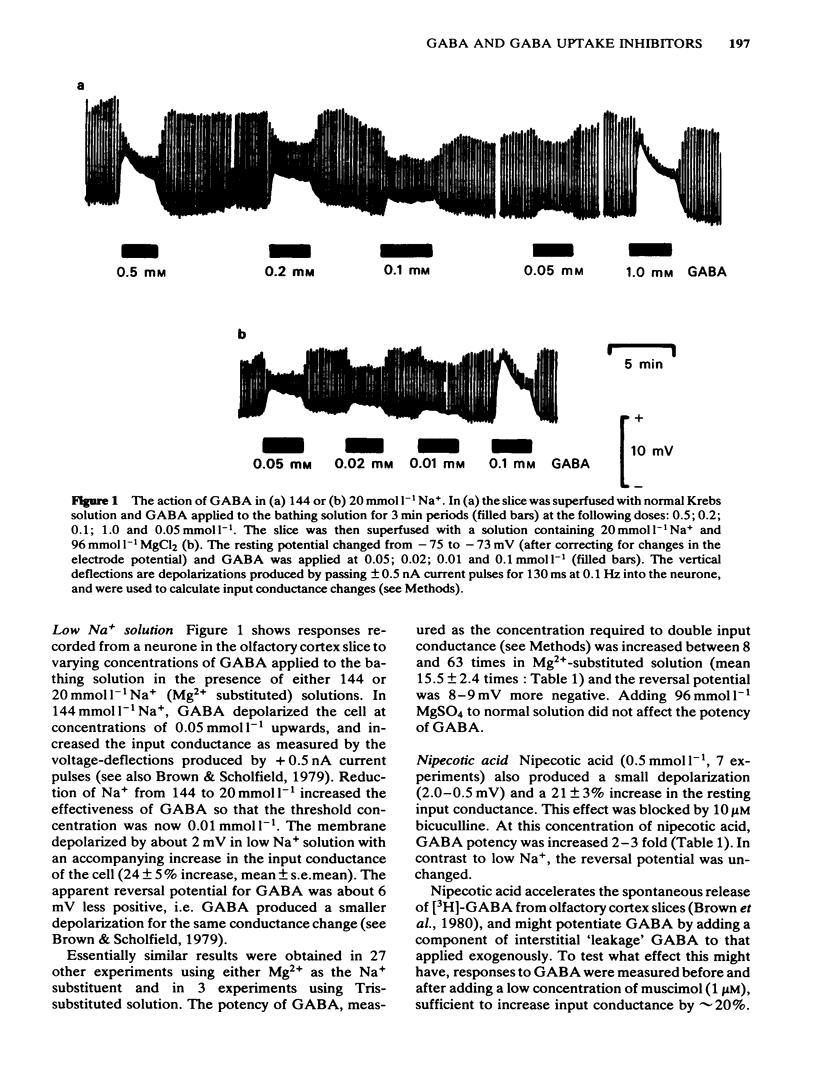
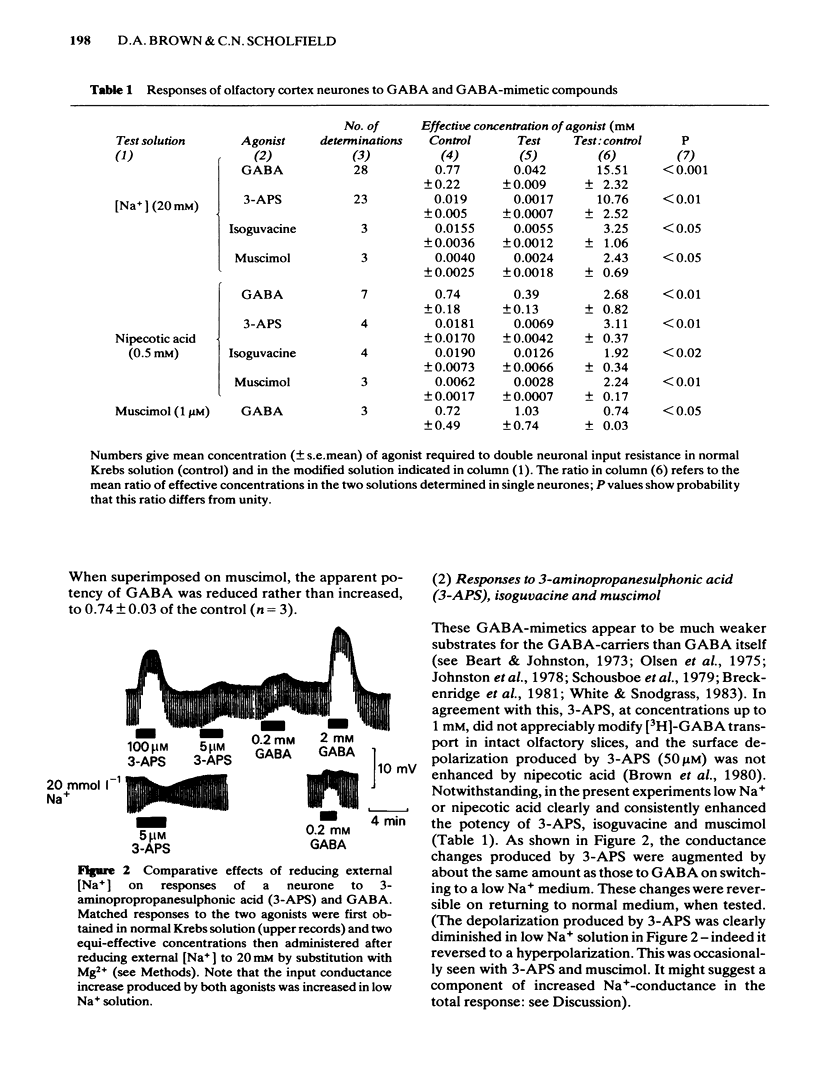
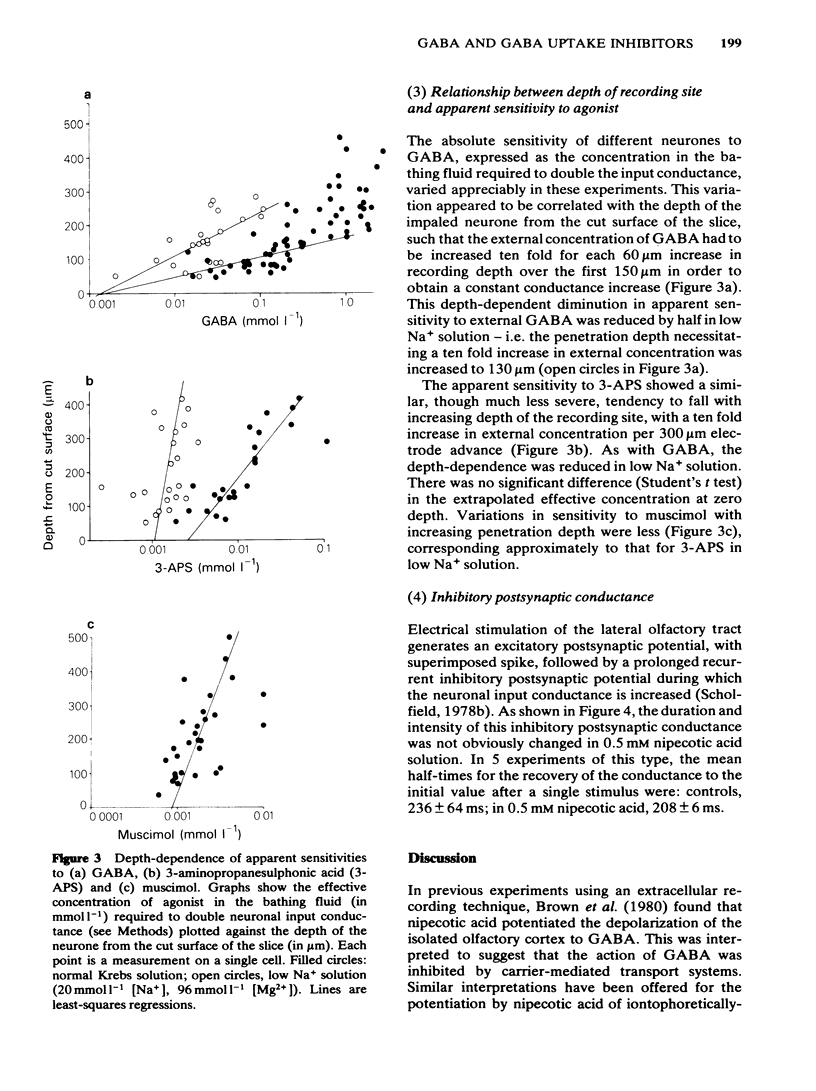
Membrane potential and input conductance were recorded in single neurones in slices of guinea-pig olfactory cortex in vitro. gamma-Aminobutyric acid (GABA) and GABA-mimetic compounds were applied by bath-perfusion. Potency was measured as the concentration required to double the input conductance. The potency of GABA was increased (i.e. the equi-effective concentrations were reduced) by 15.5 +/- 2.3 times (mean +/- s.e. mean) on reducing external [Na+] from 144 to 20 mmol l-1, by replacement with Mg2+. Corresponding potency changes for other agonists were + 10.8 +/- 2.5 for 3-aminopropanesulphonic acid (3-APS); 3.25 +/- 1.06 for isoguvacine and 2.43 +/- 0.69 for muscimol. Nipecotic acid (0.5 mM) produced the following increases in potency: GABA 2.68 +/- 0.02; 3-aminopropanesulphonic acid, 3.11 +/- 0.07; isoguvacine, 1.92 +/- 0.34; muscimol, 2.24 +/- 0.17. The concentration of GABA in the bathing fluid necessary to double input conductance increased with increasing depth of the recording site from the cut surface. The apparent potency fell 10 times for each 60 micron depth increment up to 150 micron. The recording depth also affected the apparent potency of muscimol and 3-APS but to a lesser extent. Reduction of external [Na+] reduced the depth-dependence of both GABA and 3-APS potency. No clear change in the duration of the recurrent inhibitory postsynaptic conductance could be detected in the presence of 0.5 mmol l-1 nipecotic acid.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF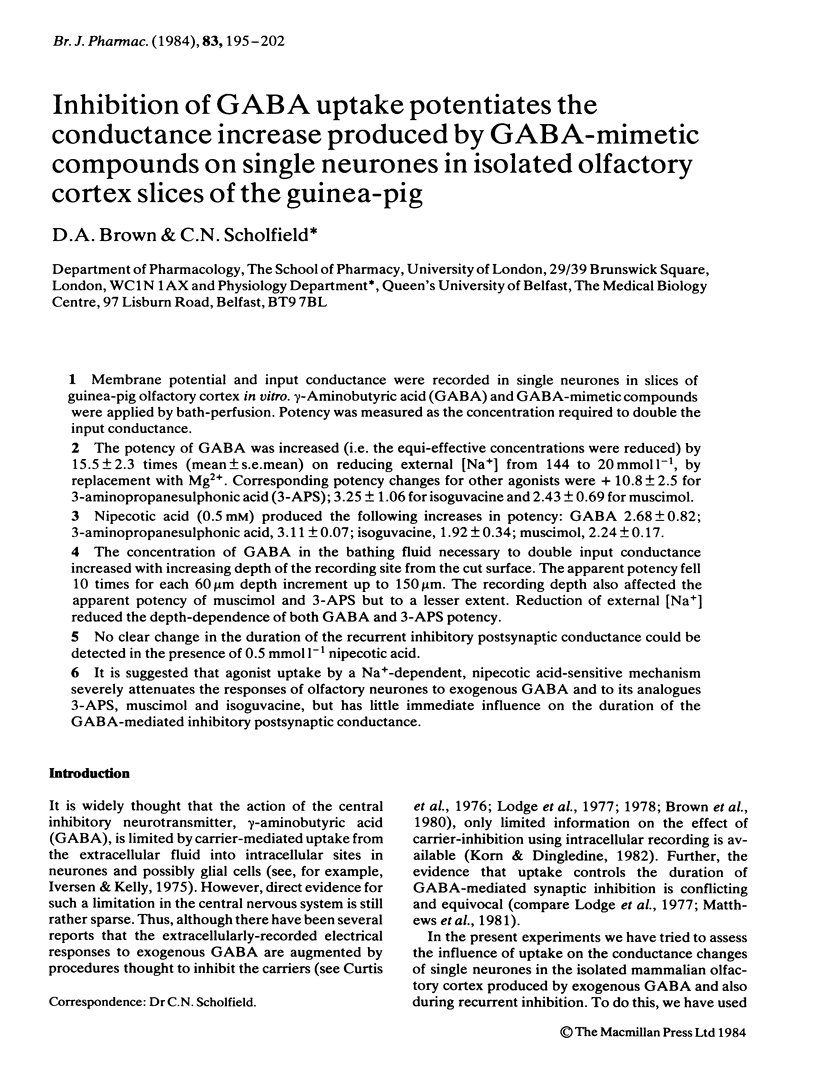



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agardh E., Ehinger B. (3H)-muscimol, (3H)-nipecotic acid and (3H)-isoguvacine as autoradiographic markers for GABA neurotransmission. J Neural Transm. 1982;54(1-2):1–18. doi: 10.1007/BF01249274. [DOI] [PubMed] [Google Scholar]
- Beart P. M., Johnston G. A. GABA uptake in rat brain slices: inhibition by GABA analogues and by various drugs. J Neurochem. 1973 Feb;20(2):319–324. doi: 10.1111/j.1471-4159.1973.tb12131.x. [DOI] [PubMed] [Google Scholar]
- Breckenridge R. J., Nicholson S. H., Nicol A. J., Suckling C. J., Leigh B., Iversen L. Inhibition of neuronal GABA uptake and glial beta-alanine uptake by synthetic GABA analogues. Biochem Pharmacol. 1981 Nov 15;30(22):3045–3049. doi: 10.1016/0006-2952(81)90491-3. [DOI] [PubMed] [Google Scholar]
- Brown D. A., Collins G. G., Galvan M. Influence of cellular transport on the interaction of amino acids with gamma-aminobutyric acid (GABA)-receptors in the isolated olfactory cortex of the guinea-pig. Br J Pharmacol. 1980 Feb;68(2):251–262. doi: 10.1111/j.1476-5381.1980.tb10414.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D. A., Scholfield C. N. Depolarization of neurones in the isolated olfactory cortex of the guinea-pig by gamma-aminobutyric acid. Br J Pharmacol. 1979 Feb;65(2):339–345. doi: 10.1111/j.1476-5381.1979.tb07835.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constanti A., Nistri A. Differential effects of sodium-free media on gamma-aminobutyrate and muscimol-evoked conductance increases recorded from lobster muscle fibres. Neuroscience. 1981;6(7):1443–1453. doi: 10.1016/0306-4522(81)90199-8. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Game C. J., Lodge D. The in vivo inactivation of GABA and other inhibitory amino acids in the cat nervous system. Exp Brain Res. 1976 Jun 30;25(4):413–428. doi: 10.1007/BF00241731. [DOI] [PubMed] [Google Scholar]
- Galvan M., Scholfield C. N. Cellular uptake of gamma-aminobutyric acid influences its potency on neurones of olfactory cortex in vitro [proceedings]. J Physiol. 1978 Nov;284:129P–130P. [PubMed] [Google Scholar]
- Iversen L. L., Kelly J. S. Uptake and metabolism of gamma-aminobutyric acid by neurones and glial cells. Biochem Pharmacol. 1975 May 1;24(9):933–938. doi: 10.1016/0006-2952(75)90422-0. [DOI] [PubMed] [Google Scholar]
- Johnston G. A., Kennedy S. M., Lodge D. Muscimol uptake, release and binding in rat brain slices. J Neurochem. 1978 Dec;31(6):1519–1523. doi: 10.1111/j.1471-4159.1978.tb06579.x. [DOI] [PubMed] [Google Scholar]
- Johnston G. A., Krogsgaard-Larsen P., Stephanson A. L., Twitchin B. Inhibition of the uptake of GABA and related amino acids in rat brain slices by the optical isomers of nipecotic acid. J Neurochem. 1976 May;26(5):1029–1032. doi: 10.1111/j.1471-4159.1976.tb06488.x. [DOI] [PubMed] [Google Scholar]
- Lodge D., Curtis D. R., Johnston G. A. Does uptake limit the actions of GABA agonists in vivo? Experiments with muscimol, isoguvacine and THIP in cat spinal cord. J Neurochem. 1978 Dec;31(6):1525–1528. doi: 10.1111/j.1471-4159.1978.tb06580.x. [DOI] [PubMed] [Google Scholar]
- Lodge D., Johnston G. A., Curtis D. R., Brand S. J. Effects of the Areca nut constituents arecaidine and guvacine on the action of GABA in the cat central nervous system. Brain Res. 1977 Nov 18;136(3):513–522. doi: 10.1016/0006-8993(77)90075-0. [DOI] [PubMed] [Google Scholar]
- Martin D. L., Smith A. A., 3rd Ions and the transport of gamma-aminobutyric acid by synaptosomes. J Neurochem. 1972 Mar;19(3):841–855. doi: 10.1111/j.1471-4159.1972.tb01398.x. [DOI] [PubMed] [Google Scholar]
- Matthews W. D., McCafferty G. P., Setler P. E. An electrophysiological model of GABA-mediated neurotransmission. Neuropharmacology. 1981 Jun;20(6):561–565. doi: 10.1016/0028-3908(81)90208-2. [DOI] [PubMed] [Google Scholar]
- Olsen R. W., Bayless J. D., Ban M. Potency of inhibitors for gamma-aminobutyric acid uptake by mouse brain subcellular particles at 0 degrees. Mol Pharmacol. 1975 Sep;11(5):558–565. [PubMed] [Google Scholar]
- Scholfield C. N. A depolarizing inhibitory potential in neurones of the olfactory cortex in vitro. J Physiol. 1978 Feb;275:547–557. doi: 10.1113/jphysiol.1978.sp012207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholfield C. N. Antagonism of gamma-aminobutyric acid and muscimol by picrotoxin, bicuculline, strychnine, bemegride, leptazol, D-tubocurarine and theophylline in the isolated olfactory cortex. Naunyn Schmiedebergs Arch Pharmacol. 1982 Mar;318(4):274–280. doi: 10.1007/BF00501165. [DOI] [PubMed] [Google Scholar]
- Scholfield C. N. Electrical properties of neurones in the olfactory cortex slice in vitro. J Physiol. 1978 Feb;275:535–546. doi: 10.1113/jphysiol.1978.sp012206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schousboe A., Thorbek P., Hertz L., Krogsgaard-Larsen P. Effects of GABA analogues of restricted conformation on GABA transport in astrocytes and brain cortex slices and on GABA receptor binding. J Neurochem. 1979 Jul;33(1):181–189. doi: 10.1111/j.1471-4159.1979.tb11720.x. [DOI] [PubMed] [Google Scholar]
- Stafstrom C. E., Schwindt P. C., Crill W. E. Negative slope conductance due to a persistent subthreshold sodium current in cat neocortical neurons in vitro. Brain Res. 1982 Mar 18;236(1):221–226. doi: 10.1016/0006-8993(82)90050-6. [DOI] [PubMed] [Google Scholar]
- White W. F., Snodgrass S. R. Isoguvacine binding, uptake, and release: relation to the GABA system. J Neurochem. 1983 Jun;40(6):1701–1708. doi: 10.1111/j.1471-4159.1983.tb08145.x. [DOI] [PubMed] [Google Scholar]


