Abstract
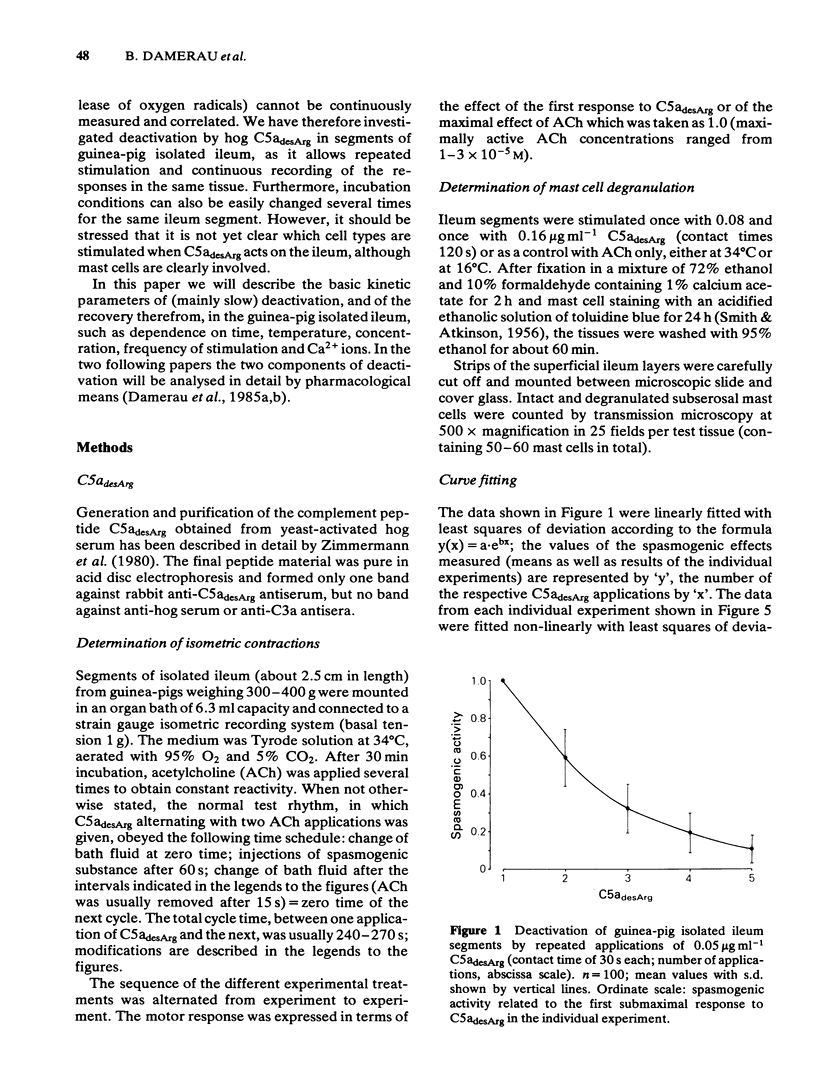
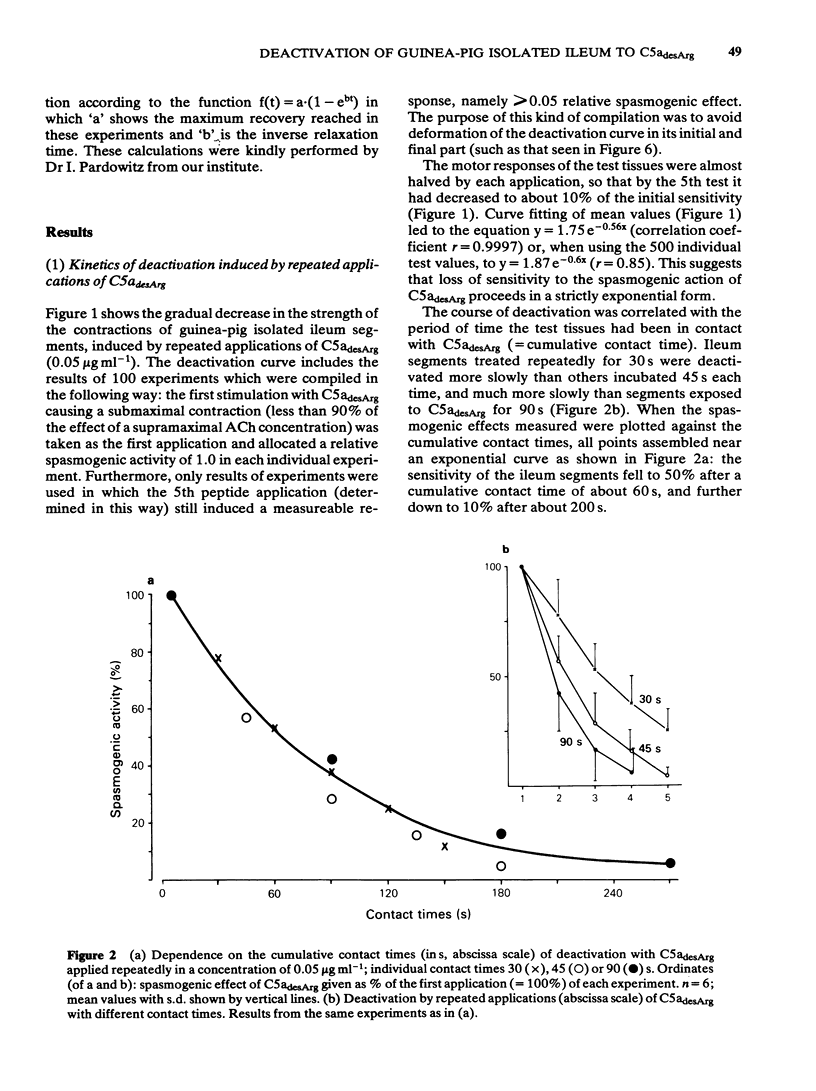
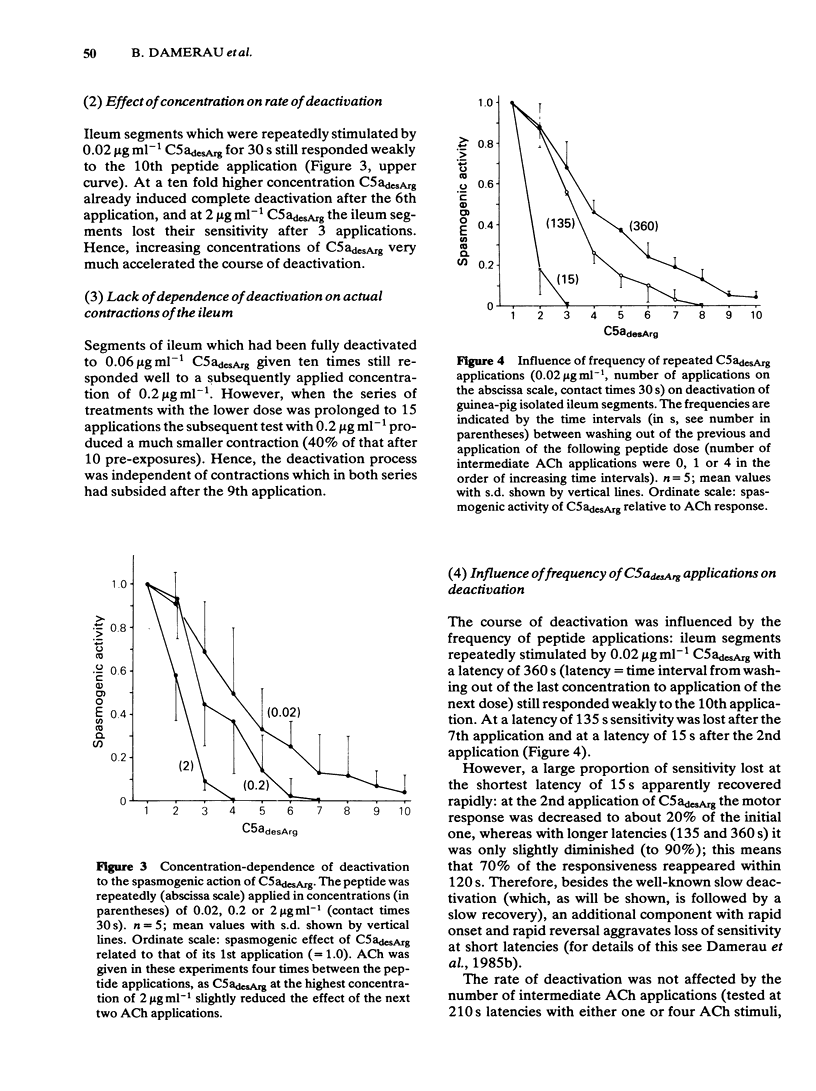
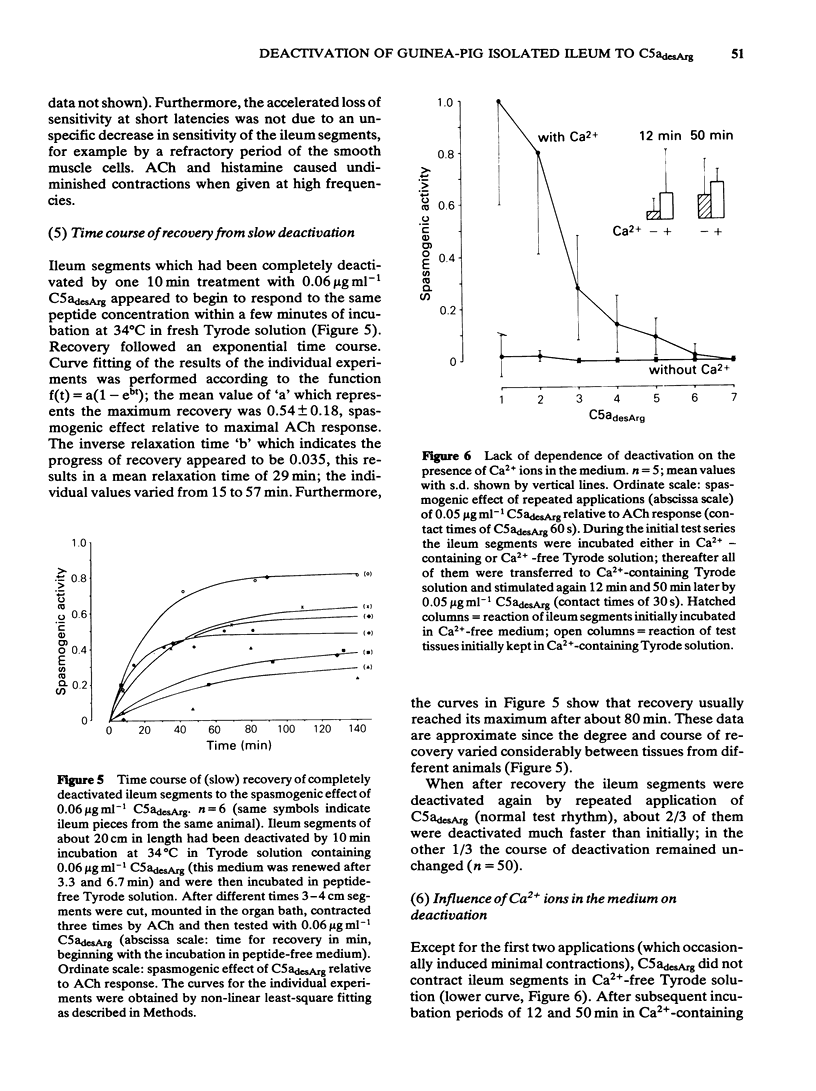
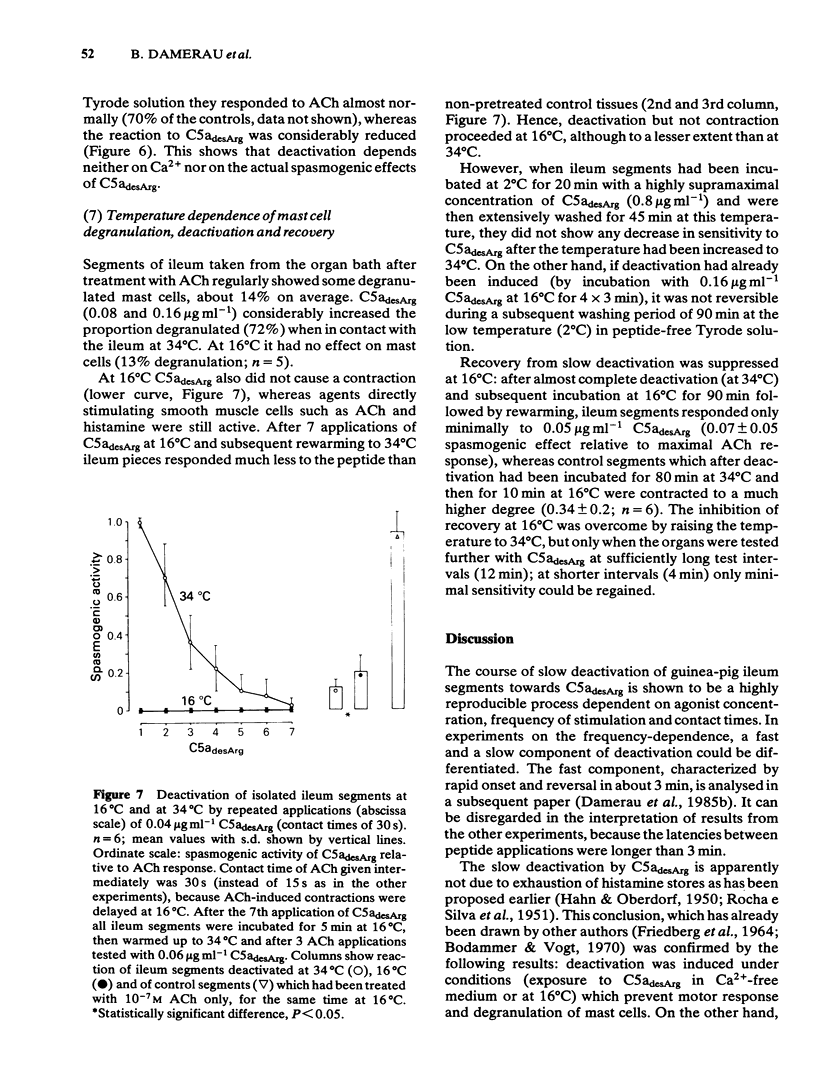
Deactivation (tachyphylaxis) of the guinea-pig isolated ileum to the spasmogenic action of the complement peptide C5adesArg was analysed. It appeared to consist of 2 components: a fast one, characterized by rapid onset of deactivation and by recovery within 2-3 min (see Damerau et al., 1985b), and a slow component, characterized by progressively increasing loss of sensitivity (until complete deactivation after several minutes) and by recovery within about 80 min. Slow deactivation shows an exponential time course; it is dependent on concentration as well as contact time with C5adesArg and occurs under conditions (incubation in Ca2+-free medium or at 16 degrees C) in which the peptide has no spasmogenic effect. Recovery from slow deactivation follows an exponential time course at 34 degrees C but is blocked at 16 degrees C; on average it reaches about half of the initial sensitivity. The results indicate that the slow deactivation is mainly due to blockade of C5a receptors by the ligand and is independent of the spasmogenic effect of C5adesArg.
Full text
PDF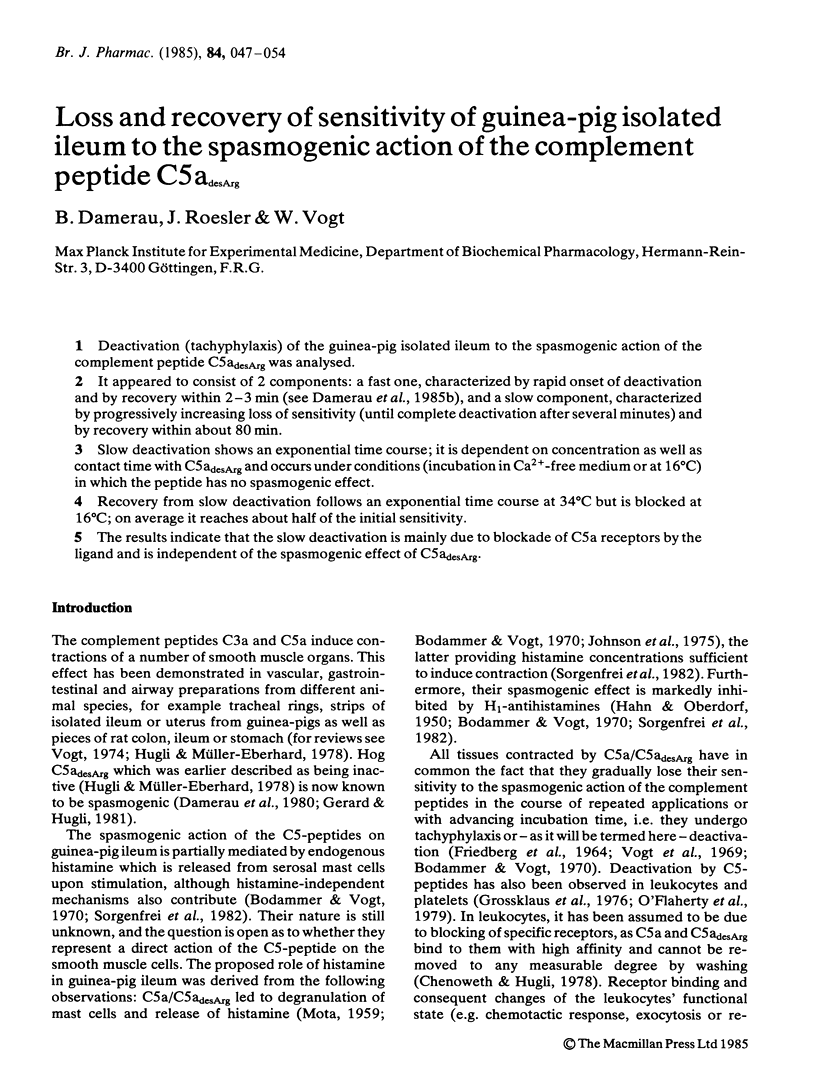


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodammer G., Vogt W. Contraction of the guinea-pig ileum induced by anaphylatoxin independent of histamine release. Int Arch Allergy Appl Immunol. 1970;39(5-6):648–657. doi: 10.1159/000230389. [DOI] [PubMed] [Google Scholar]
- Chenoweth D. E., Goodman M. G. The C5a receptor of neutrophils and macrophages. Agents Actions Suppl. 1983;12:252–273. doi: 10.1007/978-3-0348-9352-7_15. [DOI] [PubMed] [Google Scholar]
- Chenoweth D. E., Hugli T. E. Demonstration of specific C5a receptor on intact human polymorphonuclear leukocytes. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3943–3947. doi: 10.1073/pnas.75.8.3943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damerau B., Roesler J., Vogt W. Fast deactivation of guinea-pig isolated ileum to C5adesArg: a possible cyclic AMP-dependent mechanism. Br J Pharmacol. 1985 Jan;84(1):63–69. [PMC free article] [PubMed] [Google Scholar]
- Damerau B., Roesler J., Vogt W. Pharmacological characterization of the slow component of deactivation of guinea-pig isolated ileum to the spasmogenic action of C5adesArg. Br J Pharmacol. 1985 Jan;84(1):55–61. [PMC free article] [PubMed] [Google Scholar]
- Damerau B., Zimmermann B., Grünefeld E., Czorniak K., Vogt W. Biological activities of C5a and C5adesArg from hog serum. Int Arch Allergy Appl Immunol. 1980;63(4):408–414. doi: 10.1159/000232656. [DOI] [PubMed] [Google Scholar]
- FRIEDBERG K. D., ENGELHARDT G., MEINEKE F. UNTERSUCHUNGEN UEBER DIE ANAPHYLATOXIN-TACHYPHYLAXIE UND UEBER IHRE BEDEUTUNG FUER DEN ABLAUF ECHTER ANAPHYLAKTISCHER REAKTIONEN. Int Arch Allergy Appl Immunol. 1964;25:154–181. [PubMed] [Google Scholar]
- Gerard C., Hugli T. E. Identification of classical anaphylatoxin as the des-Arg form of the C5a molecule: evidence of a modulator role for the oligosaccharide unit in human des-Arg74-C5a. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1833–1837. doi: 10.1073/pnas.78.3.1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossklaus C., Damerau B., Lemgo E., Vogt W. Induction of platelet aggregation by the complement-derived peptides C3a and C5a. Naunyn Schmiedebergs Arch Pharmacol. 1976 Oct;295(1):71–76. doi: 10.1007/BF00509775. [DOI] [PubMed] [Google Scholar]
- Hugli T. E., Müller-Eberhard H. J. Anaphylatoxins: C3a and C5a. Adv Immunol. 1978;26:1–53. doi: 10.1016/s0065-2776(08)60228-x. [DOI] [PubMed] [Google Scholar]
- Johnson A. R., Hugli T. E., Müller-Eberhard H. J. Release of histamine from rat mast cells by the complement peptides C3a and C5a. Immunology. 1975 Jun;28(6):1067–1080. [PMC free article] [PubMed] [Google Scholar]
- MOTA I. The mechanism of action of anaphylatoxin. Its effect on guinea pig mast cells. Immunology. 1959 Oct;2:403–413. [PMC free article] [PubMed] [Google Scholar]
- Marshall S., Olefsky J. M. Tris (hydroxmethyl) aminomethane permits the expression of insulin-induced receptor loss in isolated rat adipocytes. Biochem Biophys Res Commun. 1981 Sep 30;102(2):646–653. doi: 10.1016/s0006-291x(81)80181-7. [DOI] [PubMed] [Google Scholar]
- Niedel J., Wilkinson S., Cuatrecasas P. Receptor-mediated uptake and degradation of 125I-chemotactic peptide by human neutrophils. J Biol Chem. 1979 Nov 10;254(21):10700–10706. [PubMed] [Google Scholar]
- O'Flaherty J. T., Showell H. S., Becker E. L., Ward P. A. Neutrophil aggregation: effect of the time and sequence of addition of calcium, magnesium and chemotactic factor. J Reticuloendothel Soc. 1979 Jan;25(1):29–38. [PubMed] [Google Scholar]
- Rossi F., De Togni P., Bellavite P., Della Bianca V., Grzeskowiak M. Relationship between the binding of N-formylmethionylleucylphenylalanine and the respiratory response in human neutrophils. Biochim Biophys Acta. 1983 Jul 29;758(2):168–175. doi: 10.1016/0304-4165(83)90298-2. [DOI] [PubMed] [Google Scholar]
- SMITH E. W., ATKINSON W. B. Simple procedure for identification and rapid counting of mast cells in tissue sections. Science. 1956 May 25;123(3204):941–942. doi: 10.1126/science.123.3204.941. [DOI] [PubMed] [Google Scholar]
- Seligmann B. E., Fletcher M. P., Gallin J. I. Adaptation of human neutrophil responsiveness to the chemoattractant N-formylmethionylleucylphenylalanine. Heterogeneity and/or negative cooperative interaction of receptors. J Biol Chem. 1982 Jun 10;257(11):6280–6286. [PubMed] [Google Scholar]
- Sklar L. A., McNeil V. M., Jesaitis A. J., Painter R. G., Cochrane C. G. A continuous, spectroscopic analysis of the kinetics of elastase secretion by neutrophils. The dependence of secretion upon receptor occupancy. J Biol Chem. 1982 May 25;257(10):5471–5475. [PubMed] [Google Scholar]
- Sorgenfrei J., Damerau B., Vogt W. Role of histamine in the spasmogenic effect of the complement peptides C3a and C5a-desArg (classical anaphylatoxin). Agents Actions. 1982 Apr;12(1-2):118–121. doi: 10.1007/BF01965121. [DOI] [PubMed] [Google Scholar]
- Sullivan S. J., Zigmond S. H. Chemotactic peptide receptor modulation in polymorphonuclear leukocytes. J Cell Biol. 1980 Jun;85(3):703–711. doi: 10.1083/jcb.85.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt W. Activation, activities and pharmacologically active products of complement. Pharmacol Rev. 1974 Jun;26(2):125–169. [PubMed] [Google Scholar]
- Vogt W., Zeman N., Garbe G. Histaminunabhängige Wirkungen von Anaphylatoxin auf glatte Muskulatur isolierter Organe. Naunyn Schmiedebergs Arch Exp Pathol Pharmakol. 1969;262(4):399–404. [PubMed] [Google Scholar]
- Weinberg J. B., Muscato J. J., Niedel J. E. Monocyte chemotactic peptide receptor. Functional characteristics and ligand-induced regulation. J Clin Invest. 1981 Sep;68(3):621–630. doi: 10.1172/JCI110296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond S. H. Consequences of chemotactic peptide receptor modulation for leukocyte orientation. J Cell Biol. 1981 Mar;88(3):644–647. doi: 10.1083/jcb.88.3.644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond S. H., Sullivan S. J., Lauffenburger D. A. Kinetic analysis of chemotactic peptide receptor modulation. J Cell Biol. 1982 Jan;92(1):34–43. doi: 10.1083/jcb.92.1.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann B., Damerau B., Vogt W. Purification and partial amino acid sequence of classical anaphylatoxin from pig serum: identification with Des-Arg-C5a. Hoppe Seylers Z Physiol Chem. 1980;361(6):915–924. doi: 10.1515/bchm2.1980.361.1.915. [DOI] [PubMed] [Google Scholar]


