Abstract
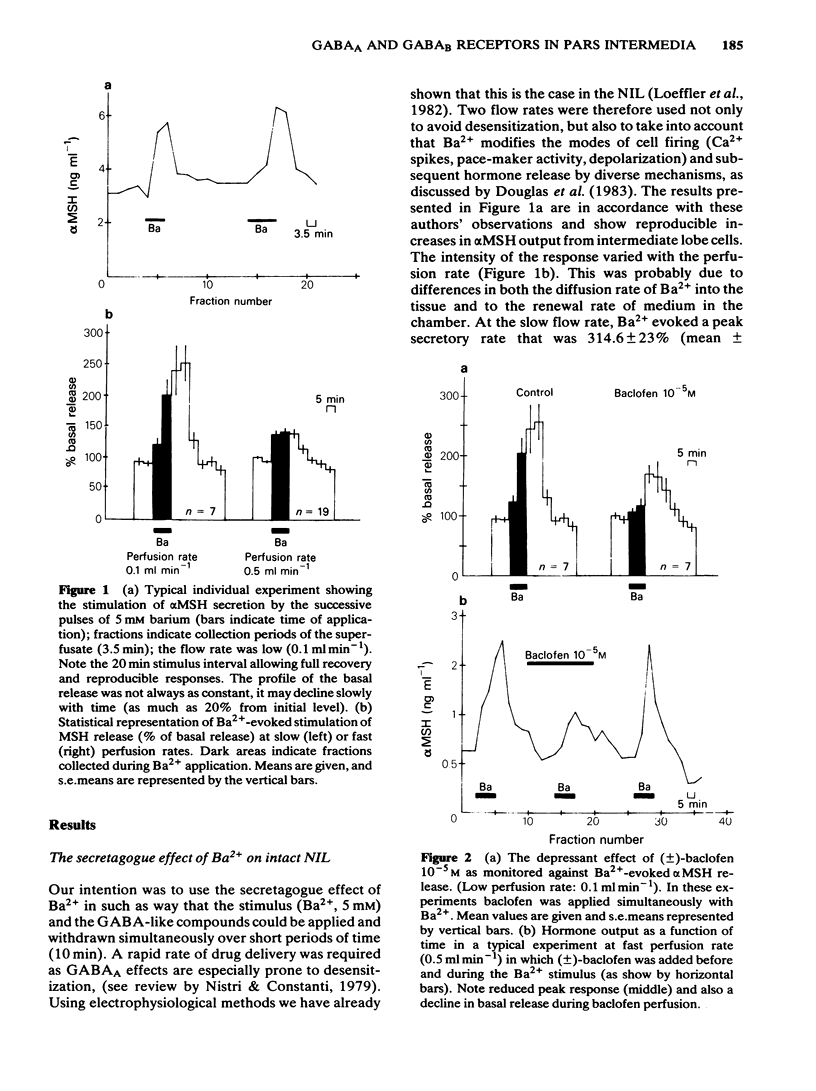
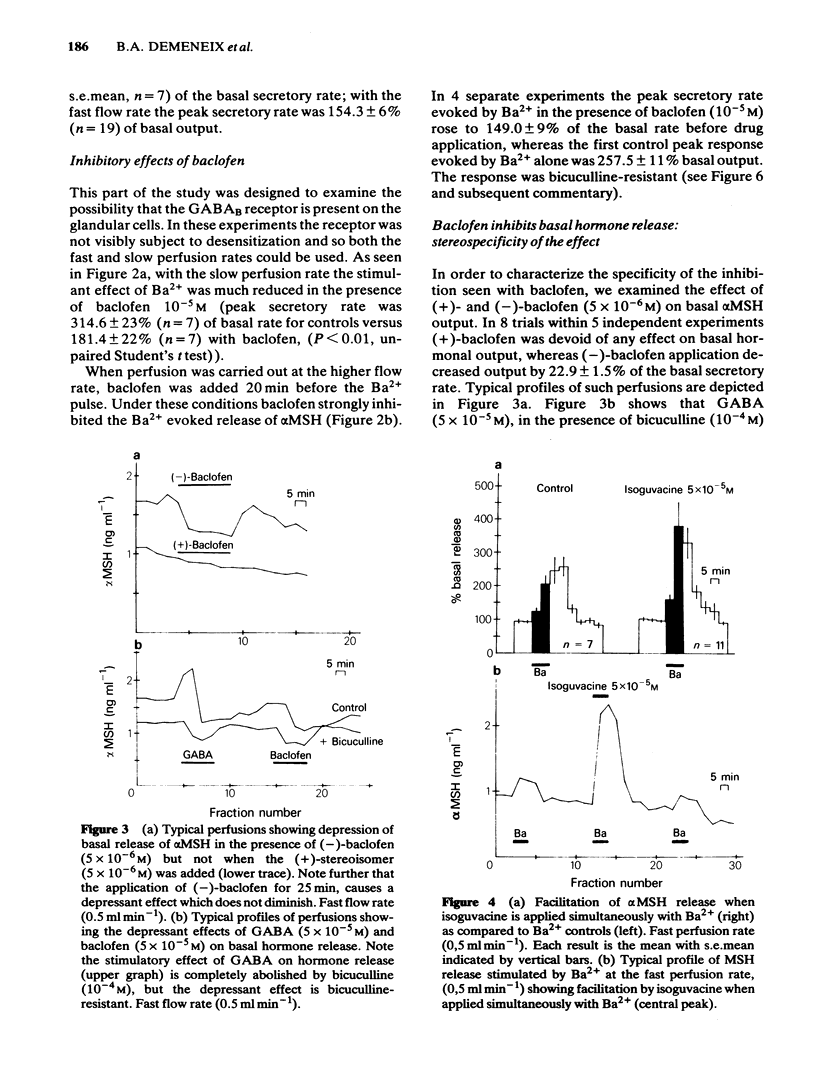
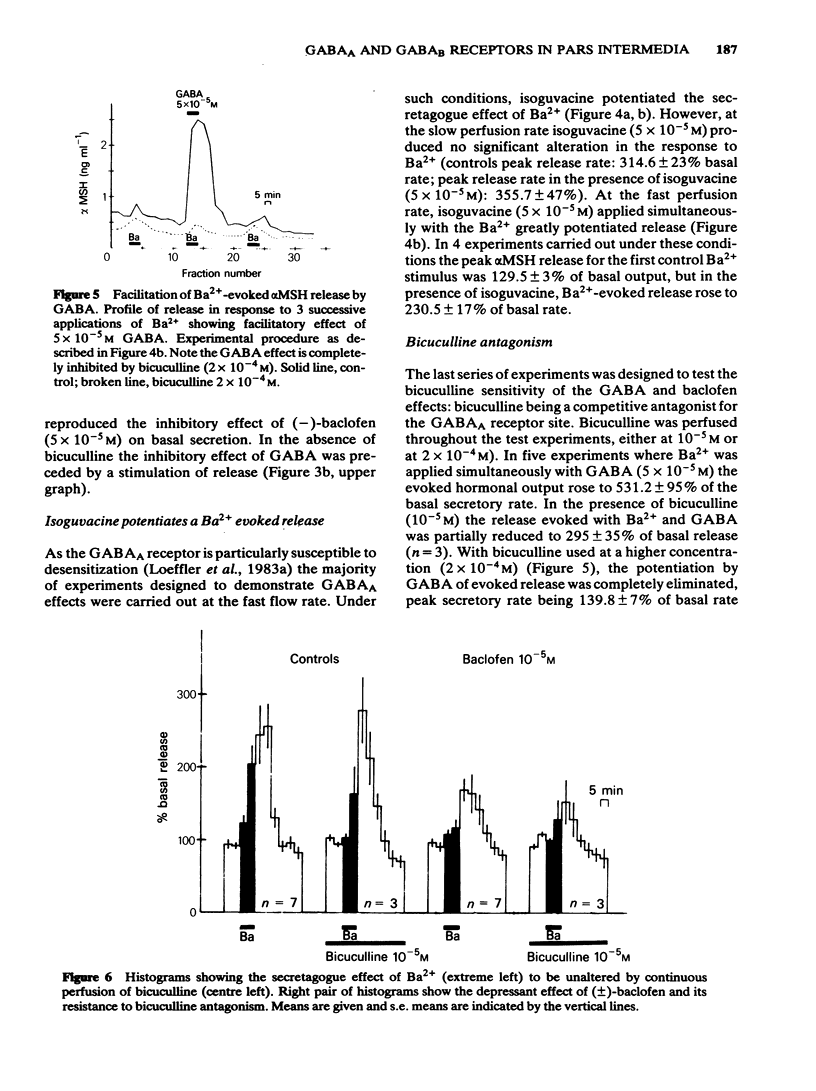
We have studied the effects of selective GABAA and GABAB agonists on alpha-melanophore stimulating hormone (alpha MSH) release from intact rat neurointermediate lobes (NIL) in vitro. Agonist effects were tested against either basal alpha MSH output or BaCl2 (5 mM)-evoked release. GABA (50 microM) produced a biphasic effect on basal release, with an enhancement followed by inhibition of release. The enhancement but not the inhibition was blocked by bicuculline methiodide (100 microM). Baclofen (10 microM), a specific GABAB agonist, reduced the basal and Ba2+-evoked hormonal release in a stereospecific manner. (-)-Baclofen (5 microM) was active whereas the (+)-isomer was inactive at the same concentration. Isoguvacine (50 microM) a specific GABAA agonist, potentiated the Ba2+-evoked release of alpha MSH. GABA (50 microM) mimicked this effect, and its action was antagonized by bicuculline methiodide (200 microM). The results suggest that both GABAA and GABAB receptors are present on the endocrine cells of the intermediate lobe.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams P. R., Brown D. A., Constanti A. Pharmacological inhibition of the M-current. J Physiol. 1982 Nov;332:223–262. doi: 10.1113/jphysiol.1982.sp014411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery N. G., Doble A., Hill D. R., Hudson A. L., Shaw J. S., Turnbull M. J., Warrington R. Bicuculline-insensitive GABA receptors on peripheral autonomic nerve terminals. Eur J Pharmacol. 1981 Apr 24;71(1):53–70. doi: 10.1016/0014-2999(81)90386-1. [DOI] [PubMed] [Google Scholar]
- Bowery N. G., Hill D. R., Hudson A. L. Characteristics of GABAB receptor binding sites on rat whole brain synaptic membranes. Br J Pharmacol. 1983 Jan;78(1):191–206. doi: 10.1111/j.1476-5381.1983.tb09380.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowery N. G., Hill D. R., Hudson A. L., Doble A., Middlemiss D. N., Shaw J., Turnbull M. (-)Baclofen decreases neurotransmitter release in the mammalian CNS by an action at a novel GABA receptor. Nature. 1980 Jan 3;283(5742):92–94. doi: 10.1038/283092a0. [DOI] [PubMed] [Google Scholar]
- Constanti A., Adams P. R., Brown D. A. Who do barium ions imitate acetylcholine? Brain Res. 1981 Feb 9;206(1):244–250. doi: 10.1016/0006-8993(81)90125-6. [DOI] [PubMed] [Google Scholar]
- Douglas W. W., Taraskevich P. S. Action potentials in gland cells of rat pituitary pars intermedia: inhibition by dopamine, an inhibitor of MSH secretion. J Physiol. 1978 Dec;285:171–184. doi: 10.1113/jphysiol.1978.sp012565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Taraskevich P. S. Calcium component to action potentials in rat pars intermedia cells. J Physiol. 1980 Dec;309:623–630. doi: 10.1113/jphysiol.1980.sp013530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Taraskevich P. S. Slowing effects of dopamine and calcium-channel blockers on frequency of sodium spikes in rat pars intermedia cells. J Physiol. 1982 May;326:201–211. doi: 10.1113/jphysiol.1982.sp014186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W., Taraskevich P. S., Tomiko S. A. Secretagogue effect of barium on output of melanocyte-stimulating hormone from pars intermedia of the mouse pituitary. J Physiol. 1983 May;338:243–257. doi: 10.1113/jphysiol.1983.sp014671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap K. Two types of gamma-aminobutyric acid receptor on embryonic sensory neurones. Br J Pharmacol. 1981 Nov;74(3):579–585. doi: 10.1111/j.1476-5381.1981.tb10467.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Désarmenien M., Feltz P., Occhipinti G., Santangelo F., Schlichter R. Coexistence of GABAA and GABAB receptors on A delta and C primary afferents. Br J Pharmacol. 1984 Feb;81(2):327–333. doi: 10.1111/j.1476-5381.1984.tb10082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Désarménien M., Santangelo F., Occhipinti G., Schlichter R., Loeffler J. P., Desaulles E., Demeneix B. A., Feltz P. Electrophysiological study of GABAA versus GABAB receptors on excitation-secretion coupling. Adv Biochem Psychopharmacol. 1983;37:93–105. [PubMed] [Google Scholar]
- Fox S., Krnjević K., Morris M. E., Puil E., Werman R. Action of baclofen on mammalian synaptic transmission. Neuroscience. 1978;3(6):495–515. doi: 10.1016/0306-4522(78)90016-7. [DOI] [PubMed] [Google Scholar]
- Frizell M. The effect of ligation combined with section on anterograde axonal transport in rabbit hypoglossal nerve. Brain Res. 1982 Oct 28;250(1):65–69. doi: 10.1016/0006-8993(82)90953-2. [DOI] [PubMed] [Google Scholar]
- Giorguieff M. F., Kemel M. L., Glowinski J., Besson M. J. Stimulation of dopamine release by GABA in rat striatal slices. Brain Res. 1978 Jan 6;139(1):115–130. doi: 10.1016/0006-8993(78)90064-1. [DOI] [PubMed] [Google Scholar]
- Hill D. R., Bowery N. G. 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABA B sites in rat brain. Nature. 1981 Mar 12;290(5802):149–152. doi: 10.1038/290149a0. [DOI] [PubMed] [Google Scholar]
- Keith L. D., Allen R. G., Stack J., Robertson L. M., Kendall J. W. Potassium-modulated secretion of immunoreactive melanocyte-stimulating hormone and endorphin from mouse neuro-intermediate lobes: evidence for stimulus-secretion uncoupling and rate sensitivity. Endocrinology. 1983 May;112(5):1886–1888. doi: 10.1210/endo-112-5-1886. [DOI] [PubMed] [Google Scholar]
- Loeffler J. P., Desaulles E., Demeneix B. A., Feltz P. Electrophysiological study with K+- and Ca2+-sensitive micropipettes of GABA receptors in the rat neurointermediate lobe in vitro. Neurosci Lett. 1982 Dec 31;34(3):271–276. doi: 10.1016/0304-3940(82)90187-2. [DOI] [PubMed] [Google Scholar]
- Nistri A., Constanti A. Pharmacological characterization of different types of GABA and glutamate receptors in vertebrates and invertebrates. Prog Neurobiol. 1979;13(2):117–235. doi: 10.1016/0301-0082(79)90016-9. [DOI] [PubMed] [Google Scholar]
- Oertel W. H., Mugnaini E., Tappaz M. L., Weise V. K., Dahl A. L., Schmechel D. E., Kopin I. J. Central GABAergic innervation of neurointermediate pituitary lobe: biochemical and immunocytochemical study in the rat. Proc Natl Acad Sci U S A. 1982 Jan;79(2):675–679. doi: 10.1073/pnas.79.2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann W., Zumstein A., Starke K. Gamma-aminobutyric acid can both inhibit and facilitate dopamine release in the caudate nucleus of the rabbit. J Neurochem. 1982 Oct;39(4):961–969. doi: 10.1111/j.1471-4159.1982.tb11483.x. [DOI] [PubMed] [Google Scholar]
- Schmitt G., Briaud B., Mialhe C., Stutinsky F. Different effects of K+ and Ca++ on alpha-MSH and ACTH release from superfused neurointermediate lobe of the rat hypophysis. Neuroendocrinology. 1979;28(5):297–301. doi: 10.1159/000122875. [DOI] [PubMed] [Google Scholar]
- Scholfield C. N. Baclofen blocks postsynaptic inhibition but not the effect of muscimol in the olfactory cortex. Br J Pharmacol. 1983 Jan;78(1):79–84. doi: 10.1111/j.1476-5381.1983.tb09365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharman D. F., Holzer P., Holzbauer M. In vitro release of endogenous catecholamines from the neural and intermediate lobe of the hypophysis. Neuroendocrinology. 1982 Mar;34(3):175–179. doi: 10.1159/000123297. [DOI] [PubMed] [Google Scholar]
- Taraskevich P. S., Douglas W. W. GABA directly affects electrophysiological properties of pituitary pars intermedia cells. Nature. 1982 Oct 21;299(5885):733–734. doi: 10.1038/299733a0. [DOI] [PubMed] [Google Scholar]
- Tomiko S. A., Taraskevich P. S., Douglas W. W. GABA acts directly on cells of pituitary pars intermedia to alter hormone output. Nature. 1983 Feb 24;301(5902):706–707. doi: 10.1038/301706a0. [DOI] [PubMed] [Google Scholar]
- Tomiko S. A., Taraskevich P. S., Douglas W. W. Potassium-induced secretion of melanocyte-stimulating hormone from isolated pars intermedia cells signals participation of voltage-dependent calcium channels in stimulus-secretion coupling. Neuroscience. 1981;6(11):2259–2267. doi: 10.1016/0306-4522(81)90015-4. [DOI] [PubMed] [Google Scholar]
- Van der Schueren B., Denef C., Cassiman J. J. Ultrastructural and functional characteristics of rat pituitary cell aggregates. Endocrinology. 1982 Feb;110(2):513–523. doi: 10.1210/endo-110-2-513. [DOI] [PubMed] [Google Scholar]
- Vincent S. R., Hökfelt T., Wu J. Y. GABA neuron systems in hypothalamus and the pituitary gland. Immunohistochemical demonstration using antibodies against glutamate decarboxylase. Neuroendocrinology. 1982 Feb;34(2):117–125. doi: 10.1159/000123288. [DOI] [PubMed] [Google Scholar]



