Abstract
Acrolein, the most reactive of the α,β-unsaturated aldehydes, is endogenously produced by lipid peroxidation, and has been found increased in the brain of patients with Alzheimer's disease. Although it is known that acrolein increases total protein carbonylation and impairs the function of selected proteins, no study has addressed which proteins are selectively carbonylated by this aldehyde. In this study we investigated the effect of increasing concentrations of acrolein (0, 0.005, 0.05, 0.5, 5, 50 μM) on protein carbonylation in gerbil synaptosomes. In addition, we applied proteomics to identify synaptosomal proteins that were selectively carbonylated by 0.5 μM acrolein. Acrolein increased total protein carbonylation in a dose-dependent manner. Proteomic analysis (2-D electrophoresis followed by mass spectrometry) revealed that tropomyosin-3-gamma isoform 2, tropomyosin-5, β-actin, mitochondrial Tu translation elongation factor (EFTumt) and voltage-dependent anion channel (VDAC) were significantly carbonylated by acrolein. Consistent with the proteomics studies that have identified specifically oxidized proteins in Alzheimer's Disease (AD) brain, the proteins identified in this study are involved in a wide variety of cellular functions including energy metabolism, neurotransmission, protein synthesis, and cytoskeletal integrity. Our results suggest that acrolein may significantly contribute for oxidative damage in AD brain.
Acrolein (2-propen-1-al), the most reactive of the α,β-unsaturated aldehydes (Esterbauer et al., 1991), is a toxic compound found in automobile exhaust gases, overheated cooking oils and a metabolite of cyclophosphamide and allyl alcohol (Halliwell and Gutteridge, 1999). Acrolein can also be endogenously produced as a product of lipid peroxidation (Adams and Klaidman, 1993, Uchida, 1999) and from polyamine metabolism (Takano et al., 2005). Acrolein reacts with proteins and DNA, forming stable Michael adducts (Esterbauer et al., 1991, Pocernich and Butterfield, 2003). As a consequence, the identification of acrolein adducts has been proposed as a biologic marker for oxidative stress (Kehrer and Biswal, 2000). Accordingly, acrolein levels have been found increased in pathological conditions associated with oxidative stress, such as spinal cord injury (Luo et al., 2005), diabetic nephropathy (Suzuki and Miyata, 1999) and Alzheimer's Disease (AD) (Markesbery and Lovell, 1998, Calingasan et al., 1999, Lovell et al., 2001).
Due to its high reactivity, acrolein may not only be a marker for oxidative stress (Uchida et al., 1998), but also plays an important role in the development of oxidative damage (Uchida, 1999, Pocernich and Butterfield, 2003) and, consequently, in the pathogenesis of selected diseases, such as AD (Montine et al., 2002, Pocernich and Butterfield, 2003, Seidler and Yeargans, 2004, Seidler and Squire, 2005).
The mechanisms by which acrolein causes oxidative damage and neurotoxicity are not completely defined, but accumulating evidence indicates that this alkenal primarily binds and depletes cellular nucleophiles, such as reduced glutathione (GSH) (Horton et al., 1997), lipoic acid (Pocernich and Butterfield, 2003) and thioredoxin (Yang et al., 2004). This view is fully supported by the findings that glutathione ethyl ester and N-acetylcysteine, glutathione precursors, protect against the oxidative damage induced by acrolein in vitro and ex vivo, respectively (Pocernich et al., 2001). The reaction of acrolein and GSH occurs spontaneously at neutral pH, (Adams and Klaidman, 1993), generating glutathionylpropionaldehyde (GS-propionaldehyde). Depletion of GSH may compromise the activity of GSH peroxidase, which uses GSH as a co-substrate to reduce hydrogen peroxide and lipid peroxides (Halliwell and Gutteridge, 1999), facilitating lipid peroxidation. In addition, there is evidence that GS-propionaldehyde can elicit O2 •− formation in the presence of xanthine oxidase (Adams and Klaidman, 1993). Both factors, i.e., production of a toxic metabolite and depletion of GSH, may trigger acrolein-induced lipoperoxidation, a biochemical event that temporally occurs after glutathione depletion by allyl alcohol (an acrolein precursor) in hepatocytes (Comporti et al., 1991).
In proteins, acrolein preferentially attacks free SH groups of cysteine residues,ε-amino groups of lysine residues and histidine residues (Esterbauer et al., 1991), resulting in an acrolein-amino acid adduct and introducing a carbonyl group to proteins (Uchida et al., 1998). Although it is known that acrolein increases total protein carbonylation (Pocernich et al., 2001) and impairs the function of selected proteins (Patel and Block, 1993, Lovell et al., 2000), no study has addressed which proteins are selectively carbonylated by this aldehyde. Therefore, in this study we evaluated the effect of increasing concentrations of acrolein on protein carbonylation in synaptosomes, aiming to determine its optimal concentration to induce protein carbonylation in our system. Once the optimal concentration of acrolein was determined, we applied proteomics to identify synaptosomal proteins that were selectively carbonylated by this alkenal.
Material and Methods
The University of Kentucky Animal Care and Use Committee approved all protocols followed. For all studies, male and female Mongolian gerbils, approximately 50 grams in size, obtained from Harlan Sprague–Dawley, were used. All animals were kept under twelve hour light/dark conditions in the University of Kentucky Sanders Brown Animal Facility, and fed standard Purina rodent laboratory chow ad libitum. The gerbils were euthanized by sodium pentobarbital, and the brain dissected quickly on ice according to the method previously described (Hensley et al., 1994). The forebrain was isolated and immediately suspended in 20 ml of ice-cold isolation buffer, [0.32 M sucrose with the protease inhibitors, 4 μg/ml leupeptin, 4 μg/ml pepstatin A, 5 μg/ml aprotinin, 20 μg/ml type II-s soybean trypsin inhibitor, 0.2 mM phenylmethylsulfonylfloride (PMSF), 2 mM EDTA, 2 mM EGTA, and 20 mM HEPES at pH 7.4] and homogenized by 12 passes of a motor-driven Teflon pestle.
Synaptosome preparation
Synaptosomes were prepared by sucrose density gradient centrifugation (Gray and Whittaker, 1962). In brief, homogenized forebrains were centrifuged at 3500 rpm for 10 min at 4°C in a Du Pont Sorvall RC5C refrigerated centrifuge. The pellet obtained was discarded, and the supernatant was spun at 13500 rpm in a similar fashion. The resulting pellet was then resuspended in a sucrose isolation buffer and layered onto discontinuous sucrose density gradients. These were spun at 22000 rpm, for 2 h at 4°C, in an SW28 rotor in a Beckman L7-55 refrigerated ultracentrifuge. Purified synaptosomes were obtained at the 1.18/1.10 M sucrose gradient interface.
Synaptosomes obtained were then washed three times with 30 ml of Locke's buffer (5 mM HEPES, 5 mM glucose, 154 mM NaCl, 5.6 mM KCl, 2.3 mM CaCl2, 1 mM MgCl2, and 3.6 mM NaHCO3 in distilled water, pH 7.4). The protein concentration of each sample was measured by the method of BCA and adjusted to 4 mg/ml. The synaptosome aliquots were then centrifuged in a refrigerated Hettich table-top microcentrifuge at 14000 rpm for 4 min. The synaptosomal pellets were treated with 0.005-50 μM acrolein in Locke's buffer, bringing the total volume to 1 ml (final protein concentration in the incubation medium: 1 mg/ml), at 37 °C for 30 min. The synaptosome suspension was then washed four times with Locke's buffer at 14000 rpm for 5 min. Samples were then analyzed for total protein carbonyl content by slot blot analysis or frozen at -80 °C until proteomics experiments were carried out. Proteomics analysis was carried out in samples treated with 0.5 μM acrolein for 30 min.
Slot blots: Protein carbonyl measurement
Protein carbonyls are an index of protein oxidation (Berlett and Stadtman, 1997) and were determined as described previously (Butterfield and Stadtman, 1997). Briefly, 5 μL of synaptosome preparations (4 mg/mL) were incubated at room temperature with 10 mM 2,4-dinitrophenylhydrazine (in 2 N HCl) in the presence of 5 μL of 12% SDS for 20 min at room temperature. The samples were neutralized with 7.5 μL of the neutralization solution (2 M Tris in 30% glycerol). Two hundred fifty nanograms of protein sample were loaded into the wells of the slot blot apparatus. Proteins were transferred directly to nitrocellulose paper under vacuum pressure and standard immunochemical techniques were performed. Membranes were blocked in the presence of 3% bovine serum albumin (BSA) in TBS-T (10 mM Tris–HCl, pH 7.5, 150 mM NaCl, 0.05% Tween 20) for 1 h, followed by incubation with rabbit polyclonal antibody anti-DNP (1:100, Chemicon) for 1 h. The membrane were washed three times with TBS-T and incubated with alkaline-phosphatase (AP)-conjugated secondary antibody for 1 h (1:4000, Sigma). The specificity of primary antibodies has been previously demonstrated by experiments performed in our laboratory (Aksenov et al., 2001). Samples were developed using SigmaFast Tablets (BCIP/NBT) substrate, and blots were scanned into Adobe Photoshop (Adobe System, Inc., Mountain View, CA, USA) and quantified with Scion Image (PC version of Macintosh compatible NIH Image).
Two-dimensional gel electrophoresis
Proteomic analysis was carried out in synaptosomes treated with 0.5 μM acrolein in Locke's buffer, at 37 °C for 30 min, as described above. The concentration of acrolein of 0.5 μM, which did not increase total protein carbonylation in our slot blot experiments, was used in the proteomics study aiming to determine the proteins that are more susceptible to protein carbonylation. By using this approach, we intended to identify selected early carbonylated proteins, which might not represent a sufficient amount to be detected in the blot analysis. In fact, previous studies have shown increased carbonylation of selected proteins in AD brains without an increase in total carbonylation (Castegna et al., 2002). The washed synaptosomes were incubated with 4 volumes of 2 N HCl for electrophoresis or incubated with 4 volumes of 20 mM DNPH (in 2 N HCl) for western blotting, in both cases at room temperature (25 °C) for 20 min followed by TCA precipitation and three washings with 1:1 (v/v) ethanol/ethyl acetate solution. Two-dimensional polyacrylamide gel electrophoresis was performed in a Bio-Rad system using 110-mm pH 3–10 immobilized pH gradients (IPG) strips and Criterion 8–16% gels (Bio-Rad), as previously described (Castegna et al., 2002a). Samples were dissolved in two-dimensional polyacrylamide gel electrophoresis sample buffer [8 M urea, 2 M thiourea, 20 mM dithiothreitol, 0.2% (v/v) biolytes 3–10, 2% CHAPS, and bromophenol blue]. In the first-dimension, 200 μg of protein were applied to a rehydrated IPG strip, and the isoelectric focusing was carried out at 20°C as follows: 300 V for 1 h, linear gradient to 800 V for 5 h and finally 20,000 V/h. Before the second dimensional separation, the gel strips were equilibrated for 10 min in 37.5 mM Tris–HCl (pH 8.8) containing 6 M urea, 2% (w/v) sodium dodecyl sulfate, 20% (v/v) glycerol, and 0.5% dithiothreitol, and then re-equilibrated for 10 min in the same buffer containing 4.5% iodoacetamide in place of dithiothreitol. Strips were placed on Criterion gels and electrophoresis ran for 65 min at 200 V.
SYPRO ruby staining
The gels were fixed in 10% methanol and 7% acetic acid for 30 min, then stained with SYPRO Ruby gel stain (Bio-Rad). The excess SYPRO Ruby stain was then removed and gels were stored in water.
Western blotting
The gels were prepared in the same manner as for 2D electrophoresis. After the second dimension, the proteins from gels were transferred to nitrocellulose papers (BioRad) using the Transblot-Blot® SD semi-Dry Transfer Cell (Bio-Rad) at 15 V for 4 h. The 2,4-dinitrophenyl hydrazone (DNP) adducts of the carbonyls of the proteins were detected on the nitrocellulose paper using a primary rabbit antibody (1:100, Chemicon) specific for DNP–protein adduct, and then a secondary goat anti-rabbit IgG alkaline phosphatase (1:4000, Sigma) antibody was applied. The resultant stain was developed by application of Sigma-Fast (BCIP/NBT) tablets.
Mass spectrometry
Mass spectra of the sample were determined by a TofSpec 2E (Micromass, UK) MALDI-TOF mass spectrometer in reflectron mode. The tryptic digest (1 μL) was mixed with 1 μL a-cyano-4-hydroxy-trans-cinnamic acid (10 mg/mL in 0.1% TFA (trifluoroacetic acid): acetonitrile, 1:1, v/v) directly on the target and dried at room temperature. The sample spot was then washed with 1 μL of 1% TFA solution for approximately 60 s. The TFA droplet was gently blown off the sample spot with compressed air. The resulting diffuse sample spot was recrystallized (refocused) using 1 μL of a solution of ethanol:acetone: 0.1% TFA (6:3:1 ratio). The spectra reported in this study are a summation of 100 laser shots. External calibration of the mass axis, used for acquisition and internal calibration, employed either trypsin autolysis ions or matrix clusters and was applied post-acquisition for accurate mass determination. The MALDI spectra used for protein identification from tryptic fragments were searched against the NCBI protein databases using the MASCOT search engine (http://www.matrixscience.com). Peptide mass fingerprinting used the assumption that peptides are monoisotopic, oxidized at methionine residues, and carbamidomethylated at cysteine residues. Up to 1 missed trypsin cleavage was allowed. Mass tolerance of 100 ppm was the window of error allowed for matching the peptide mass values. Probability-based MOWSE scores were estimated by comparison of search results against estimated random match population and were reported as −10*log10(P), where P is the probability that the identification of the protein is not correct. MOWSE scores greater than 66 were considered to be significant (P<0.05). Protein identification was consistent with the expected size and pI range based on positions in the 2D gel.
Statistical analysis
Normalized data (percent of control responses) were analyzed by one-way ANOVA, and post-hoc comparisons carried out by the Dunnet's test, when appropriate. Dose-effect relationships were assessed by partitioning the total sum of squares into trend (linear, quadratic, cubic or polynomial) components.
The analysis of the increase of protein carbonyl content induced by acrolein in synaptosomes was carried out by one-tailed paired T-test.
Results
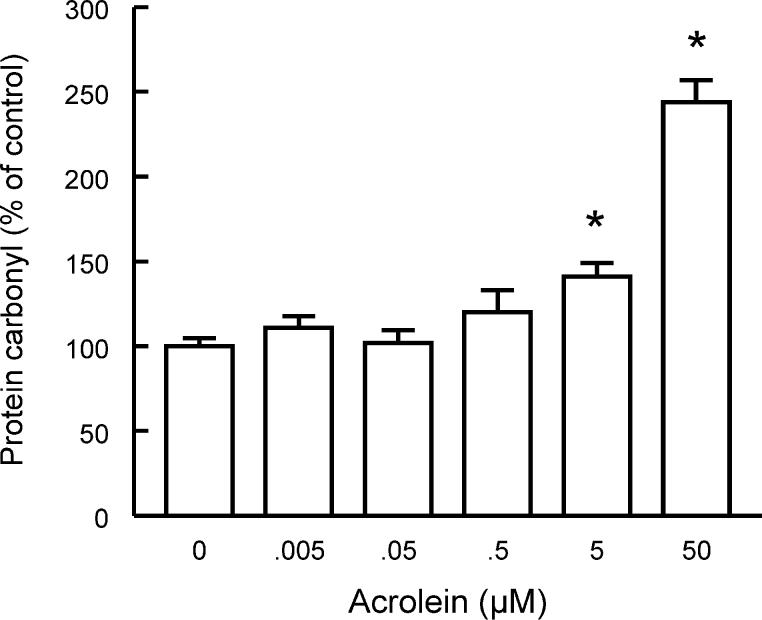
Figure 1A shows the effect of a 30-min incubation with acrolein (0.005- 50 μM) on the protein carbonyl levels of gerbil forebrain synaptosomes. Statistical analysis (oneway ANOVA) revealed a significant effect of acrolein concentrations [F(5,12)=31.22; P<0.001]. Partitioning the total sum of squares into trend components revealed a significant linear trend [F(1,12)=150; P<0.0001], indicating that acrolein increased protein carbonylation linearly with its concentration. A representative blot is shown in Figure 1B.
Fig 1.
Acrolein increases protein carbonylation in gerbil synaptosomes. Data are mean + S.E.M. for n=3 in each group. *P<0.05 compared with control group (Dunnett's test).
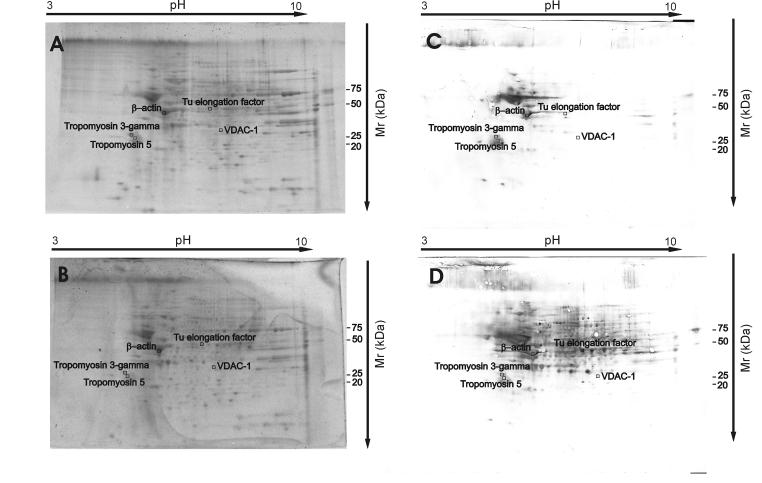
By using redox proteomics, the oxidized proteins were separated and identified. Fig. 2 shows two representative 2D gels of control and acrolein-treated synaptosomes and the corresponding 2D Western blots of oxidized proteins. Comparing the densitometric intensities of individual spots, we determined that five proteins were oxidatively modified by acrolein compared to non-exposed controls, indicated by the increased carbonyl level of these proteins (Table 1).
Fig 2.
Representative SYPRO Ruby stained 2D gels (A, B) and respective 2D-oxyblots (C,D) from control synaptosomes and synaptosomes treated with acrolein (0.5 μM).
Table 1.
Summary of the proteins identified to be increasingly carbonylated in synaptosomes treated with acrolein (0.5 μM) for 30 min.
| Protein | Mowse score |
Peptides matched |
% Coverage |
MW (kD) |
pI | Oxidation (fold-increase) |
P value |
|---|---|---|---|---|---|---|---|
| Tropomyosin 3 gamma isoform 2 |
95 | 10 | 26 | 29,2 | 4.75 | 2.3 ± 1.3 | P=0.024 |
| Tropomyosin 5 | 95 | 9 | 32 | 29,1 | 4.72 | 7.3 ± 3.8 | P=0.011 |
| Beta-actin | 85 | 10 | 32 | 39,4 | 5.78 | 8.1 ± 6.2 | P=0.017 |
| Tu translation elongation factor |
92 | 8 | 20 | 49,8 | 7.23 | 11.0 ± 2.1 | P=0.025 |
| VDAC-1 – outer mitocondrial porin 1 |
69 | 7 | 34 | 30,6 | 8.63 | 13.0 ± 4.3 | P=0.047 |
Proteins were trypsinized and analyzed by Mass Spectrometry in order to ascertain their identities. Proteins with a Mowse score >66 were considered to be identified at a statistically significant level. MW, molecular weight; pI, isoelectric point. Statistical analysis was carried out by the one-tailed paired T-test.
Mass spectrometry revealed that the five excessively oxidized proteins by acrolein were tropomyosin-3-gamma isoform 2, tropomyosin-5, β-actin, mitochondrial Tu translation elongation factor (EF-Tumt) and voltage dependent anion channel (VDAC). The mass spectra of these proteins and the database search for these proteins resulted in a single identification. The parameters for the identification of the oxidized proteins by mass spectrometry are summarized in Table 1; these protein identifications agreed with the expected MW and pI range based on their positions in the 2D gels. The high Mowse scores indicate that the probability of an incorrect identification is exceedingly remote (Butterfield et al., 2003).
Discussion
In this study we showed that acrolein induces protein carbonylation linearly with its concentration in the incubating medium, confirming and extending previous findings from our group (Pocernich and Butterfield, 2003). In addition, we identified specific proteins that are carbonylated by acrolein, as well as proteins that have their content affected by the aldehyde in synaptosomes. Mass spectrometry revealed that tropomyosin-3-gamma isoform 2, tropomyosin-5, β-actin, EF-Tumt and VDAC were oxidized. Consistent with the proteomics studies that identified specifically oxidized proteins in AD brain (Castegna et al., 2002a, Castegna et al., 2002b, Sultana et al., 2006a, Sultana et al., 2006b, Sultana et al., 2006c), the proteins identified in this study are involved in a wide variety of cellular functions including energy metabolism, neurotransmission, protein synthesis, and cytoskeletal integrity.
β-Actin was one of the proteins found to be a target of acrolein-induced oxidation. This result is in agreement with previous studies from our group that have demonstrated that although both β- and γ-actin are oxidized in synaptosomes treated with Aβ(1–42), only β-actin is significantly modified in AD brain (Aksenov et al., 2001, Boyd-Kimball et al., 2005). Therefore, since Aβ peptide induces acrolein production in neurons (Mark et al., 1997, Butterfield et al., 2002), and this aldehyde is increased in AD brain (Lovell et al., 2001), is also possible that Aβ peptide-induced β-actin oxidation is mediated by acrolein.
Actin, found in both neurons and glial cells, is a core subunit of microfilaments (MF), a cytoskeletal element involved in neuronal growth and secretion. Tropomyosins are integral components of the actin-based MF system, and provide stability for the complex. Actin MFs are particularly concentrated in presynaptic terminals, dendritic spines, and growth cones. Therefore, considering the presynaptic nature of synaptosomes, it is not surprising the high amount of actin and tropomyosin present in our gels. Since actin MFs play a role in the neuronal membrane cytoskeleton (Beck and Nelson, 1996), the oxidation of two MF proteins could lead to loss of membrane cytoskeletal structure, decreased membrane fluidity, and trafficking of synaptic proteins. Actin MF also plays a role in vesicle release and endocytosis, by maintaining vesicles in the active zone (Halpain, 2003). In fact, oxidative stress decreases vesicular release of neurotransmitters in synaptosomes (Gilman et al., 1993) and, although both proteins have been identified as prone to iron-dependent oxidation and subsequent proteasomal degradation (Drake et al., 2002), it is still unknown whether oxidation of actin and tropomyosins play a role in this deleterious effect of reactive species on neurotransmitter release.
EF-Tumt is a 46 kDa protein that binds to amino acyl-tRNA in the presence of GTP. The complex promotes the binding of the amino acyl-tRNA to the acceptor site of the ribosome, promoting protein synthesis. Thus, EF-Tumt is required for the synthesis of polypeptides encoded by the mitochondrial genome, all of which are components of the electron transport chain and ATP synthetase. Oxidation of EF-Tumt has been previously described in synaptosomes incubated with Aβ(1-42) peptide, suggesting that this protein is particularly susceptible to oxidation, and probably involved in the pathogenesis of AD (Boyd-Kimball et al., 2005). Interestingly, transgenic mice overexpressing human Cu2+/Zn2+ superoxide dismutase 1 (Tg-SOD1), a condition associated to increased reactive species production, present lower levels of EF-Tumt, mitochondrial swelling and vacuolization in the hippocampus (Shin et al., 2004). However, whether decreases in the amount of EF-Tumt or its oxidation, such as the one described in this study and previous studies from our group (Boyd-Kimball et al., 2005), are causally related to the morphologic and functional mitochondrial alterations associated to oxidative stress in TgSOD1 mice, is still undefined.
Excellent reviews about VDAC have been published in the last years (Tsujimoto and Shimizu, 2002, Shoshan-Barmatz and Gincel, 2003, Lemasters and Holmuhamedov, 2006, Tsujimoto et al., 2006), and accumulating evidence suggests that this protein plays a major role in mitochondrial function regulation, both in physiological and pathological conditions.
VDAC is a protein that allows the movement of metabolites like ATP in and out of mitochondria by passive diffusion. Accumulating evidence indicates that VDAC not only regulates mitochondrial activity in physiological conditions, such as different substrate availability and hibernation, but also plays a role in hypoxia and early stages of apoptosis, being the outer pore component of the mitochondrial permeability transition pore (Lemasters and Holmuhamedov, 2006, Tsujimoto et al., 2006). Interestingly, reactive nitrogen and oxygen species, as well as ethanol induce VDAC closure, decreasing acyl-CoA influx and ATP efflux in the mitochondria in the presence of ethanol. These alterations favor the oxidation of acetaldehyde in the mitochondria, decreasing its availability and the oxidative damage due to aldehyde production from ethanol (Lemasters and Holmuhamedov, 2006). Interestingly, VDAC was recently identified as a selective oxidized target in AD brain (Sultana et al., 2006c), and it was proposed that oxidation of this protein could prevent the interaction of BcL-xL with VDAC, leading to an increase in BAX and BAK levels that are associated with VDAC and facilitating release of cytochrome C through VDAC (Shimizu et al., 1999).
In conclusion, all the proteins we identified as oxidized by acrolein in this study are related to vital neuronal functions, such as energy production, neurotransmitter release and neuronal plasticity. In addition, acrolein oxidized proteins that are known to trigger apoptosis, a possible mechanism of death related to the accumulation of this aldehyde. The well-known association between acrolein accumulation and AD, and the identification, in this study, of common protein targets to Aβ-peptide-induced protein oxidation and proteins found oxidized in AD brains, tempt us to propose that they result from common or, at least, related processes. However, we can not rule out the possibility that these proteins may be more easily identified by our methods, a fact that would make them a common finding between our studies. It is also worth mentioning that though acrolein caused significant oxidation of selected proteins, we did not assess their activity or functioning. Therefore, further studies have to be performed to evaluate the impact of oxidation on the function of the affected proteins.
Acknowledgments
This research was supported by grants from NIH to D.A.B. [AG-10836; AG-0549]. C.F.M. is the recipient of a post-doctoral fellowship from CAPES (BEX0262/05-6), Brazil.
List of abbreviations
- AD
Alzheimer's disease
- EF-Tumt
mitochondrial Tu translation elongation factor
- VDAC
Voltage-dependent anion channel
- GSH
reduced glutathione
Footnotes
Section: Cellular Neuroscience
Editor: Dr. Menahem Segal
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adams JD, Jr., Klaidman LK. Acrolein-induced oxygen radical formation. Free Radic Biol Med. 1993;15:187–193. doi: 10.1016/0891-5849(93)90058-3. [DOI] [PubMed] [Google Scholar]
- Aksenov MY, Aksenova MV, Butterfield DA, Geddes JW, Markesbery WR. Protein oxidation in the brain in Alzheimer's disease. Neuroscience. 2001;103:373–383. doi: 10.1016/s0306-4522(00)00580-7. [DOI] [PubMed] [Google Scholar]
- Beck KA, Nelson WJ. The spectrin-based membrane skeleton as a membrane protein-sorting machine. Am J Physiol. 1996;270:C1263–1270. doi: 10.1152/ajpcell.1996.270.5.C1263. [DOI] [PubMed] [Google Scholar]
- Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem. 1997;272:20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- Boyd-Kimball D, Castegna A, Sultana R, Poon HF, Petroze R, Lynn BC, Klein JB, Butterfield DA. Proteomic identification of proteins oxidized by Abeta(1-42) in synaptosomes: implications for Alzheimer's disease. Brain Res. 2005;1044:206–215. doi: 10.1016/j.brainres.2005.02.086. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Boyd-Kimball D, Castegna A. Proteomics in Alzheimer's disease: insights into potential mechanisms of neurodegeneration. J Neurochem. 2003;86:1313–1327. doi: 10.1046/j.1471-4159.2003.01948.x. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Castegna A, Lauderback CM, Drake J. Evidence that amyloid beta-peptide-induced lipid peroxidation and its sequelae in Alzheimer's disease brain contribute to neuronal death. Neurobiol Aging. 2002;23:655–664. doi: 10.1016/s0197-4580(01)00340-2. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Stadtman ER. Protein oxidation processes in aging brain. Advances in Cell Aging and Gerontology. 1997;2:161–191. [Google Scholar]
- Calingasan NY, Uchida K, Gibson GE. Protein-bound acrolein: a novel marker of oxidative stress in Alzheimer's disease. J Neurochem. 1999;72:751–756. doi: 10.1046/j.1471-4159.1999.0720751.x. [DOI] [PubMed] [Google Scholar]
- Castegna A, Aksenov M, Aksenova M, Thongboonkerd V, Klein JB, Pierce WM, Booze R, Markesbery WR, Butterfield DA. Proteomic identification of oxidatively modified proteins in Alzheimer's disease brain. Part I: creatine kinase BB, glutamine synthase, and ubiquitin carboxy-terminal hydrolase L-1. Free Radic Biol Med. 2002a;33:562–571. doi: 10.1016/s0891-5849(02)00914-0. [DOI] [PubMed] [Google Scholar]
- Castegna A, Aksenov M, Thongboonkerd V, Klein JB, Pierce WM, Booze R, Markesbery WR, Butterfield DA. Proteomic identification of oxidatively modified proteins in Alzheimer's disease brain. Part II: dihydropyrimidinase-related protein 2, alpha-enolase and heat shock cognate 71. J Neurochem. 2002b;82:1524–1532. doi: 10.1046/j.1471-4159.2002.01103.x. [DOI] [PubMed] [Google Scholar]
- Comporti M, Maellaro E, Del Bello B, Casini AF. Glutathione depletion: its effects on other antioxidant systems and hepatocellular damage. Xenobiotica. 1991;21:1067–1076. doi: 10.3109/00498259109039546. [DOI] [PubMed] [Google Scholar]
- Drake SK, Bourdon E, Wehr NB, Levine RL, Backlund PS, Yergey AL, Rouault TA. Numerous proteins in Mammalian cells are prone to iron-dependent oxidation and proteasomal degradation. Dev Neurosci. 2002;24:114–124. doi: 10.1159/000065693. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- Gilman SC, Bonner MJ, Pellmar TC. Effect of oxidative stress on excitatory amino acid release by cerebral cortical synaptosomes. Free Radic Biol Med. 1993;15:671–675. doi: 10.1016/0891-5849(93)90172-q. [DOI] [PubMed] [Google Scholar]
- Gray EG, Whittaker VP. The isolation of nerve endings from brain: an electron-microscopic study of cell fragments derived by homogenization and centrifugation. J Anat. 1962;96:79–88. [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Oxford University Press; Oxford: 1999. [Google Scholar]
- Halpain S. Actin in a supporting role. Nat Neurosci. 2003;6:101–102. doi: 10.1038/nn0203-101. [DOI] [PubMed] [Google Scholar]
- Hensley K, Carney J, Hall N, Shaw W, Butterfield DA. Electron paramagnetic resonance investigations of free radical-induced alterations in neocortical synaptosomal membrane protein infrastructure. Free Radic Biol Med. 1994;17:321–331. doi: 10.1016/0891-5849(94)90018-3. [DOI] [PubMed] [Google Scholar]
- Horton ND, Mamiya BM, Kehrer JP. Relationships between cell density, glutathione and proliferation of A549 human lung adenocarcinoma cells treated with acrolein. Toxicology. 1997;122:111–122. doi: 10.1016/s0300-483x(97)00086-3. [DOI] [PubMed] [Google Scholar]
- Kehrer JP, Biswal SS. The molecular effects of acrolein. Toxicol Sci. 2000;57:6–15. doi: 10.1093/toxsci/57.1.6. [DOI] [PubMed] [Google Scholar]
- Lemasters JJ, Holmuhamedov E. Voltage-dependent anion channel (VDAC) as mitochondrial governator--thinking outside the box. Biochim Biophys Acta. 2006;1762:181–190. doi: 10.1016/j.bbadis.2005.10.006. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Xie C, Markesbery WR. Acrolein, a product of lipid peroxidation, inhibits glucose and glutamate uptake in primary neuronal cultures. Free Radic Biol Med. 2000;29:714–720. doi: 10.1016/s0891-5849(00)00346-4. [DOI] [PubMed] [Google Scholar]
- Lovell MA, Xie C, Markesbery WR. Acrolein is increased in Alzheimer's disease brain and is toxic to primary hippocampal cultures. Neurobiol Aging. 2001;22:187–194. doi: 10.1016/s0197-4580(00)00235-9. [DOI] [PubMed] [Google Scholar]
- Luo J, Uchida K, Shi R. Accumulation of acrolein-protein adducts after traumatic spinal cord injury. Neurochem Res. 2005;30:291–295. doi: 10.1007/s11064-005-2602-7. [DOI] [PubMed] [Google Scholar]
- Mark RJ, Lovell MA, Markesbery WR, Uchida K, Mattson MP. A role for 4-hydroxynonenal, an aldehydic product of lipid peroxidation, in disruption of ion homeostasis and neuronal death induced by amyloid beta-peptide. J Neurochem. 1997;68:255–264. doi: 10.1046/j.1471-4159.1997.68010255.x. [DOI] [PubMed] [Google Scholar]
- Markesbery WR, Lovell MA. Four-hydroxynonenal, a product of lipid peroxidation, is increased in the brain in Alzheimer's disease. Neurobiol Aging. 1998;19:33–36. doi: 10.1016/s0197-4580(98)00009-8. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Neely MD, Quinn JF, Beal MF, Markesbery WR, Roberts LJ, Morrow JD. Lipid peroxidation in aging brain and Alzheimer's disease. Free Radic Biol Med. 2002;33:620–626. doi: 10.1016/s0891-5849(02)00807-9. [DOI] [PubMed] [Google Scholar]
- Patel JM, Block ER. Acrolein-induced injury to cultured pulmonary artery endothelial cells. Toxicol Appl Pharmacol. 1993;122:46–53. doi: 10.1006/taap.1993.1170. [DOI] [PubMed] [Google Scholar]
- Pocernich CB, Butterfield DA. Acrolein inhibits NADH-linked mitochondrial enzyme activity: implications for Alzheimer's disease. Neurotox Res. 2003;5:515–520. doi: 10.1007/BF03033161. [DOI] [PubMed] [Google Scholar]
- Pocernich CB, Cardin AL, Racine CL, Lauderback CM, Butterfield DA. Glutathione elevation and its protective role in acrolein-induced protein damage in synaptosomal membranes: relevance to brain lipid peroxidation in neurodegenerative disease. Neurochem Int. 2001;39:141–149. doi: 10.1016/s0197-0186(01)00012-2. [DOI] [PubMed] [Google Scholar]
- Seidler NW, Squire TJ. Abeta-polyacrolein aggregates: novel mechanism of plastic formation in senile plaques. Biochem Biophys Res Commun. 2005;335:501–504. doi: 10.1016/j.bbrc.2005.07.111. [DOI] [PubMed] [Google Scholar]
- Seidler NW, Yeargans GS. Albumin-bound polyacrolein: implications for Alzheimer's disease. Biochem Biophys Res Commun. 2004;320:213–217. doi: 10.1016/j.bbrc.2004.05.154. [DOI] [PubMed] [Google Scholar]
- Shimizu S, Narita M, Tsujimoto Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature. 1999;399:483–487. doi: 10.1038/20959. [DOI] [PubMed] [Google Scholar]
- Shin JH, London J, Le Pecheur M, Hoger H, Pollak D, Lubec G. Aberrant neuronal and mitochondrial proteins in hippocampus of transgenic mice overexpressing human Cu/Zn superoxide dismutase 1. Free Radic Biol Med. 2004;37:643–653. doi: 10.1016/j.freeradbiomed.2004.05.019. [DOI] [PubMed] [Google Scholar]
- Shoshan-Barmatz V, Gincel D. The voltage-dependent anion channel: characterization, modulation, and role in mitochondrial function in cell life and death. Cell Biochem Biophys. 2003;39:279–292. doi: 10.1385/CBB:39:3:279. [DOI] [PubMed] [Google Scholar]
- Sultana R, Boyd-Kimball D, Poon HF, Cai J, Pierce WM, Klein JB, Markesbery WR, Zhou XZ, Lu KP, Butterfield DA. Oxidative modification and down-regulation of Pin1 in Alzheimer's disease hippocampus: A redox proteomics analysis. Neurobiol Aging. 2006a;27:918–925. doi: 10.1016/j.neurobiolaging.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Sultana R, Perluigi M, Butterfield DA. Redox proteomics identification of oxidatively modified proteins in Alzheimer's disease brain and in vivo and in vitro models of AD centered around Abeta (1-42) J Chromatogr B Analyt Technol Biomed Life Sci. 2006b;833:3–11. doi: 10.1016/j.jchromb.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Sultana R, Poon HF, Cai J, Pierce WM, Merchant M, Klein JB, Markesbery WR, Butterfield DA. Identification of nitrated proteins in Alzheimer's disease brain using a redox proteomics approach. Neurobiol Dis. 2006c;22:76–87. doi: 10.1016/j.nbd.2005.10.004. [DOI] [PubMed] [Google Scholar]
- Suzuki D, Miyata T. Carbonyl stress in the pathogenesis of diabetic nephropathy. Intern Med. 1999;38:309–314. doi: 10.2169/internalmedicine.38.309. [DOI] [PubMed] [Google Scholar]
- Takano K, Ogura M, Yoneda Y, Nakamura Y. Oxidative metabolites are involved in polyamine-induced microglial cell death. Neuroscience. 2005;134:1123–1131. doi: 10.1016/j.neuroscience.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y, Nakagawa T, Shimizu S. Mitochondrial membrane permeability transition and cell death. Biochim Biophys Acta. 2006 doi: 10.1016/j.bbabio.2006.03.017. in press. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y, Shimizu S. The voltage-dependent anion channel: an essential player in apoptosis. Biochimie. 2002;84:187–193. doi: 10.1016/s0300-9084(02)01370-6. [DOI] [PubMed] [Google Scholar]
- Uchida K. Current status of acrolein as a lipid peroxidation product. Trends Cardiovasc Med. 1999;9:109–113. doi: 10.1016/s1050-1738(99)00016-x. [DOI] [PubMed] [Google Scholar]
- Uchida K, Kanematsu M, Sakai K, Matsuda T, Hattori N, Mizuno Y, Suzuki D, Miyata T, Noguchi N, Niki E, Osawa T. Protein-bound acrolein: potential markers for oxidative stress. Proc Natl Acad Sci U S A. 1998;95:4882–4887. doi: 10.1073/pnas.95.9.4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Wu X, Choi YE, Kern JC, Kehrer JP. Effect of acrolein and glutathione depleting agents on thioredoxin. Toxicology. 2004;204:209–218. doi: 10.1016/j.tox.2004.06.056. [DOI] [PubMed] [Google Scholar]