Abstract
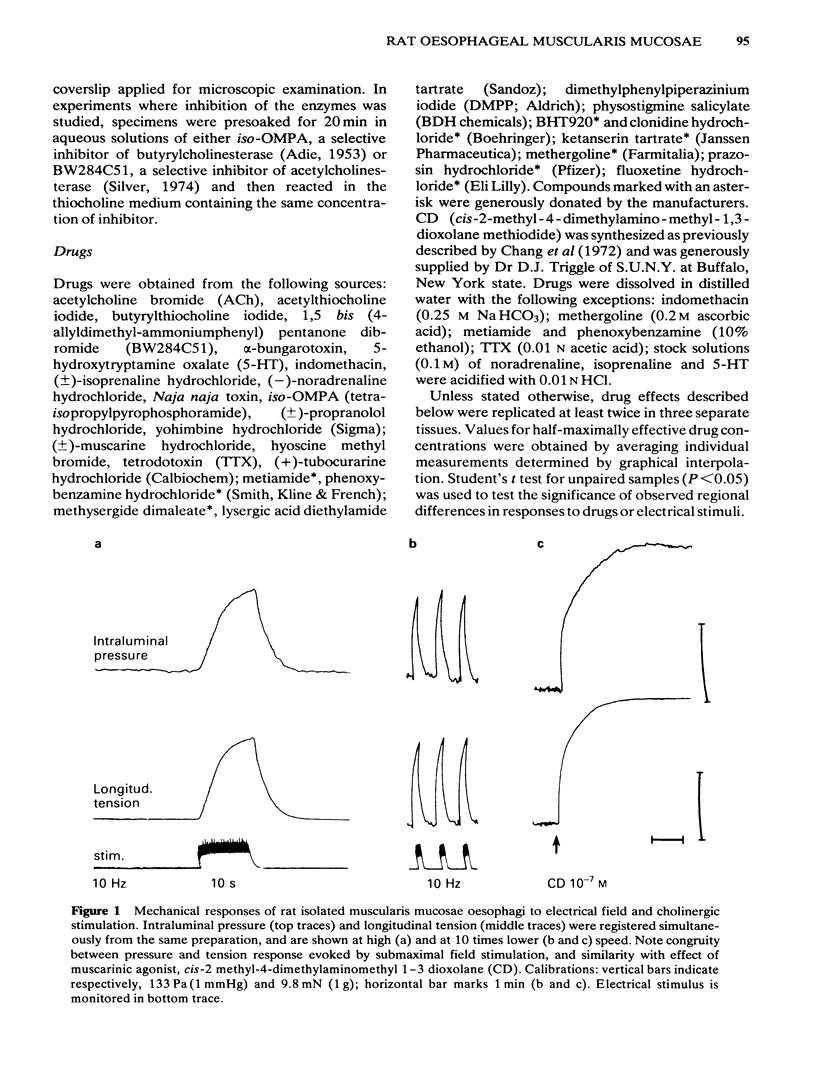
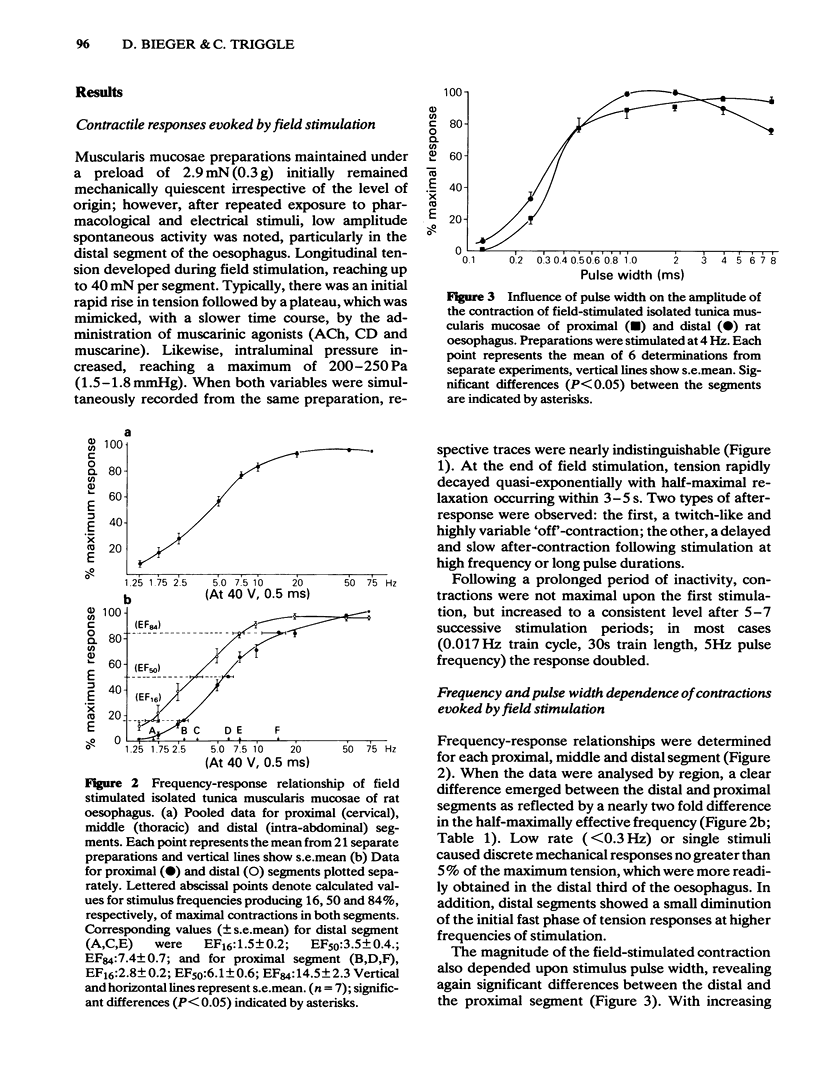
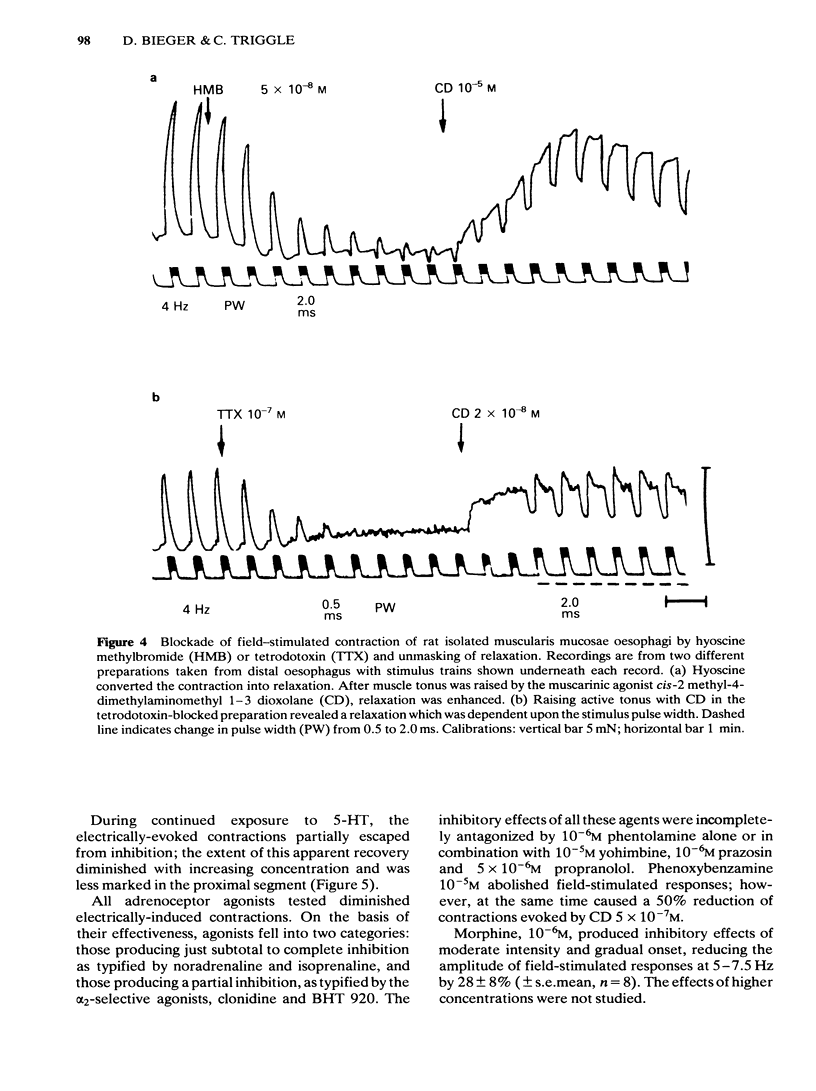
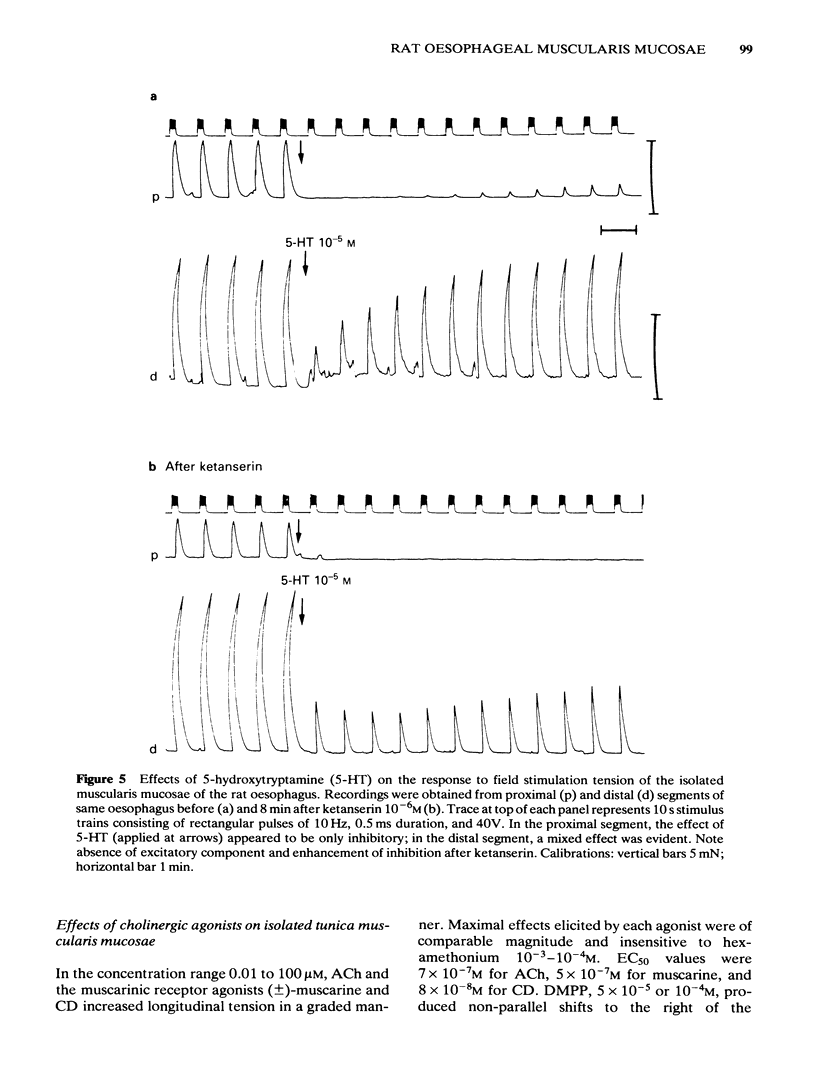
Electrical stimulation applied to vagal oesophageal branches of the isolated curarized oesophagus, or via field electrodes to the isolated tunica muscularis mucosae (TMM), increased longitudinal tension and intraluminal pressure in a frequency-dependent manner. Differences between cervical and distal TMM segments were noted in frequency-response relationships, as well as in the pulse-width dependence of contractions to field stimulation. Vagally- and field-stimulated contractions were eliminated by tetrodotoxin or hyoscine, indicating their mediation by cholinergic neurones. The field-stimulated postganglionic responses were resistant to hexamethonium or (+)-tubocurarine and weakly inhibited by morphine. Vagally- and field-stimulated TMM contractions were mimicked by muscarinic agonists, augmented by acetylcholinesterase inhibitors, and inhibited more effectively by beta-than by alpha 1-or alpha 2-adrenoceptor agonists. 5-Hydroxytryptamine (5-HT) exerted excitatory and/or inhibitory effects: TMM in situ responded with a hexamethonium-resistant, ketanserin-sensitive transient increase in tension comparable to that produced by field stimulation. In the isolated TMM, moderate excitatory responses were limited to the distal portion with inhibition predominating in the remaining proximal portion. 5-HT-induced inhibitory effects on field-stimulated tension responses were paralleled by relaxant effects on muscarinic agonist-induced tonic contractile responses, both of which were resistant to 5-HT-receptor antagonists including ketanserin, lysergic acid diethylamide (LSD), methysergide or methergoline. Field stimulation at a low frequency and pulse durations greater than 1.0 ms produced a relaxation response in preparations exposed to tetrodotoxin or hyoscine, provided that active muscle tonus was present. The relaxation in response to field stimulation was insensitive to antagonists of 5-HT, catecholamines, histamine, or indomethacin, suggesting a non-neurogenic origin. Histochemical examination of the isolated TMM preparation for cholinesterases revealed the presence of an extensive submucosal ganglionic plexus. It is concluded that: (i) intrinsic cholinergic neurones of the submucosal plexus form the final common pathway for extrinsic vagal and local (myenteric) projections to the TMM; (ii) the neural basis, if any, of non-cholinergic non-adrenergic inhibitory mechanisms remains to be established; (iii) the TMM may assist in generating propulsive oesophageal motility.
Full text
PDF


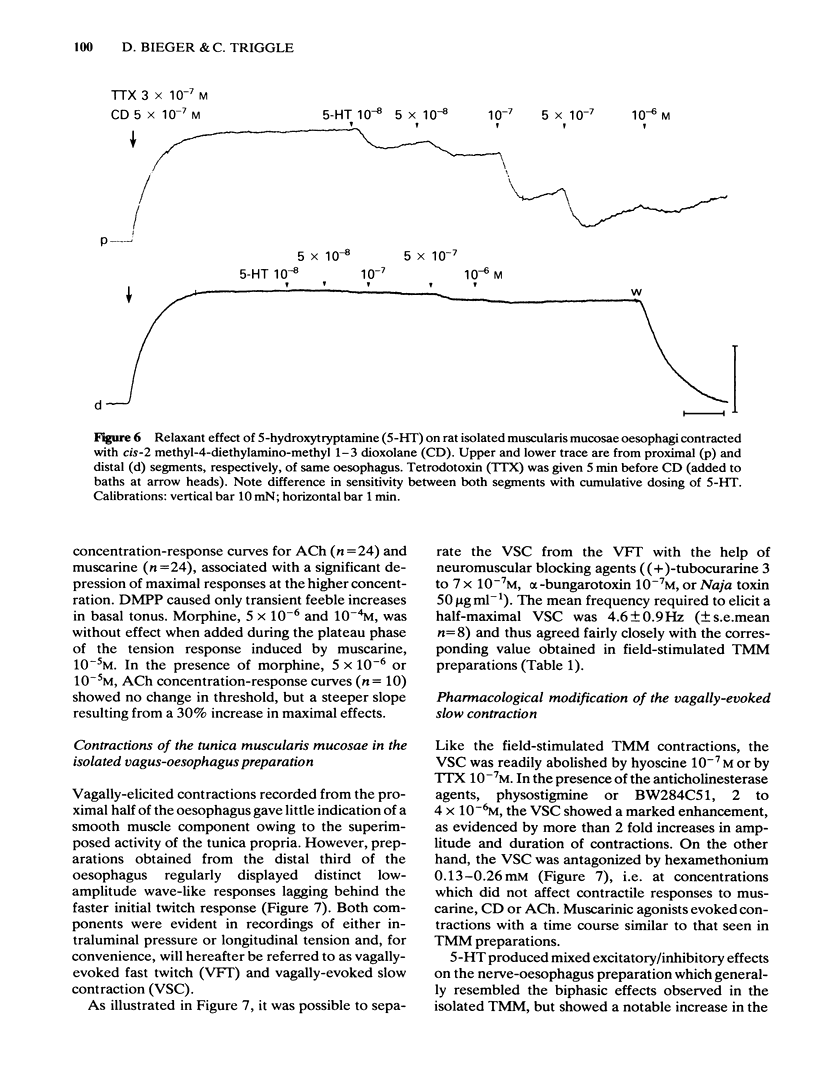
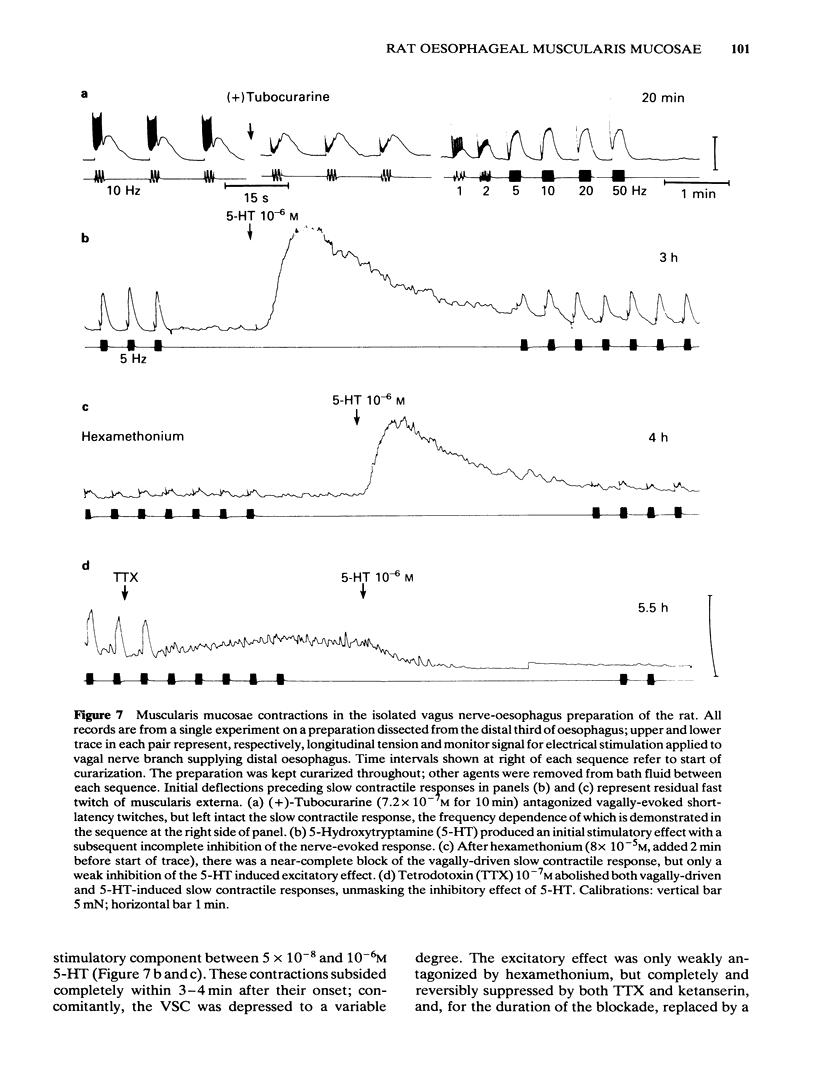
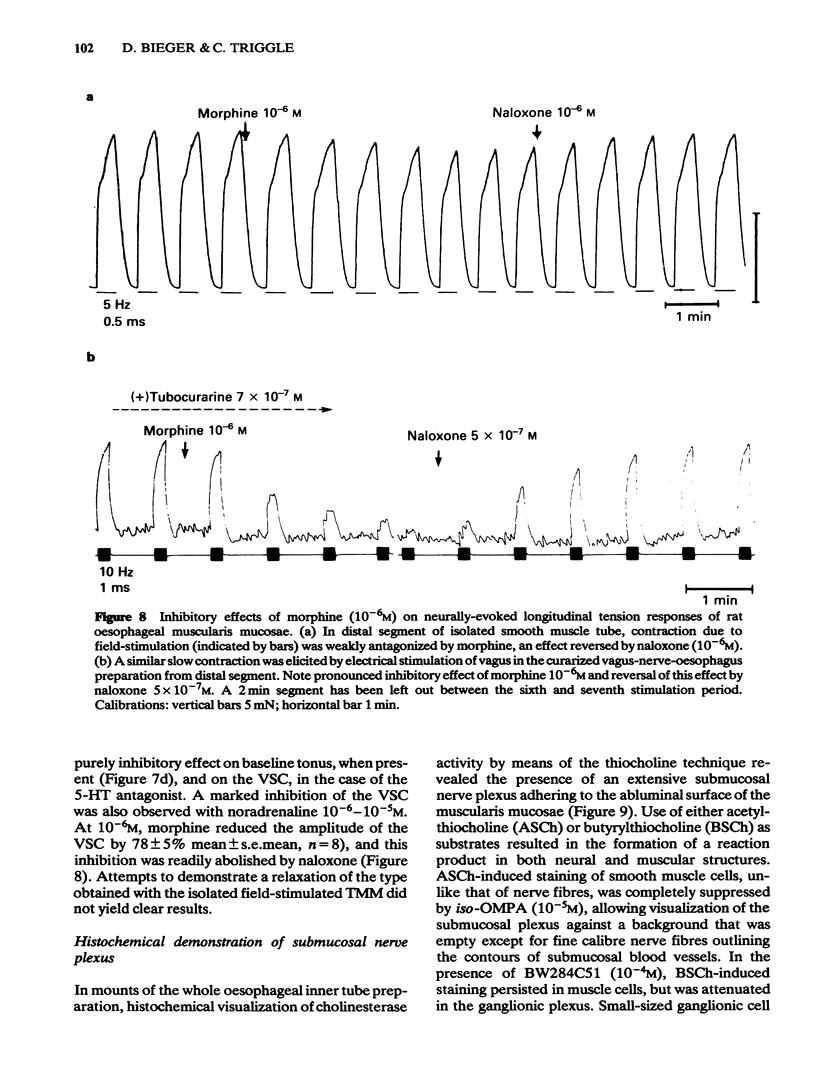
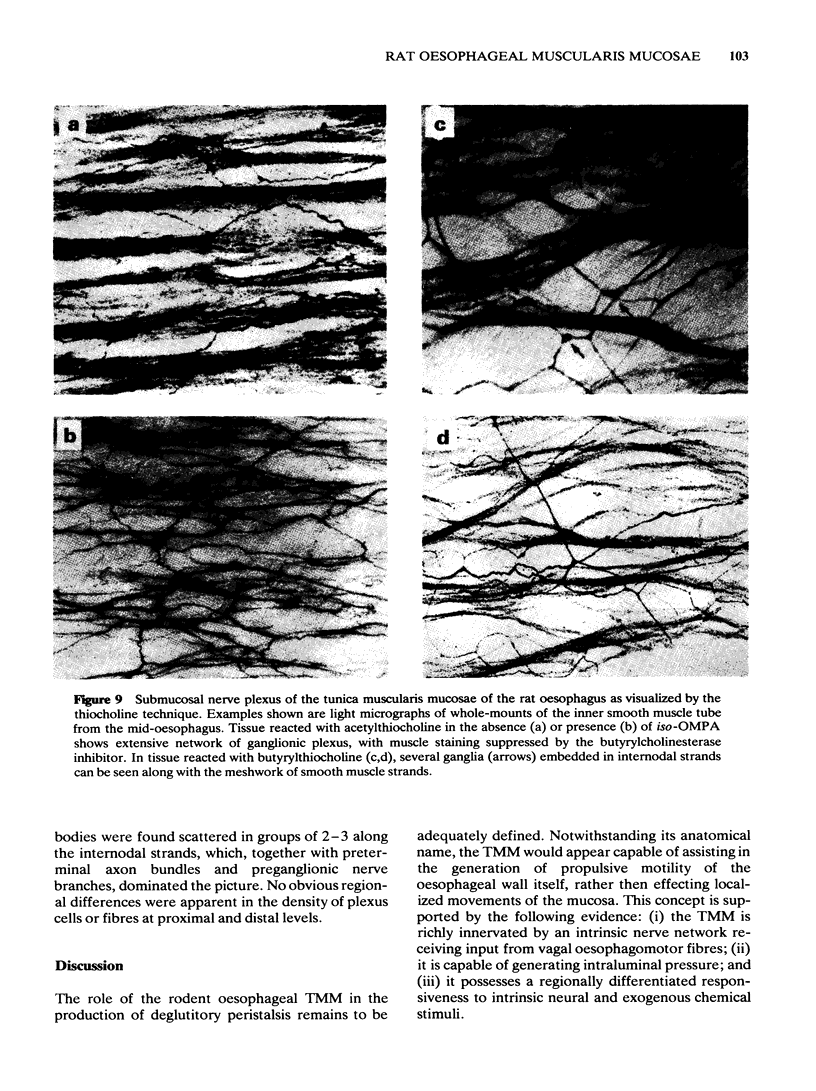



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALDRIDGE W. N. The differentiation of true and pseudo cholinesterase by organophosphorus compounds. Biochem J. 1953 Jan;53(1):62–67. doi: 10.1042/bj0530062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartlet A. L. Actions of 5-hydroxytryptamine and histamine on the neural structures and muscularis mucosae of the guinea-pig oesophagus. Br J Pharmacol Chemother. 1968 May;33(1):184–192. doi: 10.1111/j.1476-5381.1968.tb00487.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bieger D. Role of bulbar serotonergic neurotransmission in the initiation of swallowing in the rat. Neuropharmacology. 1981 Nov;20(11):1073–1083. doi: 10.1016/0028-3908(81)90099-x. [DOI] [PubMed] [Google Scholar]
- Chang K. J., Deth R. C., Triggle D. J. Structural parameters determining cholinergic and anticholinergic activities in a series of 1,3-dioxolanes. J Med Chem. 1972 Mar;15(3):243–247. doi: 10.1021/jm00273a010. [DOI] [PubMed] [Google Scholar]
- Christensen J. Pharmacology of the esophageal motor funciton. Annu Rev Pharmacol. 1975;15:243–258. doi: 10.1146/annurev.pa.15.040175.001331. [DOI] [PubMed] [Google Scholar]
- Decktor D. L., Ryan J. P. Transmembrane voltage of opossum esophageal smooth muscle and its response to electrical stimulation of intrinsic nerves. Gastroenterology. 1982 Feb;82(2):301–308. [PubMed] [Google Scholar]
- Ebeigbe A. B., Gantzos R. D., Webb R. C. Relaxation of rat tail artery to electrical stimulation. Life Sci. 1983 Jul 25;33(4):303–309. doi: 10.1016/s0024-3205(83)80001-0. [DOI] [PubMed] [Google Scholar]
- Goyal R. K., Gidda J. S. Relation between electrical and mechanical activity in esophageal smooth muscle. Am J Physiol. 1981 Apr;240(4):G305–G311. doi: 10.1152/ajpgi.1981.240.4.G305. [DOI] [PubMed] [Google Scholar]
- Kamikawa Y., Shimo Y. Cholinergic and adrenergic innervations of the muscularis mucosae in guinea-pig esophagus. Arch Int Pharmacodyn Ther. 1979 Apr;238(2):220–232. [PubMed] [Google Scholar]
- Kamikawa Y., Shimo Y. Indirect action of 5-hydroxytryptamine on the isolated muscularis mucosae of the guinea-pig oesophagus. Br J Pharmacol. 1983 Jan;78(1):103–110. doi: 10.1111/j.1476-5381.1983.tb09368.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamikawa Y., Shimo Y. Pharmacological characterization of the opioid receptor in the submucous plexus of the guinea-pig oesophagus. Br J Pharmacol. 1983 Apr;78(4):693–699. doi: 10.1111/j.1476-5381.1983.tb09422.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamikawa Y., Shimo Y., Uchida K. Inhibitory actions of catecholamines on electrically induced contractions of the submucous plexus-longitudinal muscularis mucosae preparation of the guinea-pig oesophagus. Br J Pharmacol. 1982 Jun;76(2):271–277. doi: 10.1111/j.1476-5381.1982.tb09217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. J. Deglutition. Physiol Rev. 1982 Jan;62(1):129–184. doi: 10.1152/physrev.1982.62.1.129. [DOI] [PubMed] [Google Scholar]
- Rooke T., Cohen R. A., Verbeuren T. J., Vanhoutte P. M. Non-neurogenic inhibitory effect of electrical impulses in isolated canine coronary arteries. Eur J Pharmacol. 1982 May 21;80(2-3):251–254. doi: 10.1016/0014-2999(82)90063-2. [DOI] [PubMed] [Google Scholar]
- Schultzberg M., Hökfelt T., Nilsson G., Terenius L., Rehfeld J. F., Brown M., Elde R., Goldstein M., Said S. Distribution of peptide- and catecholamine-containing neurons in the gastro-intestinal tract of rat and guinea-pig: immunohistochemical studies with antisera to substance P, vasoactive intestinal polypeptide, enkephalins, somatostatin, gastrin/cholecystokinin, neurotensin and dopamine beta-hydroxylase. Neuroscience. 1980;5(4):689–744. doi: 10.1016/0306-4522(80)90166-9. [DOI] [PubMed] [Google Scholar]
- Schulze K., Hajjar J. J., Christensen J. Regional differences in potassium content of smooth muscle from opossum esophagus. Am J Physiol. 1978 Dec;235(6):E709–E713. doi: 10.1152/ajpendo.1978.235.6.E709. [DOI] [PubMed] [Google Scholar]
- Shute C. C., Lewis P. R. The ascending cholinergic reticular system: neocortical, olfactory and subcortical projections. Brain. 1967 Sep;90(3):497–520. doi: 10.1093/brain/90.3.497. [DOI] [PubMed] [Google Scholar]
- Van Nueten J. M., Janssen P. A., Van Beek J., Xhonneux R., Verbeuren T. J., Vanhoutte P. M. Vascular effects of ketanserin (R 41 468), a novel antagonist of 5-HT2 serotonergic receptors. J Pharmacol Exp Ther. 1981 Jul;218(1):217–230. [PubMed] [Google Scholar]
- Wong D. T., Bymaster F. P., Horng J. S., Molloy B. B. A new selective inhibitor for uptake of serotonin into synaptosomes of rat brain: 3-(p-trifluoromethylphenoxy). N-methyl-3-phenylpropylamine. J Pharmacol Exp Ther. 1975 Jun;193(3):804–811. [PubMed] [Google Scholar]



