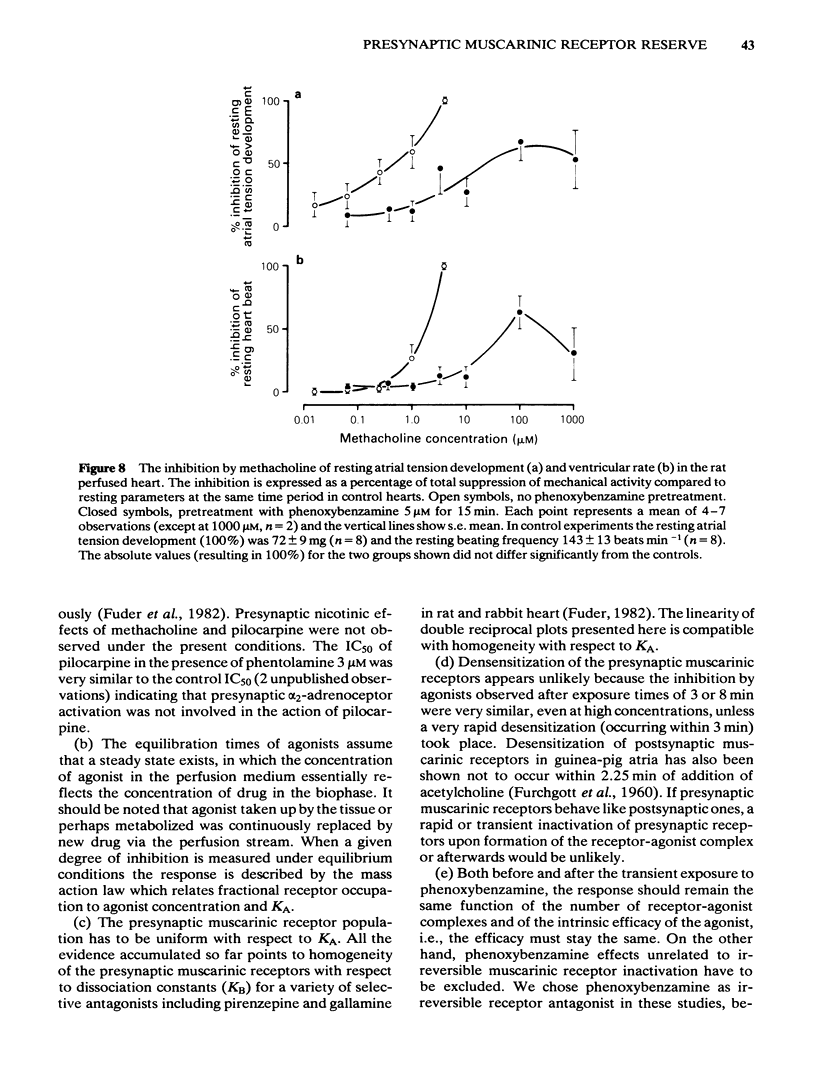
Abstract
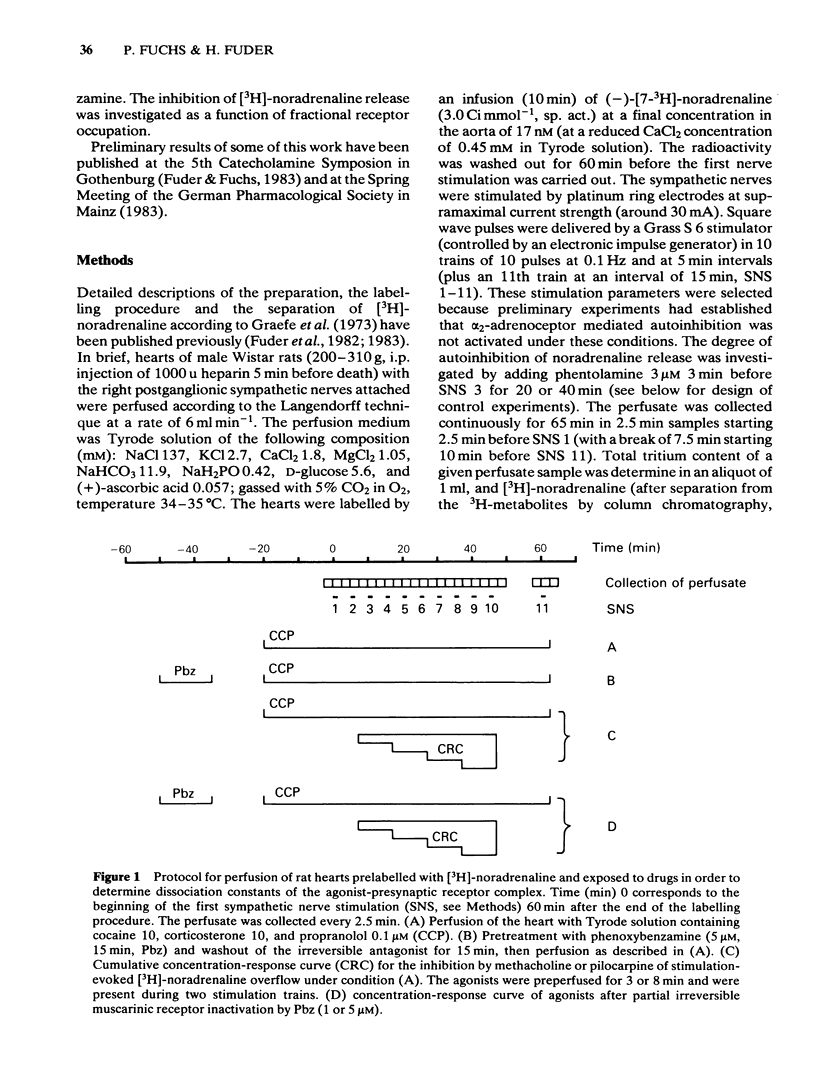
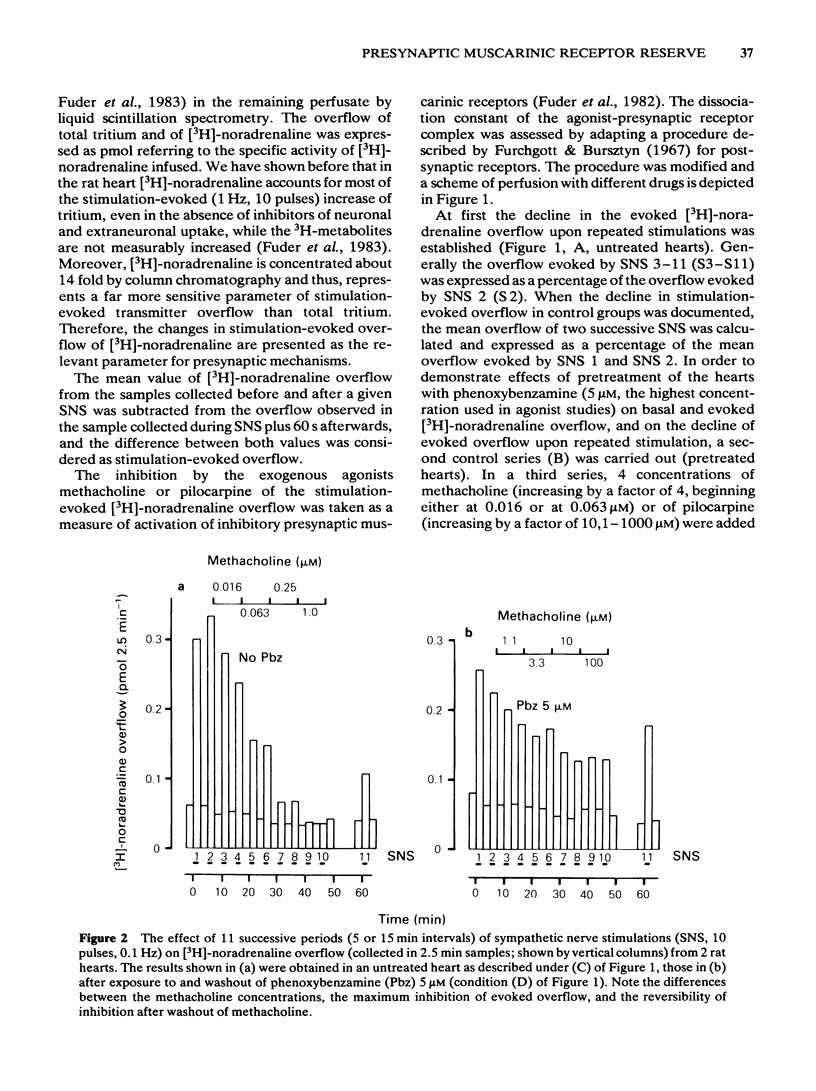
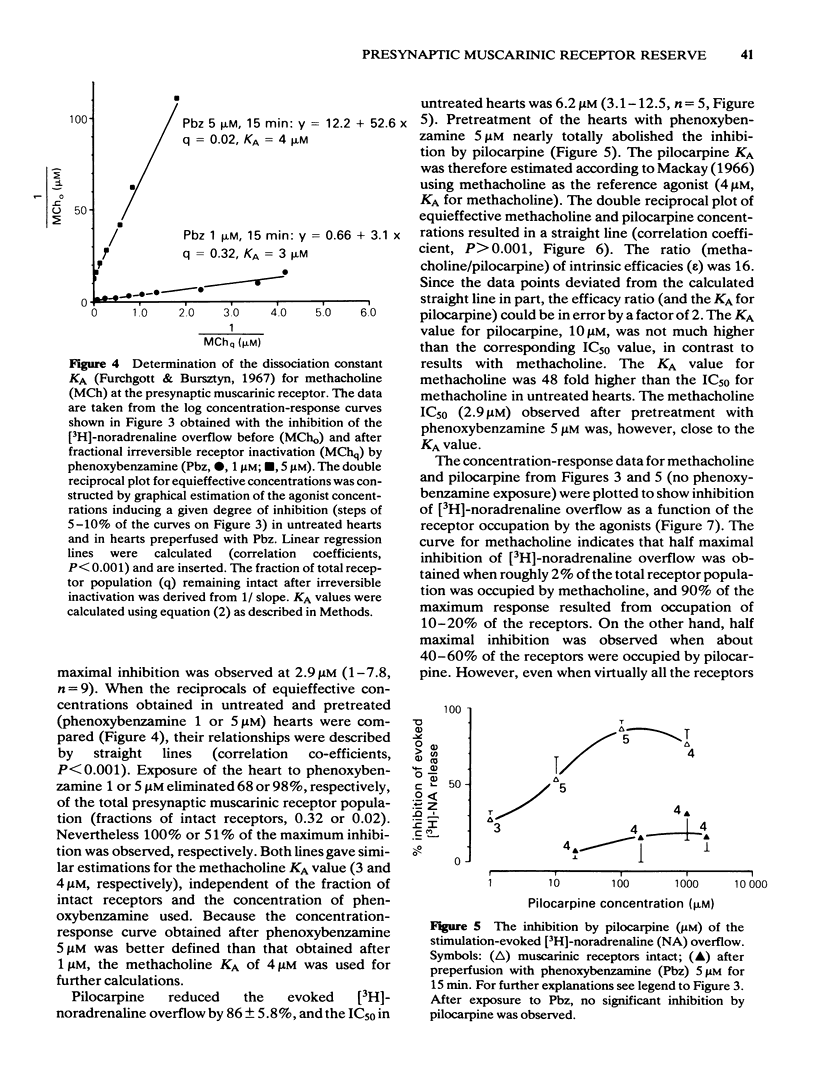
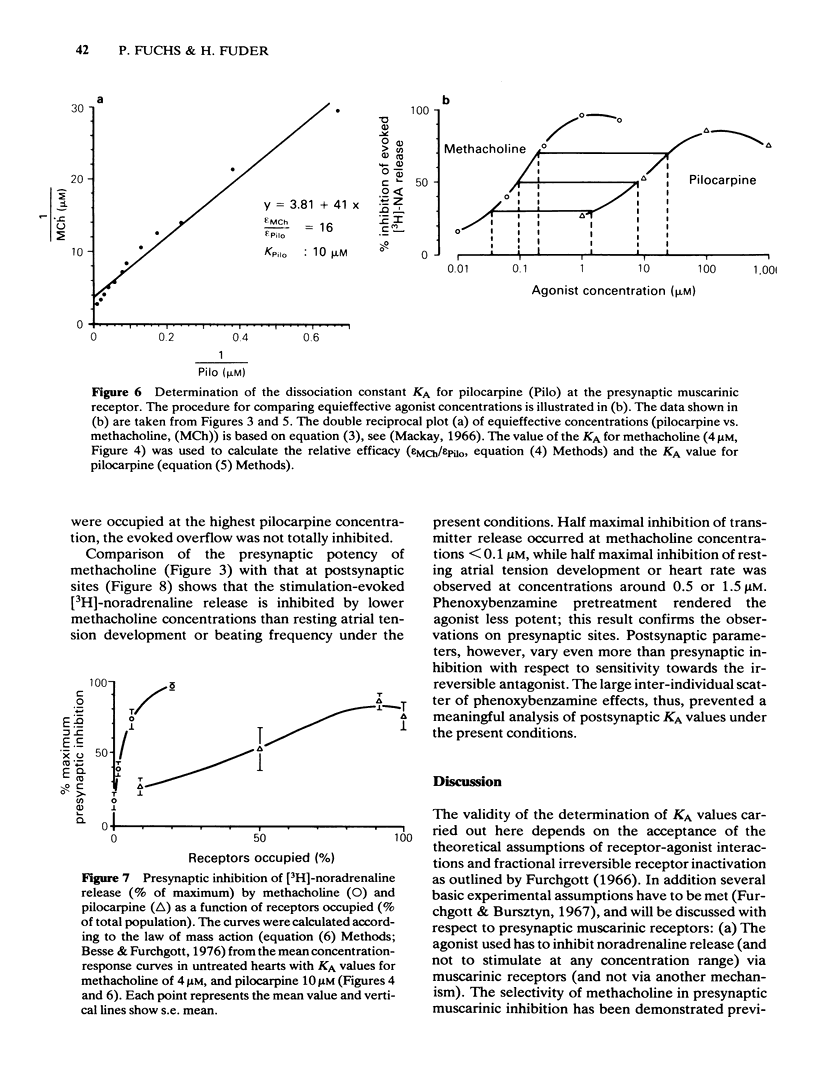
Rat isolated perfused hearts with the right sympathetic nerves attached were loaded with [3H]-noradrenaline. The nerves were stimulated with up to 11 trains of 10 pulses at 0.1 Hz. The evoked increases of [3H]-noradrenaline overflow into the perfusate were measured in the presence of cocaine, corticosterone and propranolol. Activation of presynaptic muscarinic receptors by methacholine or pilocarpine inhibited the evoked transmitter release in a reversible and concentration-dependent manner. Preperfusion with phenoxybenzamine (5 microM) for 15 min (followed by a washout of 35 min) changed neither resting nor evoked overflow of [3H]-noradrenaline. The concentration-response curve of methacholine was shifted to the right after exposure of the hearts to phenoxybenzamine (1 microM) without depression of the maximum effect. Pretreatment with phenoxybenzamine (5 microM) reduced the maximum inhibition of release by about 50%. Analysis of the data gave a dissociation constant for the agonist-receptor complex (KA) of 4.0 microM and a receptor reserve of roughly 70%. Half-maximal inhibition of [3H]-noradrenaline release occurred when about 2% of the total receptor population was occupied. Comparison of the concentration-response data for methacholine and pilocarpine revealed a relative efficacy (methacholine/pilocarpine) of 16, a KA of 10 microM for pilocarpine and no receptor reserve for this agonist. The results show that KA values for methacholine and pilocarpine obtained at presynaptic receptors are similar to those obtained at postsynaptic muscarinic receptors. This is in agreement with the idea that muscarinic receptors located on postganglionic adrenergic nerves are not different from those located on effector sites of non-neuronal tissue.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akesson C., Swanson C., Patil P. N. Muscarinic receptors of rabbit irides. Naunyn Schmiedebergs Arch Pharmacol. 1983 Mar;322(2):104–110. doi: 10.1007/BF00512382. [DOI] [PubMed] [Google Scholar]
- Besse J. C., Furchgott R. F. Dissociation constants and relative efficacies of agonists acting on alpha adrenergic receptors in rabbit aorta. J Pharmacol Exp Ther. 1976 Apr;197(1):66–78. [PubMed] [Google Scholar]
- Birdsall N. J., Burgen A. S., Hulme E. C. The binding of agonists to brain muscarinic receptors. Mol Pharmacol. 1978 Sep;14(5):723–736. [PubMed] [Google Scholar]
- El-Fakahany E., Richelson E. Phenoxybenzamine and dibenamine interactions with calcium channel effectors of the muscarinic receptor. Mol Pharmacol. 1981 Nov;20(3):519–525. [PubMed] [Google Scholar]
- FURCHGOTT R. F., SLEATOR W., Jr, DE GUBAREFF T. Effects of acetylcholine and epinephrine on the contractile strength and action potential of electrically driven guinea pig atria. J Pharmacol Exp Ther. 1960 Aug;129:405–416. [PubMed] [Google Scholar]
- Fuder H., Muscholl E., Spemann R. The determination of presynaptic pA2 values of yohimbine and phentolamine on the perfused rat heart under conditions of negligible autoinhibition. Br J Pharmacol. 1983 May;79(1):109–119. doi: 10.1111/j.1476-5381.1983.tb10502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuder H., Rink D., Muscholl E. Sympathetic Nerve Stimulation on the perfused rat heart. Affinities of N-methylatropine and pirenzepine at pre- and postsynaptic muscarine receptors. Naunyn Schmiedebergs Arch Pharmacol. 1982 Feb;318(3):210–219. doi: 10.1007/BF00500482. [DOI] [PubMed] [Google Scholar]
- Fuder H. The affinity of pirenzepine and other antimuscarinic compounds for pre- and postsynaptic muscarine receptors of the isolated rabbit and rat heart. Scand J Gastroenterol Suppl. 1982;72:79–85. [PubMed] [Google Scholar]
- Graffe K. H., Stefano F. J., Langer S. Z. Preferential metabolism of (-) 3 H-norepinephrine through the deaminated glycol in the rat vas deferens. Biochem Pharmacol. 1973 May 15;22(10):1147–1160. doi: 10.1016/0006-2952(73)90231-1. [DOI] [PubMed] [Google Scholar]
- Mackay D. A general analysis of the receptor-drug interaction. Br J Pharmacol Chemother. 1966 Jan;26(1):9–16. doi: 10.1111/j.1476-5381.1966.tb01805.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscholl E., Muth A. The effect of physostigmine on the vagally induced muscarinic inhibition of noradrenaline release from the isolated perfused rabbit atria. Naunyn Schmiedebergs Arch Pharmacol. 1982 Aug;320(2):160–169. doi: 10.1007/BF00506316. [DOI] [PubMed] [Google Scholar]
- Nordström O., Alberts P., Westlind A., Undén A., Bartfai T. Presynaptic antagonist-postsynaptic agonist at muscarinic cholinergic synapses. N-methyl-N-(1-methyl-4-pyrrolidino-2-butynyl)acetamide. Mol Pharmacol. 1983 Jul;24(1):1–5. [PubMed] [Google Scholar]
- Starke K. Alpha-adrenoceptor subclassification. Rev Physiol Biochem Pharmacol. 1981;88:199–236. [PubMed] [Google Scholar]
- Wallenstein S., Zucker C. L., Fleiss J. L. Some statistical methods useful in circulation research. Circ Res. 1980 Jul;47(1):1–9. doi: 10.1161/01.res.47.1.1. [DOI] [PubMed] [Google Scholar]
- Waud D. R. On the measurement of the affinity of partial agonists for receptors. J Pharmacol Exp Ther. 1969 Nov;170(1):117–122. [PubMed] [Google Scholar]
- Waud D. R. Pharmacological receptors. Pharmacol Rev. 1968 Jun;20(2):49–88. [PubMed] [Google Scholar]
- Weitzell R., Tanaka T., Starke K. Pre- and postsynaptic effects of yohimbine stereoisomers on noradrenergic transmission in the pulmonary artery of the rabbit. Naunyn Schmiedebergs Arch Pharmacol. 1979 Aug;308(2):127–136. doi: 10.1007/BF00499054. [DOI] [PubMed] [Google Scholar]


