Abstract
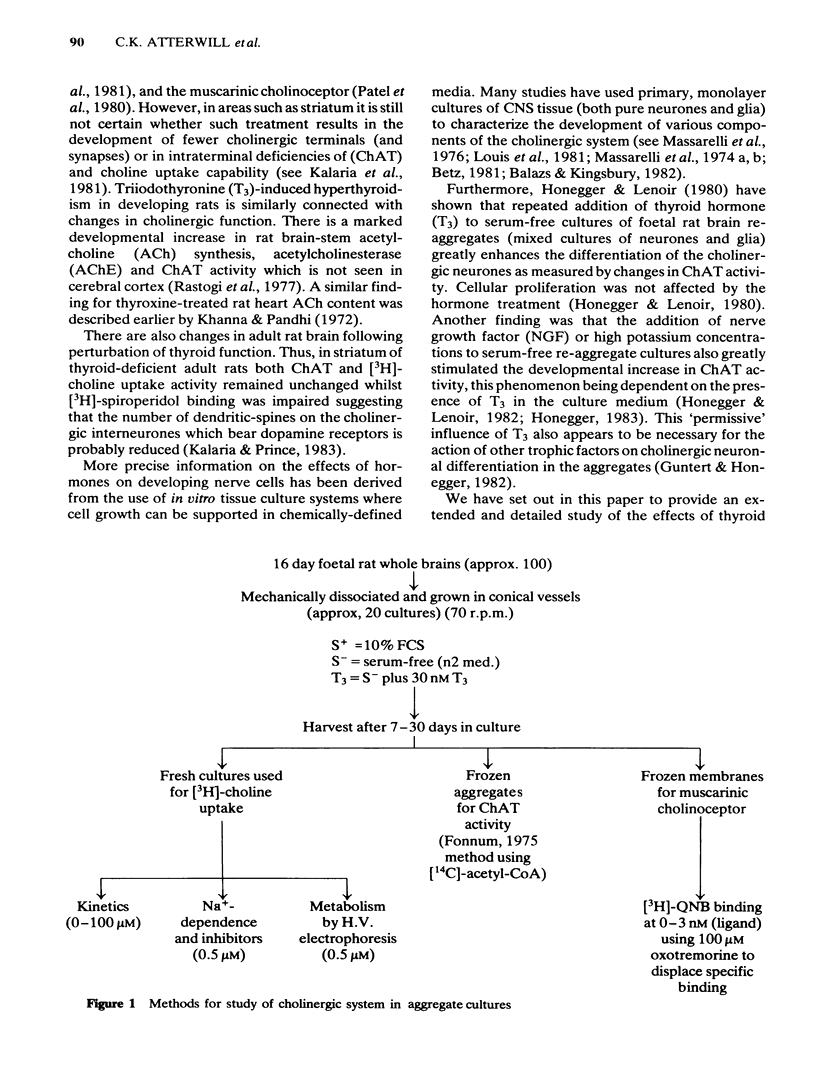
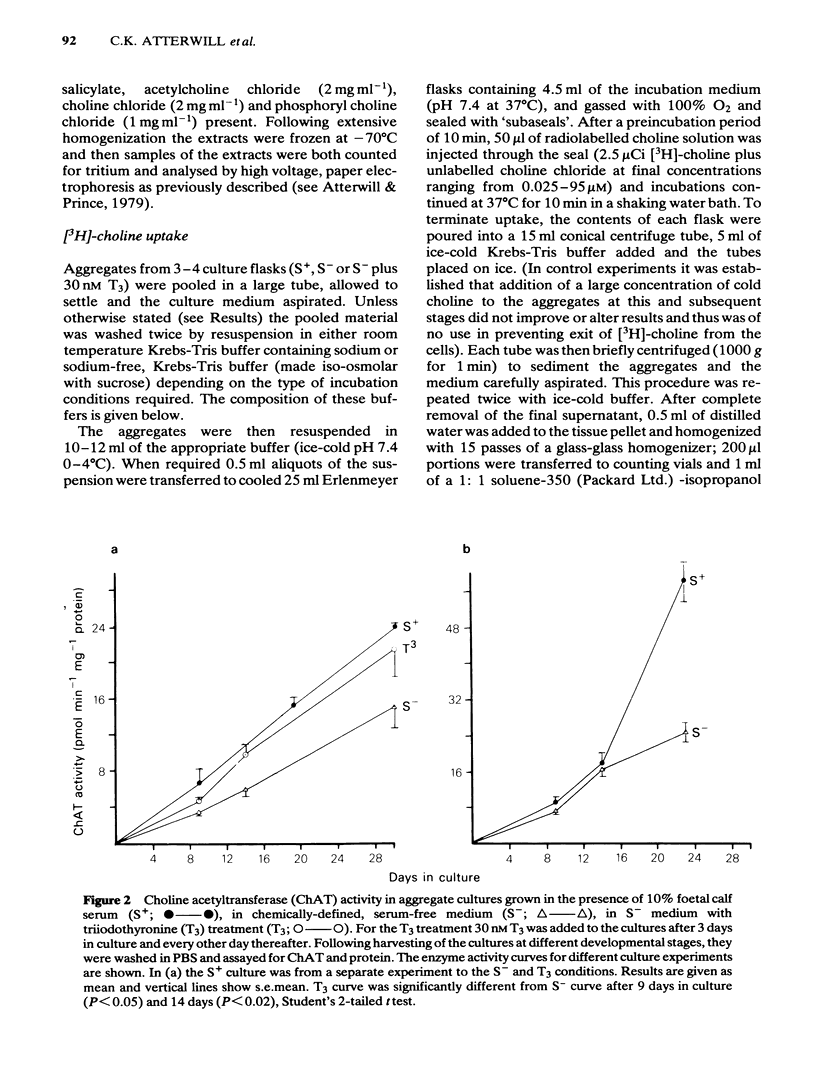
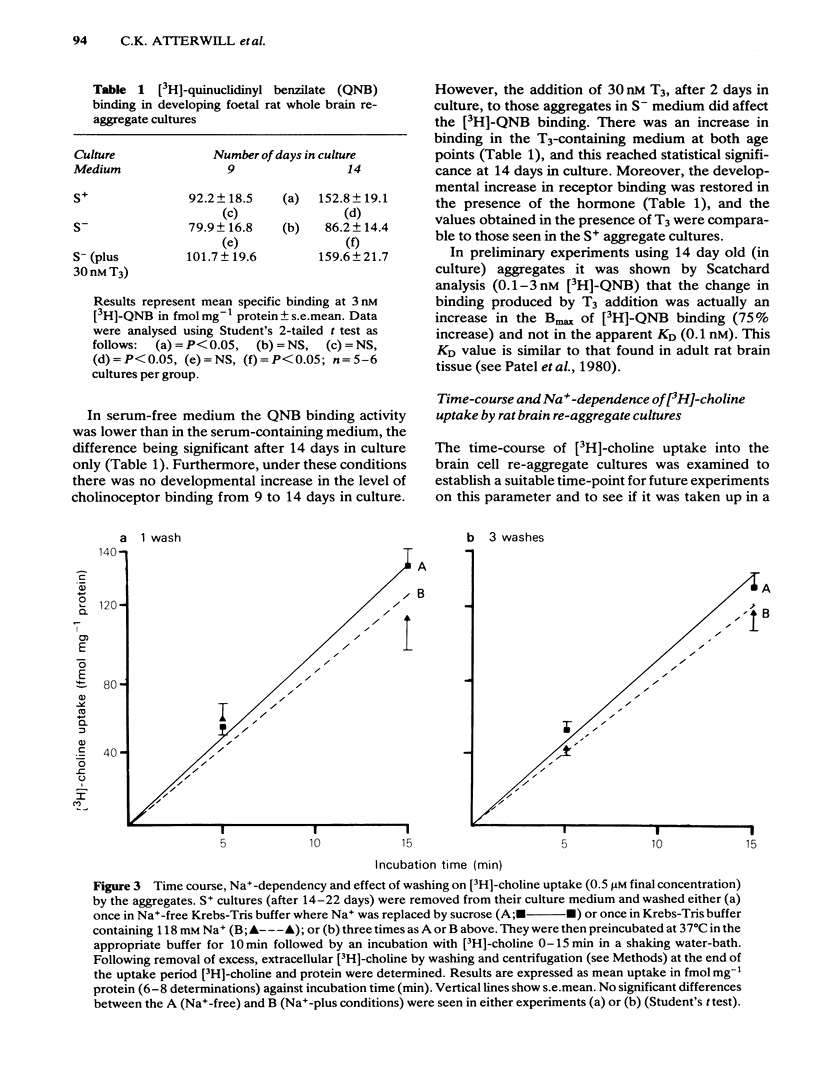
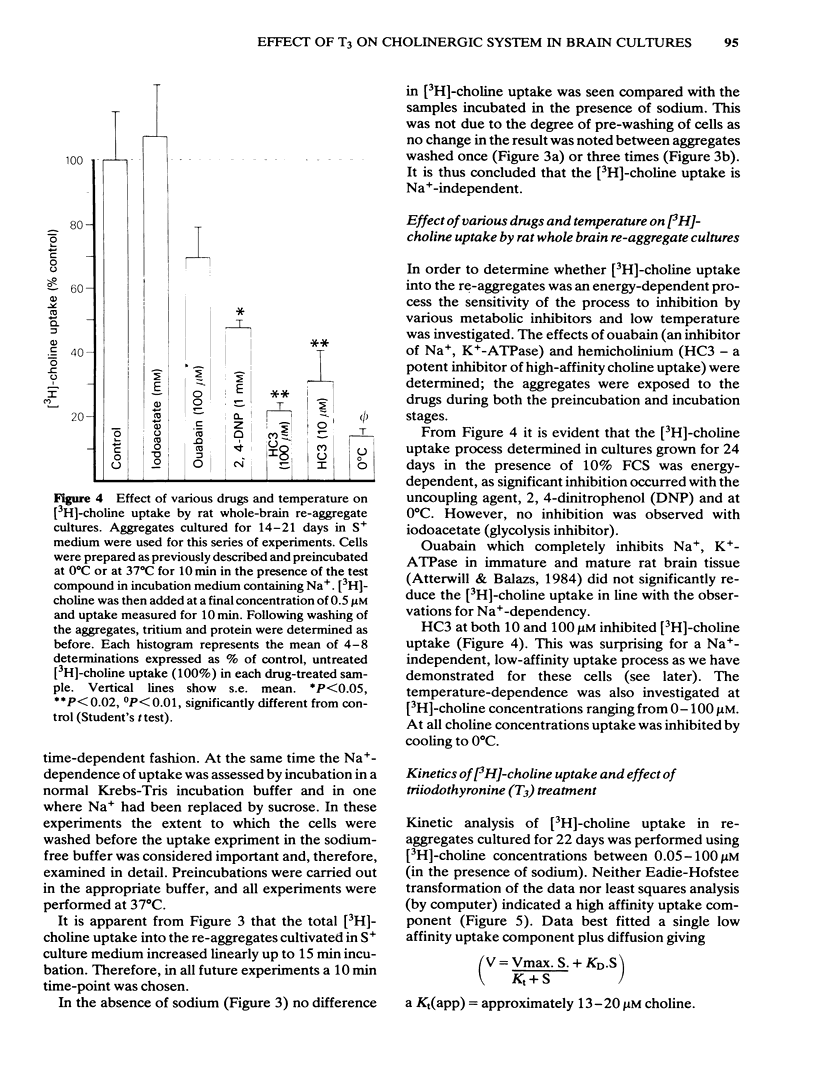
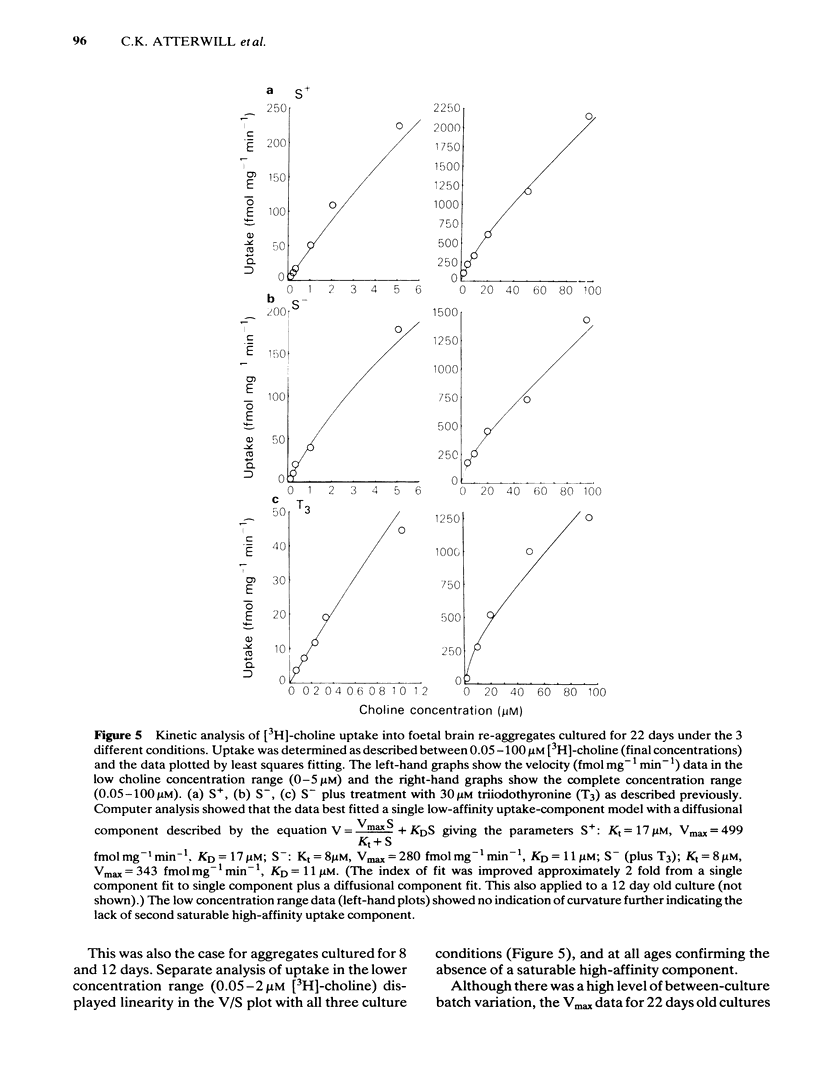
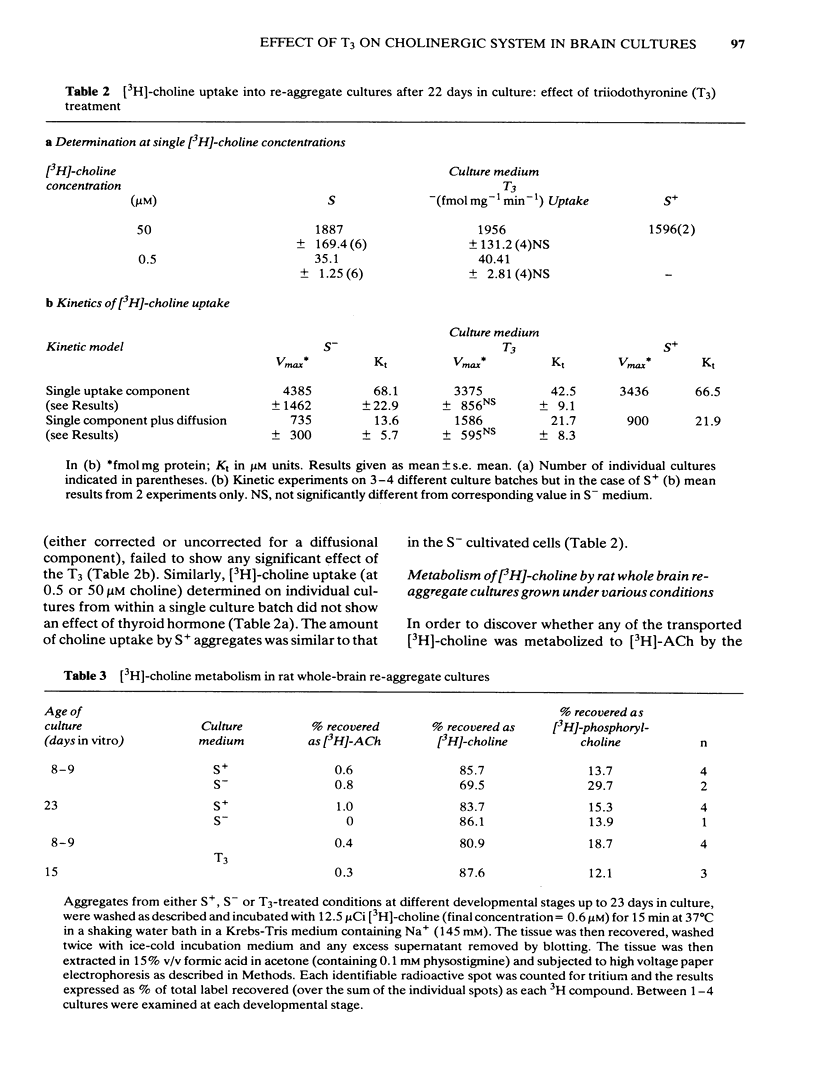
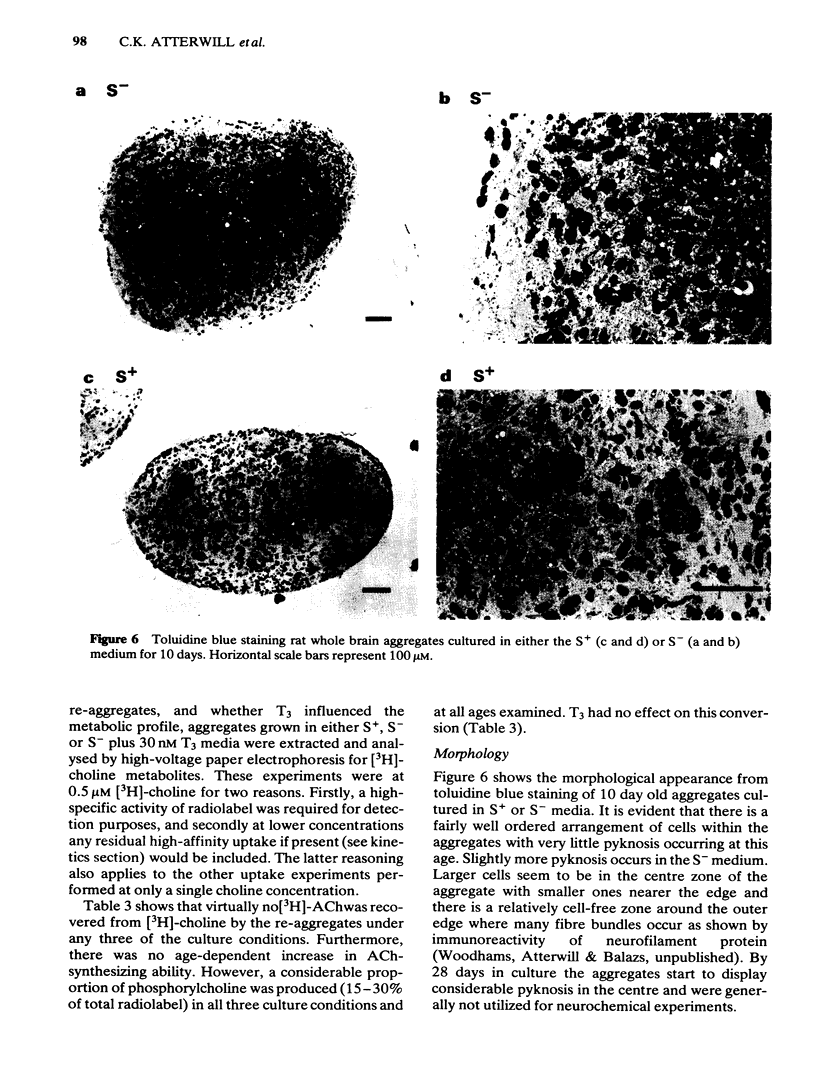
Development has been studied in re-aggregate cultures derived from the 16 day foetal rat brain and the effects of triiodothyronine (T3) investigated. Cultures were maintained in either a medium containing 10% serum (S+), or in serum-free culture medium (S-) or in serum-free medium containing 30nM T3. The muscarinic cholinoceptor, measured by specific binding of [3H]-quinuclidinyl benzitate ([3H]-QNB) at 9 and 14 days in vitro, was at a lower level in the serum-free cultured cells compared with those in serum-containing culture medium (S+). In cultures in the latter medium, receptor concentration at day 14 was of a similar magnitude to that in rat brain at an equivalent postnatal age. Binding increased with development from 9 to 14 days in vitro in the S+ medium but not in the S- medium. T3 treatment caused an 85% increase in [3H]-QNB binding compared with the cultures in S- medium at day 14 to a level equivalent to that found in the cells grown in S+ medium. This increase was reflected in the Bmax but not in the KD (approx. 0.1nM). Choline acetyltransferase (ChAT) activity developed more slowly in the S- medium than in the S+ medium where the specific activity approximated values obtained in vivo. T3 treatment of cultures grow in S- medium significantly enhanced the developmental rate of increase of ChAT activity. The characteristics of [3H]-choline uptake and metabolism in the cultures was examined. Uptake was strictly Na+-independent but was energy-dependent, and inhibited by 2, 4'-dinitrophenol (2, 4'-DNP) and cooling (0-4 degrees C). Neither iodoacetate nor ouabain had any effect on the amount of uptake. Hemicholinium (HC3) was a potent inhibitor of uptake (70% inhibition at 10 microM HC3). Metabolism studies showed virtually no conversion to [3H]-acetylcholine ([3H]-ACH) in reaggregates grown in either the S+, S- or T3 containing media. However, a small amount of [3H]-choline was incorporated into phosphorylcholine. T3 treatment had no effect on this metabolic profile. The kinetics of [3H]-choline uptake by the re-aggregates was also studied in the re-aggregate cultures (after 12 and 22 days in vitro) using [3H]-choline at 0.05-100 microM. Both Eadie-Hofstee transformation and least-squares analysis of the data showed that the uptake comprised only a single low-affinity component with an apparent Kt = approx. 50 microM. Unlike ChAT and [3H]-QNB binding, there appeared to be no difference between the uptake in the different culture conditions.(ABSTRACT TRUNCATED AT 400 WORDS)
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appel S. H. A unifying hypothesis for the cause of amyotrophic lateral sclerosis, parkinsonism, and Alzheimer disease. Ann Neurol. 1981 Dec;10(6):499–505. doi: 10.1002/ana.410100602. [DOI] [PubMed] [Google Scholar]
- Atterwill C. K., Cunningham V. J., Balázs R. Characterization of Na+,K+-ATPase in cultured and separated neuronal and glial cells from rat cerebellum. J Neurochem. 1984 Jul;43(1):8–18. doi: 10.1111/j.1471-4159.1984.tb06672.x. [DOI] [PubMed] [Google Scholar]
- Atterwill C. K., Prince A. K. Multiple forms of choline acetyltransferase and the high affinity uptake of choline in brain of developing and adult rats. J Neurochem. 1978 Sep;31(3):719–725. doi: 10.1111/j.1471-4159.1978.tb07846.x. [DOI] [PubMed] [Google Scholar]
- Atweh S., Simon J. R., Kuhar M. J. Utilization of sodium-dependent high affinity choline uptake in vitro as a measure of the activity of cholinergic neurons in vivo. Life Sci. 1975 Nov 15;17(10):1535–1544. doi: 10.1016/0024-3205(75)90174-5. [DOI] [PubMed] [Google Scholar]
- Betz H. Choline acetyltransferase activity in chick retina cultures: effect of membrane depolarizing agents. Brain Res. 1981 Oct 26;223(1):190–194. doi: 10.1016/0006-8993(81)90822-2. [DOI] [PubMed] [Google Scholar]
- Burgess E. J., Atterwill C. K., Prince A. K. Choline acetyltransferase and the high affinity uptake of choline in corpus striatum of reserpinised rats. J Neurochem. 1978 Oct;31(4):1027–1033. doi: 10.1111/j.1471-4159.1978.tb00143.x. [DOI] [PubMed] [Google Scholar]
- Ebel A., Massarelli R., Sensenbrenner M., Mandel P. Choline acetyltransferase and acetylcholinesterase activities in chicken brain hemispheres in vivo and in cell culture. Brain Res. 1974 Aug 23;76(3):461–472. doi: 10.1016/0006-8993(74)90822-1. [DOI] [PubMed] [Google Scholar]
- Fonnum F. A rapid radiochemical method for the determination of choline acetyltransferase. J Neurochem. 1975 Feb;24(2):407–409. doi: 10.1111/j.1471-4159.1975.tb11895.x. [DOI] [PubMed] [Google Scholar]
- Francescangeli E., Goracci G., Piccinin G. L., Mozzi R., Woelk H., Porcellati G. The metabolism of labelled choline in neuronal and glial cells of the rabbit in vivo. J Neurochem. 1977 Jan;28(1):171–176. doi: 10.1111/j.1471-4159.1977.tb07723.x. [DOI] [PubMed] [Google Scholar]
- Higgins A. J., Neal M. J. Potassium activation of [3H]-choline accumulation by isolated sympathetic ganglia of the rat. Br J Pharmacol. 1982 Dec;77(4):573–580. doi: 10.1111/j.1476-5381.1982.tb09334.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honegger P., Lenoir D. Nerve growth factor (NGF) stimulation of cholinergic telencephalic neurons in aggregating cell cultures. Brain Res. 1982 Feb;255(2):229–238. doi: 10.1016/0165-3806(82)90023-2. [DOI] [PubMed] [Google Scholar]
- Honegger P., Lenoir D. Triodothyronine enhancement of neuronal differentiation in aggregating fetal rat brain cells cultured in a chemically defined medium. Brain Res. 1980 Oct 20;199(2):425–434. doi: 10.1016/0006-8993(80)90699-x. [DOI] [PubMed] [Google Scholar]
- Honegger P. Nerve growth factor-sensitive brain neurons in culture. Monogr Neural Sci. 1983;9:36–42. doi: 10.1159/000406876. [DOI] [PubMed] [Google Scholar]
- Honegger P., Richelson E. Biochemical differentiation of mechanically dissociated mammalian brain in aggregating cell culture. Brain Res. 1976 Jun 11;109(2):335–354. doi: 10.1016/0006-8993(76)90534-5. [DOI] [PubMed] [Google Scholar]
- Honegger P., Richelson E. Neurotransmitter synthesis, storage and release by aggregating cell cultures of rat brain. Brain Res. 1979 Feb 16;162(1):89–101. doi: 10.1016/0006-8993(79)90758-3. [DOI] [PubMed] [Google Scholar]
- Ishida I., Deguchi T. Regulation of choline acetyltransferase in primary cell cultures of spinal cord by neurotransmitter L-norepinephrine. Brain Res. 1983 Mar;283(1):13–23. doi: 10.1016/0165-3806(83)90077-9. [DOI] [PubMed] [Google Scholar]
- Khanna N. K., Pandhi P. Effect of treatment with thyroxine on the acetylcholine content of rat heart. Jpn J Pharmacol. 1972 Apr;22(2):261–262. doi: 10.1254/jjp.22.261. [DOI] [PubMed] [Google Scholar]
- Kuhar M. J., Sethy V. H., Roth R. H., Aghajanian G. K. Choline: selective accumulation by central cholinergic neurons. J Neurochem. 1973 Feb;20(2):581–593. doi: 10.1111/j.1471-4159.1973.tb12157.x. [DOI] [PubMed] [Google Scholar]
- Ladinsky H., Consolo S., Peri G., Garattini S. Acetylcholine, choline and choline acetyltransferase activity in the developing brain of normal and hypothyroid rats. J Neurochem. 1972 Aug;19(8):1947–1952. doi: 10.1111/j.1471-4159.1972.tb01483.x. [DOI] [PubMed] [Google Scholar]
- Louis J. C., Pettmann B., Courageot J., Rumigny J. F., Mandel P., Sensenbrenner M. Developmental changes in cultured neurones from chick embryo cerebral hemispheres. An ultrastructural and neurochemical study. Exp Brain Res. 1981;42(1):63–72. doi: 10.1007/BF00235730. [DOI] [PubMed] [Google Scholar]
- Martin K. Concentrative accumulation of choline by human erythrocytes. J Gen Physiol. 1968 Apr;51(4):497–516. doi: 10.1085/jgp.51.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massarelli R., Ciesielski-Treska J., Ebel A., Mandel P. Choline uptake in glial cell cultures. Brain Res. 1974 Dec 6;81(2):361–363. doi: 10.1016/0006-8993(74)90953-6. [DOI] [PubMed] [Google Scholar]
- Massarelli R., Stefanovic V., Mandel P. Cholinesterase activity and choline uptake in intact nerve cell cultures. Brain Res. 1976 Aug 6;112(1):103–112. doi: 10.1016/0006-8993(76)90337-1. [DOI] [PubMed] [Google Scholar]
- Massarelli R., Syapin P. J., Noble E. P. Increased uptake of choline by neural cell cultures chronically exposed to ethanol. Life Sci. 1976 Feb 15;18(4):397–403. doi: 10.1016/0024-3205(76)90216-2. [DOI] [PubMed] [Google Scholar]
- Massarelli R., Yong T. Y., Froissart C., Robert J. Choline uptake and metabolism by nerve cell cultures. Prog Brain Res. 1979;49:89–96. doi: 10.1016/s0079-6123(08)64624-5. [DOI] [PubMed] [Google Scholar]
- Messer A. Primary monolayer cultures of the rat corpus striatum: morphology and properties related to acetylcholine and gamma-aminobutyrate. Neuroscience. 1981;6(12):2677–2687. doi: 10.1016/0306-4522(81)90112-3. [DOI] [PubMed] [Google Scholar]
- Patel A. J., Smith R. M., Kingsbury A. E., Hunt A., Balázs R. Effects of thyroid state on brain development: muscarinic acetylcholine and GABA receptors. Brain Res. 1980 Oct 6;198(2):389–402. doi: 10.1016/0006-8993(80)90752-0. [DOI] [PubMed] [Google Scholar]
- Rastogi R. B., Hrdina P. D., Dubas T., Singhal R. L. Alterations of brain acetylcholine metabolism during neonatal hyperthyroidism. Brain Res. 1977 Mar 4;123(1):188–192. doi: 10.1016/0006-8993(77)90655-2. [DOI] [PubMed] [Google Scholar]
- Repke H., Maderspach K. Muscarinic acetylcholine receptors on culture glia cells. Brain Res. 1982 Jan 28;232(1):206–211. doi: 10.1016/0006-8993(82)90627-8. [DOI] [PubMed] [Google Scholar]
- Richelson E., Thompson E. J. Transport of neurotransmitter precursors into cultured cells. Nat New Biol. 1973 Feb 14;241(111):201–204. doi: 10.1038/newbio241201a0. [DOI] [PubMed] [Google Scholar]
- Siman R. G., Klein W. L. Cholinergic activity regulates muscarinic receptors in central nervous system cultures. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4141–4145. doi: 10.1073/pnas.76.8.4141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siman R. G., Klein W. L. Differential regulation of muscarinic and nicotinic receptors by cholinergic stimulation in cultured avian retina cells. Brain Res. 1983 Feb 28;262(1):99–108. doi: 10.1016/0006-8993(83)90473-0. [DOI] [PubMed] [Google Scholar]
- Sorimachi M., Kataoka K. High affinity choline uptake: an early index of cholinergic innervation in rat brain. Brain Res. 1975 Aug 29;94(2):325–336. doi: 10.1016/0006-8993(75)90065-7. [DOI] [PubMed] [Google Scholar]
- Sung C. P., Johnstone R. M. Evidence for active transport of choline in rat kidney cortex slices. Can J Biochem. 1965 Jul;43(7):1111–1118. doi: 10.1139/o65-124. [DOI] [PubMed] [Google Scholar]
- Suszkiw J. B., Pilar G. Selective localization of a high affinity choline uptake system and its role in ACh formation in cholinergic nerve terminals. J Neurochem. 1976 Jun;26(6):1133–1138. doi: 10.1111/j.1471-4159.1976.tb06996.x. [DOI] [PubMed] [Google Scholar]
- Toru M., Aprison M. H. Brain acetylcholine studies: a new extraction procedure. J Neurochem. 1966 Dec;13(12):1533–1544. doi: 10.1111/j.1471-4159.1966.tb04318.x. [DOI] [PubMed] [Google Scholar]
- Trapp B. D., Honegger P., Richelson E., Webster H. D. Morphological differentiation of mechanically dissociated fetal rat brain in aggregating cell cultures. Brain Res. 1979 Jan 5;160(1):117–130. doi: 10.1016/0006-8993(79)90605-x. [DOI] [PubMed] [Google Scholar]



