Abstract
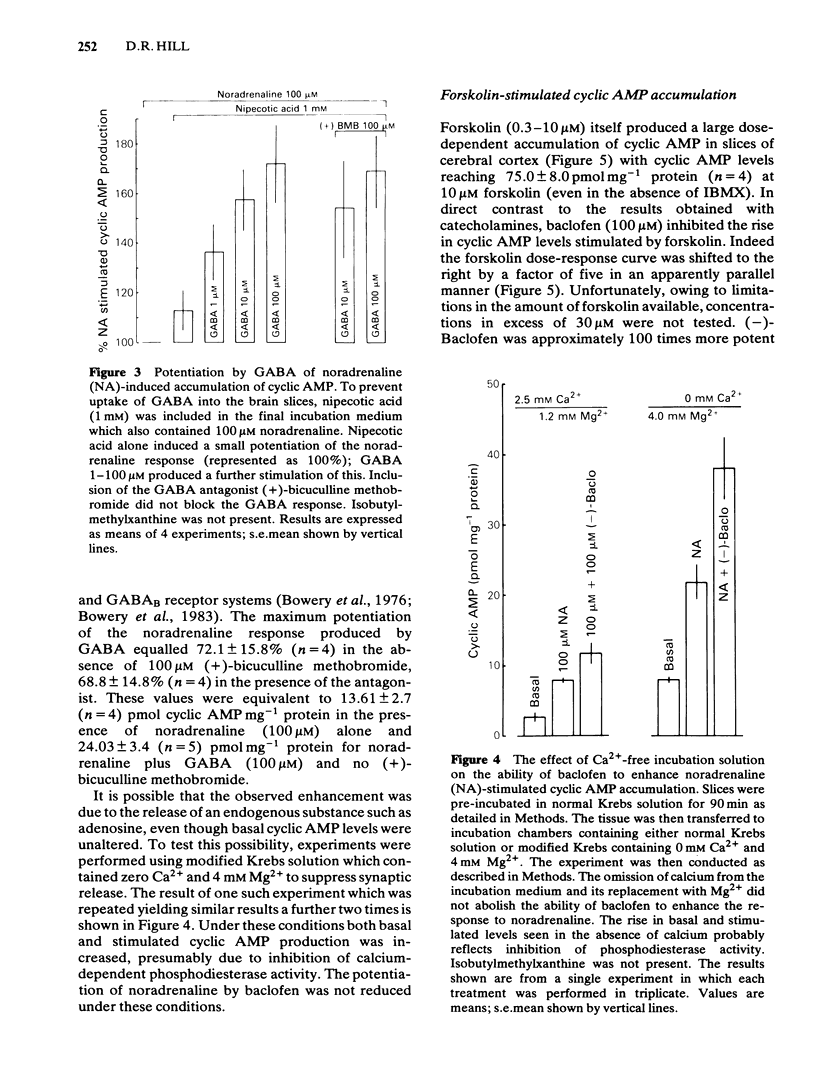
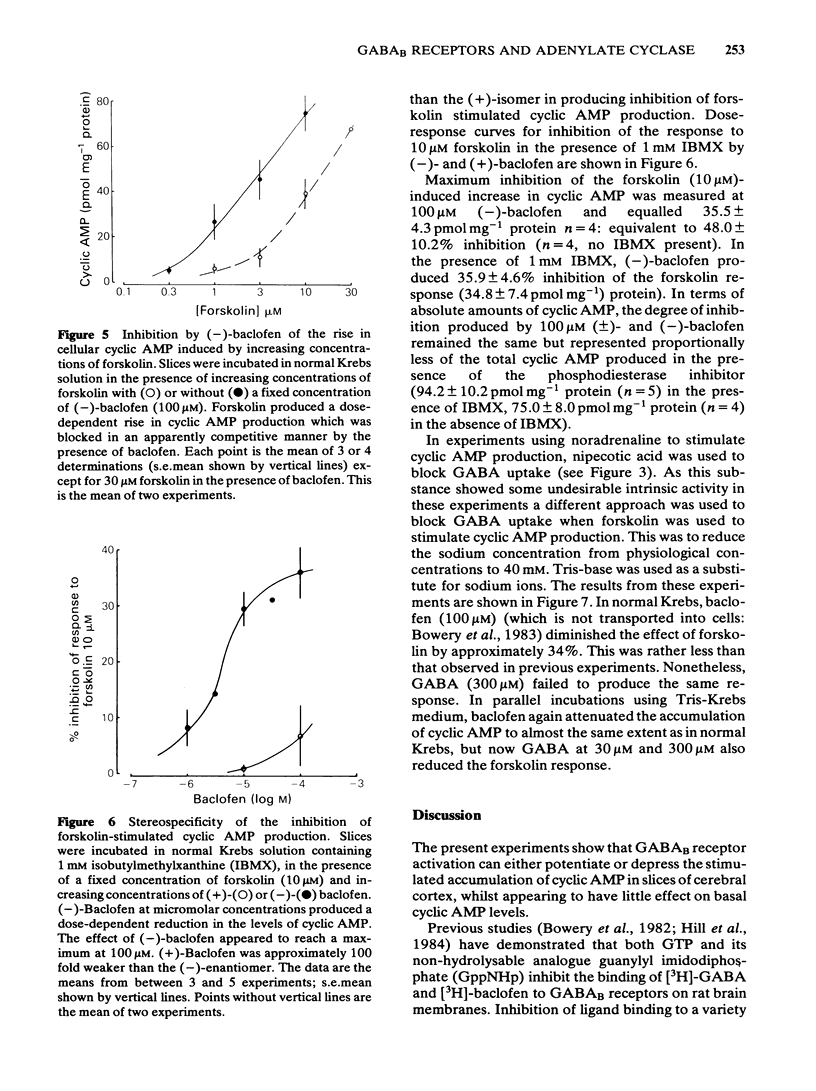
An investigation of the effects of gamma-aminobutyric acid (GABA) and the selective GABAB receptor agonist, baclofen, on basal and stimulated adenosine 3':5'-cyclic monophosphate (cyclic AMP) levels in slices of rat cerebral cortex has been carried out. Neither GABA nor baclofen produced any significant change in basal cyclic AMP levels. By contrast noradrenaline and forskolin both produced dose-dependent increases in cellular cyclic AMP accumulation. GABA (in the presence of nipecotic acid) and baclofen both potentiated the maximal response to noradrenaline with baclofen (100 microM) increasing the level of cyclic AMP produced by noradrenaline (100 microM) by 133%. GABA (0.3-100 microM) was rather less effective than baclofen, increasing the response to noradrenaline by 70% at 100 microM. (-)-Baclofen was the active isomer with (+)-baclofen failing to potentiate noradrenaline responses. Bicuculline-methobromide (100 microM) failed to block the action of either GABA or baclofen. The enhancement of adrenoceptor-stimulated cyclic AMP accumulation persisted in the presence of a phosphodiesterase inhibitor (1 mM 3-isobutyl-1-methylxanthine) and also in Ca2+-free solution. When forskolin was used to stimulate adenylate cyclase, the effect of baclofen was to inhibit the rise in cyclic AMP levels. Thus (-)-baclofen (100 microM) shifted the dose-response curve to forskolin to the right 5 fold in an apparently parallel fashion. The effect was again stereospecific for the (-)-isomer of baclofen. When GABA uptake was reduced using low sodium (40 mM) incubation medium, GABA also attenuated the rise in cyclic AMP induced by 10 microM forskolin. GABA produced little effect in normal Krebs solution.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowery N. G., Hill D. R., Hudson A. L. Characteristics of GABAB receptor binding sites on rat whole brain synaptic membranes. Br J Pharmacol. 1983 Jan;78(1):191–206. doi: 10.1111/j.1476-5381.1983.tb09380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery N. G., Hill D. R., Hudson A. L., Doble A., Middlemiss D. N., Shaw J., Turnbull M. (-)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature. 1980 Jan 3;283(5742):92–94. doi: 10.1038/283092a0. [DOI] [PubMed] [Google Scholar]
- Brown B. L., Albano J. D., Ekins R. P., Sgherzi A. M. A simple and sensitive saturation assay method for the measurement of adenosine 3':5'-cyclic monophosphate. Biochem J. 1971 Feb;121(3):561–562. doi: 10.1042/bj1210561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherubini E., North R. A. Inhibition of calcium spikes and transmitter release by gamma-aminobutyric acid in the guinea-pig myenteric plexus. Br J Pharmacol. 1984 May;82(1):101–105. doi: 10.1111/j.1476-5381.1984.tb16446.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Felix D., Johnston G. A. Bicuculline, an antagonist of GABA and synaptic inhibition in the spinal cord of the cat. Brain Res. 1971 Sep 10;32(1):69–96. doi: 10.1016/0006-8993(71)90156-9. [DOI] [PubMed] [Google Scholar]
- Curtis D. R., Duggan A. W., Felix D., Johnston G. A. GABA, bicuculline and central inhibition. Nature. 1970 Jun 27;226(5252):1222–1224. doi: 10.1038/2261222a0. [DOI] [PubMed] [Google Scholar]
- Daly J. W. Adenosine receptors: targets for future drugs. J Med Chem. 1982 Mar;25(3):197–207. doi: 10.1021/jm00345a001. [DOI] [PubMed] [Google Scholar]
- Daly J. W., Padgett W., Nimitkitpaisan Y., Creveling C. R., Cantacuzene D., Kirk K. L. Fluoronorepinephrines: specific agonists for the activation of alpha and beta adrenergic-sensitive cyclic AMP-generating systems in brain slices. J Pharmacol Exp Ther. 1980 Mar;212(3):382–389. [PubMed] [Google Scholar]
- Dunlap K. Two types of gamma-aminobutyric acid receptor on embryonic sensory neurones. Br J Pharmacol. 1981 Nov;74(3):579–585. doi: 10.1111/j.1476-5381.1981.tb10467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Désarmenien M., Feltz P., Occhipinti G., Santangelo F., Schlichter R. Coexistence of GABAA and GABAB receptors on A delta and C primary afferents. Br J Pharmacol. 1984 Feb;81(2):327–333. doi: 10.1111/j.1476-5381.1984.tb10082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredholm B. B., Jonzon B., Lindgren E., Lindström K. Adenosine receptors mediating cyclic AMP production in the rat hippocampus. J Neurochem. 1982 Jul;39(1):165–175. doi: 10.1111/j.1471-4159.1982.tb04715.x. [DOI] [PubMed] [Google Scholar]
- Gold M. R., Martin A. R. gamma-Aminobutyric acid and glycine activate Cl- channels having different characteristics in CNS neurones. Nature. 1984 Apr 12;308(5960):639–641. doi: 10.1038/308639a0. [DOI] [PubMed] [Google Scholar]
- Hill D. R., Bowery N. G. 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABA B sites in rat brain. Nature. 1981 Mar 12;290(5802):149–152. doi: 10.1038/290149a0. [DOI] [PubMed] [Google Scholar]
- Hill D. R., Bowery N. G., Hudson A. L. Inhibition of GABAB receptor binding by guanyl nucleotides. J Neurochem. 1984 Mar;42(3):652–657. doi: 10.1111/j.1471-4159.1984.tb02732.x. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Kelly J. S. Uptake and metabolism of gamma-aminobutyric acid by neurones and glial cells. Biochem Pharmacol. 1975 May 1;24(9):933–938. doi: 10.1016/0006-2952(75)90422-0. [DOI] [PubMed] [Google Scholar]
- Iversen L. L., Neal M. J. The uptake of [3H]GABA by slices of rat cerebral cortex. J Neurochem. 1968 Oct;15(10):1141–1149. doi: 10.1111/j.1471-4159.1968.tb06831.x. [DOI] [PubMed] [Google Scholar]
- Jones G. P., Neal M. J. Selective inhibition of neuronal GABA uptake by cis-1,3-aminocyclohexane carboxylic acid. Nature. 1976 Nov 18;264(5583):281–284. doi: 10.1038/264281a0. [DOI] [PubMed] [Google Scholar]
- Krogsgaard-Larsen P., Falch E., Schousboe A., Curtis D. R., Lodge D. Piperidine-4-sulphonic acid, a new specific GABA agonist. J Neurochem. 1980 Mar;34(3):756–759. doi: 10.1111/j.1471-4159.1980.tb11211.x. [DOI] [PubMed] [Google Scholar]
- Krogsgaard-Larsen P., Johnston G. A., Lodge D., Curtis D. R. A new class of GABA agonist. Nature. 1977 Jul 7;268(5615):53–55. doi: 10.1038/268053a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Leblanc G. G., Ciaranello R. D. alpha-Noradrenergic potentiation of neurotransmitter-stimulated cAMP production in rat striatal slices. Brain Res. 1984 Feb 13;293(1):57–65. doi: 10.1016/0006-8993(84)91452-5. [DOI] [PubMed] [Google Scholar]
- Magistretti P. J., Schorderet M. VIP and noradrenaline act synergistically to increase cyclic AMP in cerebral cortex. Nature. 1984 Mar 15;308(5956):280–282. doi: 10.1038/308280a0. [DOI] [PubMed] [Google Scholar]
- Nathanson J. A. Cyclic nucleotides and nervous system function. Physiol Rev. 1977 Apr;57(2):157–256. doi: 10.1152/physrev.1977.57.2.157. [DOI] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. A bicuculline-resistant inhibitory post-synaptic potential in rat hippocampal pyramidal cells in vitro. J Physiol. 1984 Mar;348:239–254. doi: 10.1113/jphysiol.1984.sp015107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Direct hyperpolarizing action of baclofen on hippocampal pyramidal cells. 1984 Mar 29-Apr 4Nature. 308(5958):450–452. doi: 10.1038/308450a0. [DOI] [PubMed] [Google Scholar]
- Obata K., Ito M., Ochi R., Sato N. Pharmacological properties of the postsynaptic inhibition by Purkinje cell axons and the action of gamma-aminobutyric acid on deiters NEURONES. Exp Brain Res. 1967;4(1):43–57. doi: 10.1007/BF00235216. [DOI] [PubMed] [Google Scholar]
- Olianas M. C., Onali P., Neff N. H., Costa E. Adenylate cyclase activity of synaptic membranes from rat striatum. Inhibition by muscarinic receptor agonists. Mol Pharmacol. 1983 Mar;23(2):393–398. [PubMed] [Google Scholar]
- Rodbell M. The role of hormone receptors and GTP-regulatory proteins in membrane transduction. Nature. 1980 Mar 6;284(5751):17–22. doi: 10.1038/284017a0. [DOI] [PubMed] [Google Scholar]
- Seamon K. B., Padgett W., Daly J. W. Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3363–3367. doi: 10.1073/pnas.78.6.3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werz M. A., MacDonald R. L. Opioid peptides selective for mu- and delta-opiate receptors reduce calcium-dependent action potential duration by increasing potassium conductance. Neurosci Lett. 1983 Dec 2;42(2):173–178. doi: 10.1016/0304-3940(83)90402-0. [DOI] [PubMed] [Google Scholar]
- Wojcik W. J., Neff N. H. Adenosine A1 receptors are associated with cerebellar granule cells. J Neurochem. 1983 Sep;41(3):759–763. doi: 10.1111/j.1471-4159.1983.tb04805.x. [DOI] [PubMed] [Google Scholar]
- Wojcik W. J., Neff N. H. gamma-aminobutyric acid B receptors are negatively coupled to adenylate cyclase in brain, and in the cerebellum these receptors may be associated with granule cells. Mol Pharmacol. 1984 Jan;25(1):24–28. [PubMed] [Google Scholar]



