Abstract
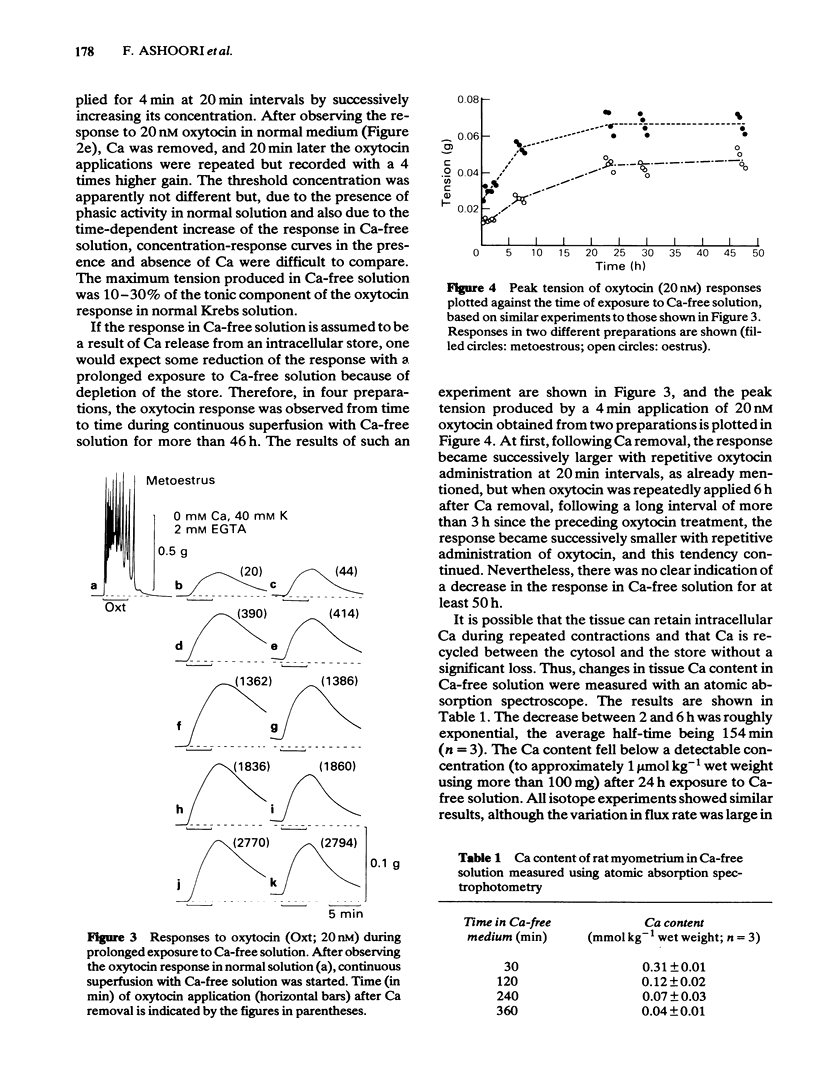
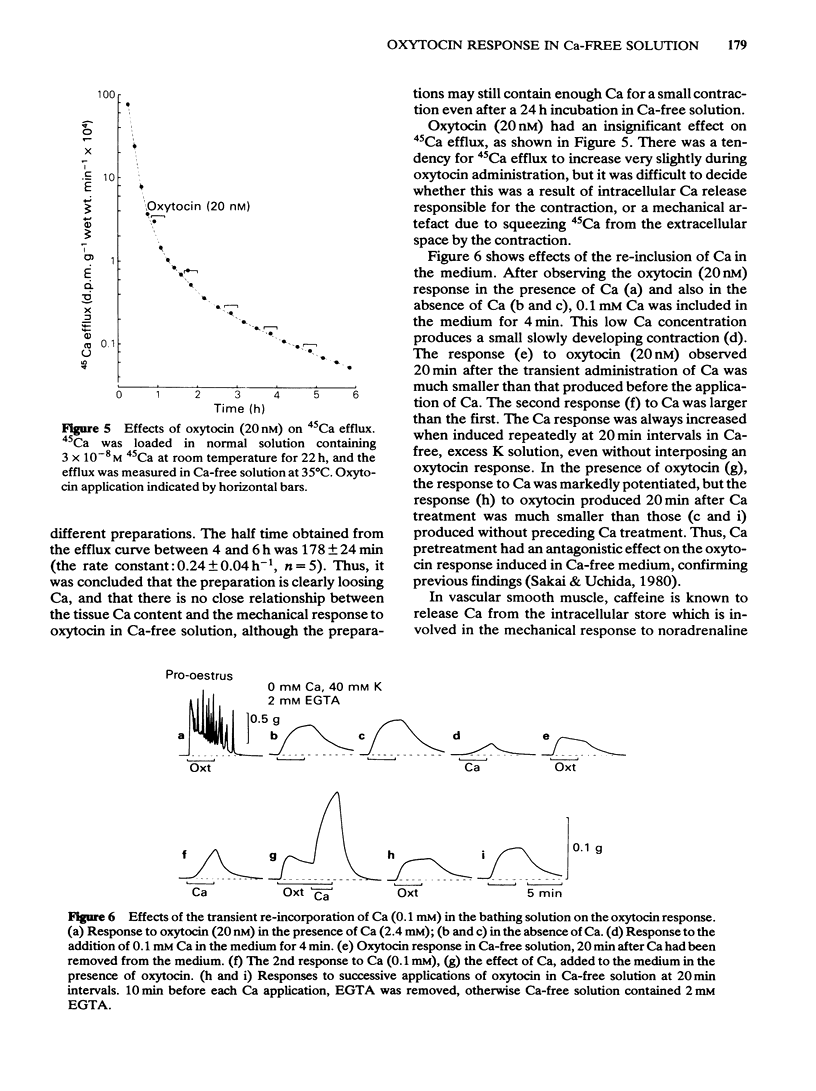
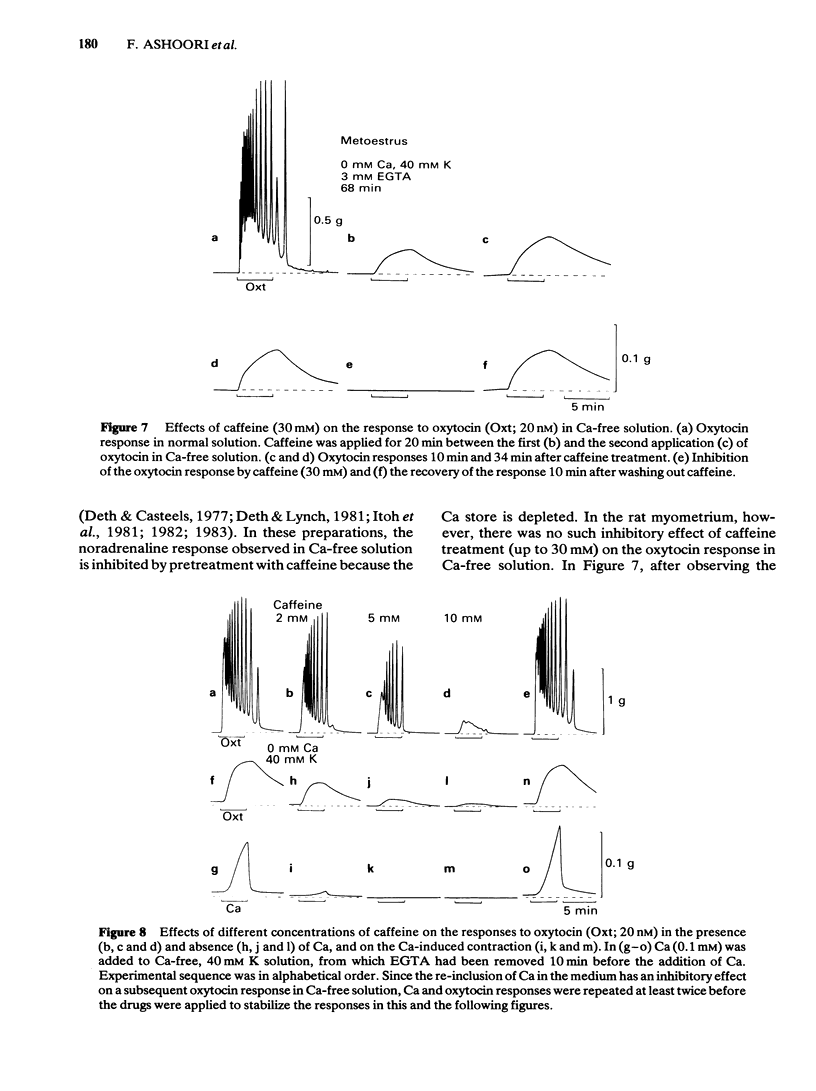
The contractile response of the longitudinal muscle of non-pregnant rat myometrium to oxytocin (0.2-20 nM) consisted of a phasic and a tonic component. Ca-removal abolished the phasic component but a tonic contraction could be evoked without reduction of amplitude for 50 h. Exceptionally, the tonic contraction also disappeared gradually in Ca-free medium containing 2 mM EGTA. When oxytocin was repeatedly applied in the absence of Ca, the response became at first progressively larger before reaching a steady state. Transient addition of Ca to the medium reduced the size of the subsequent oxytocin contraction. In Ca-free medium, the tissue lost Ca slowly, but it still contained 40 mumol kg-1 after 6 h and roughly 1 mumol kg-1 wet weight after 24 h exposure. 45Ca efflux was marginally increased by oxytocin (20 nM). Caffeine (5-30 mM) produced no contraction, but slightly reduced the resting tension and strongly inhibited the oxytocin response both in the presence and in the absence of Ca. Caffeine also blocked the contraction induced by Ca added to Ca-free 40 mM K solution. However, pretreatment with caffeine (30 mM) had no effect on the following oxytocin response. A calmodulin antagonist, trifluoperazine (1-10 microM) suppressed strongly the Ca-induced contraction, but had only a weak effect on the oxytocin response in Ca-free medium. Chlorpromazine (10-100 microM) and fluphenazine (10-30 microM) had similar effects.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano M., Suzuki Y., Hidaka H. Effects of various calmodulin antagonists on contraction of rabbit aortic strips. J Pharmacol Exp Ther. 1982 Jan;220(1):191–196. [PubMed] [Google Scholar]
- Ashoori F., Tomita T. Mechanical response to noradrenaline in calcium-free solution in the rat vas deferens. J Physiol. 1983 May;338:165–178. doi: 10.1113/jphysiol.1983.sp014667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casteels R., Raeymaekers L., Suzuki H., Van Eldere J. Tension response and 45Ca release in vascular smooth muscle incubated in Ca-free solution. Pflugers Arch. 1981 Dec;392(2):139–145. doi: 10.1007/BF00581262. [DOI] [PubMed] [Google Scholar]
- Conti M. A., Adelstein R. S. The relationship between calmodulin binding and phosphorylation of smooth muscle myosin kinase by the catalytic subunit of 3':5' cAMP-dependent protein kinase. J Biol Chem. 1981 Apr 10;256(7):3178–3181. [PubMed] [Google Scholar]
- Deth R. C., Lynch C. J. Mobilization of a common source of smooth muscle Ca2+ by norepinephrine and methylxanthines. Am J Physiol. 1981 May;240(5):C239–C247. doi: 10.1152/ajpcell.1981.240.5.C239. [DOI] [PubMed] [Google Scholar]
- Deth R., Casteels R. A study of releasable Ca fractions in smooth muscle cells of the rabbit aorta. J Gen Physiol. 1977 Apr;69(4):401–416. doi: 10.1085/jgp.69.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deth R., van Breemen C. Agonist induced release of intracellular Ca2+ in the rabbit aorta. J Membr Biol. 1977 Jan 28;30(4):363–380. doi: 10.1007/BF01869677. [DOI] [PubMed] [Google Scholar]
- Droogmans G., Raeymaekers L., Casteels R. Electro- and pharmacomechanical coupling in the smooth muscle cells of the rabbit ear artery. J Gen Physiol. 1977 Aug;70(2):129–148. doi: 10.1085/jgp.70.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fermum R., Meisel P., Klinner U. Versuche zum Wirkungsmechanismus von Gefässspasmolytika. 3. Wirkung von Nitroprussid-Natrium, Nitroglyzerin, Prenylamin und Verapamil an der Fluorid-induzierten Kontraktur isolierter Koronararterien. Acta Biol Med Ger. 1977;36(2):245–255. [PubMed] [Google Scholar]
- Hartshorne D. J., Siemankowski R. F. Regulation of smooth muscle actomyosin. Annu Rev Physiol. 1981;43:519–530. doi: 10.1146/annurev.ph.43.030181.002511. [DOI] [PubMed] [Google Scholar]
- Heaslip R. J., Rahwan R. G. Evidence for the existence of two distinct pools of intracellular calcium in the rat aorta accessible to mobilization by norepinephrine. J Pharmacol Exp Ther. 1982 Apr;221(1):7–13. [PubMed] [Google Scholar]
- Itoh T., Kajiwara M., Kitamura K., Kuriyama H. Roles of stored calcium on the mechanical response evoked in smooth muscle cells of the porcine coronary artery. J Physiol. 1982 Jan;322:107–125. doi: 10.1113/jphysiol.1982.sp014026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kuriyama H., Suzuki H. Differences and similarities in the noradrenaline- and caffeine-induced mechanical responses in the rabbit mesenteric artery. J Physiol. 1983 Apr;337:609–629. doi: 10.1113/jphysiol.1983.sp014645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh T., Kuriyama H., Suzuki H. Excitation--contraction coupling in smooth muscle cells of the guinea-pig mesenteric artery. J Physiol. 1981 Dec;321:513–535. doi: 10.1113/jphysiol.1981.sp014000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaki H., Murakami K., Nakagawa H., Ozaki H., Urakawa N. Effects of calmodulin antagonists on tension and cellular calcium content in depolarized vascular and intestinal smooth muscles. Br J Pharmacol. 1982 Dec;77(4):661–666. doi: 10.1111/j.1476-5381.1982.tb09344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osa T. The inhibitory action of caffeine on the smooth muscles of mouse myometrium and guinea pig ileum. Jpn J Physiol. 1973 Apr;23(2):199–216. doi: 10.2170/jjphysiol.23.199. [DOI] [PubMed] [Google Scholar]
- Rüegg J. C., Paul R. J. Vascular smooth muscle. Calmodulin and cyclic AMP-dependent protein kinase after calcium sensitivity in porcine carotid skinned fibers. Circ Res. 1982 Mar;50(3):394–399. doi: 10.1161/01.res.50.3.394. [DOI] [PubMed] [Google Scholar]
- Rüegg J. C., Sparrow M. P., Mrwa U. Cyclic-AMP mediated relaxation of chemically skinned fibers of smooth muscle. Pflugers Arch. 1981 May;390(2):198–201. doi: 10.1007/BF00590207. [DOI] [PubMed] [Google Scholar]
- Sakai K., Higuchi K., Yamaguchi T., Uchida M. Oxytocin-induced Ca-free contraction of rat uterine smooth muscle: effects of preincubation with EGTA and drugs. Gen Pharmacol. 1982;13(5):393–400. doi: 10.1016/0306-3623(82)90104-5. [DOI] [PubMed] [Google Scholar]
- Sakai K., Uchida M. A calcium reversal phenomenon: differentiation of excitatory and inhibitory roles of Ca in uterine smooth muscle contraction. Jpn J Pharmacol. 1980 Jun;30(3):394–396. doi: 10.1254/jjp.30.394. [DOI] [PubMed] [Google Scholar]
- Sakai K., Yamaguchi T., Uchida M. Oxytocin-induced Ca-free contraction of rat uterine smooth muscle: effects of divalent cations and drugs. Arch Int Pharmacodyn Ther. 1981 Mar;250(1):40–54. [PubMed] [Google Scholar]
- Somlyo A. P., Somlyo A. V., Shuman H., Endo M. Calcium and monovalent ions in smooth muscle. Fed Proc. 1982 Oct;41(12):2883–2890. [PubMed] [Google Scholar]
- van Breemen C., Siegel B. The mechanism of alpha-adrenergic activation of the dog coronary artery. Circ Res. 1980 Mar;46(3):426–429. doi: 10.1161/01.res.46.3.426. [DOI] [PubMed] [Google Scholar]


