Abstract
Drug resistance caused by overexpression of P-glycoprotein (P-gp), the MDR1 (ABCB1) gene product, limits the therapeutic outcome. Expression of MDR1 can be induced by divergent stimuli, and involves a number of transcriptional factors. We found that the expression of CtBP1 (C-terminal-binding protein 1), a transcriptional co-regulator, was increased (~4 – fold) in human multidrug resistant (MDR) cancer cell lines, NCI/ADR-RES and A2780/DX, as compared to their sensitive counterparts. Silencing of CtBP1 expression by RNAi decreased the MDR1 mRNA and P-gp. Knockdown of CtBP1 also enhanced the sensitivity of MDR cells to chemotherapeutic drugs that are transported by P-gp and increased intracellular drug accumulation. In a reporter gene assay, co-transfection of MDR1 promoter constructs with a CtBP1 expression vector resulted in a ~2–4-fold induction of MDR1 promoter activity. CtBP1 appeared to contribute to the activation of MDR1 transcription through directly interacting with the MDR1 promoter, as evidenced by its physical binding to the promoter region of the MDR1 gene in chromatin immunoprecipitation and electromobility shift assays. Histone modifications at the MDR1 promoter, such as mono-methylation, di-methylation, and acetylation of histone H3, were not found to be affected by silencing of CtBP1 expression. Our results reveal a novel role for CtBP1 as an activator of MDR1 gene transcription, and suggest that CtBP1 might be one of the key transcription factors involved in the induction of MDR1 gene. Therefore, CtBP1 may represent a potentially new target for inhibiting drug resistance mediated by overexpression of the MDR1 gene.
Keywords: Multidrug resistance, MDR1 gene, P-glycoprotein, CtBP1, transcription, cancer
1. Introduction
Overexpression of the human multidrug transporter P-glycoprotein (P-gp), the product of the MDR1 (ABCB1) gene, confers cancer cell resistance to a variety of chemotherapeutic drugs, and constitutes one of the major obstacles to successful treatment of numerous types of malignancies. P-gp is a 170 ~ 190 kDa transmembrane phospho-glycoprotein that belongs to the ATP-binding cassette superfamily. The ability of P-gp to transport many structurally and functionally diverse cytotoxic compounds such as palitaxel, doxorubicin and vinblastine results in lowered intracellular drug concentration and decreased drug efficacy (1). Multidrug resistance (MDR) mediated by the overexpression of P-gp can be intrinsic, or induced by a variety of chemical or physical insults such as cytotoxic agents, arsenite, heat shock, and UV irradiation (2–7). Nevertheless, the molecular mechanism(s) underlying the induction of MDR1 expression has not been fully defined. The transcriptional activation of the MDR1 gene is a highly regulated complex event and is associated with several signaling pathways. For example, our laboratory demonstrated that activation of the phospholipase C/Raf/mitogen-activated protein kinase pathway stimulates the transcription of the MDR1 gene and expression of P-gp, and this pathway can be activated by heat shock, PDGF and EGF (8). The regulation of the MDR1 gene transcription also requires involvement of a number of transcriptional factors and co-regulators, including HIF-1, p53, NF-Y, c-fos, c-jun, NF-kB, etc. (9, 10). The elucidation of the roles of these factors in MDR1 gene transcription has led to a better understanding of the regulation of the expression of this gene, and helped develop new strategies to inhibit or prevent the induction of MDR1 expression.
In the current study, we demonstrated a novel role for the C-terminal binding protein 1 (CtBP1), a transcriptional co-regulator that favors oncogenesis and tumor cell survival (11), in the activation of MDR1 expression. CtBP1, a 48 kDa protein that was originally shown to bind to the C-terminal region of the human adenovirus E1A proteins (12), is present in both the nucleus and cytoplasm, and functions mainly as a transcriptional corepressor (13). Vertebrates, including human, have two CtBP homologs: CtBP1 and CtBP2; in invertebrates, only a single CtBP gene has been identified. CtBP family members share amino acid sequence homology with D-2-hydroxy acid dehydrogenases, and possess an NAD (nicotinamide adenine dinucleotide) - regulated dehydrogenase activity that is believed to be required for its function as a transcriptional corepressor (13). CtBP can interact with a number of DNA-binding transcriptional repressors that contain Pro-X-Asp-Leu-Ser (PXDLS) motifs, such as SLUG, Knirps, snail and ZEB, thereby repressing the transcription of a variety of genes (14–16). CtBP may also interact with certain regulatory proteins that lack PXDLS motif, including histone deacetylase 1 (HDAC1), HDAC2 and HDAC5 (17–19), to form multicomponent transcriptional complexes. Furthermore, recent study shows that CtBP can not only repress, but also activate gene transcription. It has been shown that in addition to repressing a subset of Wnt target genes in the absence of Wnt stimulation, CtBP also causes transcriptional activation of several genes upon Wnt stimulation (20). We report here that CtBP1 participates in transcriptional activation of the MDR1 gene through direct interaction with the MDR1 promoter, and inhibition of CtBP1 expression can enhance sensitivity of MDR cancer cells to certain chemotherapeutic drugs.
2. Methods and Materials
2.1. Cell lines and culture
The MDR cancer cell line, NCI/ADR-RES (previously named MCF-7/AdrR), and the sensitive line, MCF-7, were provided by Dr. Kenneth Cowan of the Eppley Institute for Research in Cancer (Omaha, NE). They were maintained in RPMI 1640 medium containing 10% fetal bovine serum, 100 units/ml penicillin, and 100 μg/ml streptomycin at 37°C in a humidified atmosphere containing 5% CO2/95% air. The MDR human ovarian carcinoma cell line, A2780DX, and its parental, sensitive line, A2780, were kindly supplied by Dr. Youcef Rustum (Roswell Park Cancer Institute, Buffalo, NY) and were grown in DMEM medium containing 10% fetal bovine serum under the identical condition as described above except that for A2780DX, 2 μM of doxorubicin was added to the medium for the maintenance of the MDR phenotype. The human colon carcinoma cell line, HCT-15, was purchased from the American Type Culture Collection (Manassas, VA) and maintained as instructed. Cells were checked routinely and found to be free of contamination by Mycoplasma or fungi. All the cell lines were discarded after 3 months and new lines obtained from frozen stocks.
2.2. siRNA and transfection
The siRNA targeting CtBP1 and non-targeting siRNA were purchased from Dharmacon, Inc (Lafayette, CO). The CtBP1-targeted siRNA duplexes with the following sense and antisense sequences were used: GAGCAGGCAUCCAUCGAGAUU-5′ (sense) and 5′-PUCUCGAUGGAUGCCUGCUCUU (antisense); GGAUAGAGACCACGCCAGUUU-5′ (sense) and 5′-PACUGGCGUGGUCUCUAUCCUU (antisense). Cells in exponential phase of growth were plated in 6-well plates at 5 × 105 cells/well, grown for 24 h, and then transfected with CtBP1-targeted siRNA or non-targeting siRNA at a final concentration of 100nM using oligofectamine and OPTI-MEM I reduced serum medium (Invitrogen Life Technologies, Inc., Carlsbad, CA), according to the manufacturer’s protocol. The concentrations of siRNAs were chosen based on dose-response studies. Silencing effect was examined 72 h after transfection.
2.3. Reverse transcription-PCR
Total RNA was extracted from cells using TRIzol reagent (Invitrogen Life Technologies, Inc., Carlsbad, CA), and quantified by UV absorbance spectroscopy. The reverse transcription reaction was performed using the Superscript First-Strand Synthesis System (Invitrogen Life Technologies, Inc.) in a final volume of 20 μl containing 5 μg of total RNA, 200 ng of random hexamers, 1 × reverse transcription buffer, 2.5 mM MgCl2, 1 mM deoxynucleotide triphosphate mixture, 10 mM DTT, RNaseOUT recombinant ribonuclease inhibitor, 50 units of Superscript reverse transcriptase, and diethylpyrocarbonate-treated water. After incubation at 42°C for 50min, the reverse transcription reaction was terminated by heating at 85°C for 5 min. The newly synthesized cDNA was amplified by PCR. The reaction mixture contained 2 μl of cDNA template, 1.5 mM MgCl2, 2.5 units of Taq polymerase, and 0.5 μM of MDR1 primer (5′-ATATCAGCAGCCCACATCAT-3′; 5′-GAAGCACTGGGATGTCCGGT-3′), or CtBP1 primer (5′-CTGGAGAAGTTCAAAGCC-3′; 5′-CCATCCGACAAGTAAGGG-3′). GAPDH primer (5′-GCCAAAAGGGTCATCATCTC-3′; 5′-GTAGAGGCAGGGATGATGTTC-3′) was used as an internal control. Amplication cycles were: 94°C for 3 min, then 33 cycles at 94°C for 1 min, 58°C for 1 min, 72°C for 1.5 min, followed by 72°C for 10 min. Aliquots of PCR product were electrophoresed on 1.5% agarose gels, and PCR fragments were visualized by ethidium bromide staining.
2.4. Western blot analysis
Cells were washed twice with PBS containing 1 mM phenylmethylsulphonyl fluoride, and then lysed in mammalian protein extraction buffer (Pierce Biotechnology Inc., Rockford, IL). The lysates were transferred to Eppendorf tubes and clarified by centrifugation at 12,000 X g for 30 min at 4°C. Identical amounts (50 μg protein) of cell lysates were resolved by 6% SDS-PAGE, and then transferred to nitrocellulose. The membranes were incubated in blocking solution consisting of 5% powered milk in PBST (PBS plus 0.1% Tween 20) at room temperature for 1 h, then immunoblotted with monoclonal anti-P-gp antibody C219 (Calbiochem, San Diego, CA), anti-CtBP1 antibody (Upstate, Lake Placid, NY), or antitubulin antibody (Sigma-Aldrich, St. Louis, MO). Detection by enzyme-linked chemiluminescence was performed according to the manufacturer’s protocol (ECL; Amersham Pharmacia Biotech, Piscataway, NJ). Quantification of protein bands was carried out using the Molecular Analyst software (Bio-Rad Laboratories, Hercules, CA).
2.5. Luciferase reporter gene assay
A2780 and MCF-7 cells were first seeded in 6-well tissue culture plates at a density of 1–2×105 cells/well and cultured for 24 h; cells were then co-transfected with 1.0 μg MDR1 promoter/luciferase deletion constructs pMDR1 series (21) and 0.5 μg pcDNA3.0-CtBP1 plasmid (a gift from Dr. Yang Shi of Harvard Medical School, Boston, MA) or pcDNA3.0 plasmid using Lipofectamine. Renilla luciferase reporter construct, pRL-TK (Promega, Madison, WI), was used as an internal control for transfection efficiency. Forty hours later, luciferase activity of protein extracts was measured on a TD-20/20 luminometer (Turner Designs, Sunnyvale, CA). Luciferase activity of the MDR1 promoter was normalized to the protein content and the activity of Renilla luciferase expression vector.
2.6. Chromatin immunoprecipitation (ChIP) assay
ChIP assays were carried out according to the manufacturer’s protocol (Active motif, Carlsbad CA). Briefly, cells in 150-mm tissue culture dishes were fixed with 1% formaldehyde and incubated for 10 min at 37°C. The cells were then washed twice with ice-cold PBS, harvested, and re-suspended in ice-cold TNT lysis buffer (20 mM Tris-HCl, pH 7.4, 200 mM NaCl, 1% Triton X-100, 1 mM PMSF and 1% aprotinin). The lysates were sonicated to shear the DNA to fragments of 200–500 bp, and subjected to immunoprecipitation with antibodies (1 μg) specific for CtBP1, mono-Me-K9 H3, di-Me-K9 H3, or Ac-K9 H3 (Upstate, Lake Placid, NY). The antibody/protein complexes were collected by Protein G beads and washed 3 times with ChIP wash buffer (5% SDS, 1 mM EDTA, 0.5% bovine serum albumin and 40 mM NaHPO4, pH 7.2). The immune complexes were eluted with 1% SDS and 1 M NaHCO3, and the cross-links were reversed by incubation at 65°C for 4 h in the presence of 200 mM NaCl and RNase A. The samples were then treated with proteinase K for 2 h, and the DNA was purified by mini-column, ethanol precipitation, and re-suspended in 100 μl of H2O. The primer corresponding to the MDR1 promoter region -164 and -11 upstream of the transcription start site (sense: 5′-CCTCCTGGAAATTCAACCTG-3′; antisense: 5′-TGTGGCAAAGAGAGCGAAG-3′) (22) was used for PCR to detect the presence of the MDR1 promoter DNA.
2.7. Electrophoretic mobility shift assay (EMSA)
EMSAs were performed using a LightShift™ Chemiluminescent EMSA Kit (Pierce Biotechnology Inc., Rockford, IL). The binding reactions were carried out as previously described (21). Briefly, nuclear extracts (10 μg) made with the compartmental protein extraction kit (Chemicon, Temecula, CA) were pre-incubated for 15 min at room temperature in the presence or absence of 200-fold molar excess of unlabeled competitor DNA, in 100 mM KCl, 10 mM HEPES (pH 7.9), 2.5 mM MgCl2, 0.5 mM dithiothreitol, 4% glycerol, 25 μg/ml of poly(dI-dC) in a total volume of 20 μl. After addition of 15 fmol biotin 3′-end-labeled double-stranded oligonucleotide probe, the reaction mixtures were incubated at room temperature for another 20 min. At the end of incubation, samples were resolved on a native 4% polyacrylamide gel, transferred to a nylon membrane, and UV cross-linked. The biotin end-labeled oligonucleotides were detected by a chemiluminescent reaction with streptavidin-horseradish peroxidase and visualized by autoradiography. The nucleotide sequence of the double-stranded oligonucleotides corresponds to the promoter region -75 and -50 of the MDR1 gene and is as follows: TGGCTGGGCAGGAACAGCGCCGGGGC.
2.8. Drug sensitivity assay
CtBP1-targeted siRNA or non-targeting siRNA transfected NCI/ADR-RES or A2780DX cells were plated in 96-well plates in growth medium and incubated at 37°C in a humidified 5% CO2 atmosphere for 60 h in the presence of varying concentrations of vinblastine, doxorubicin, or hydroxyurea. At the end of incubation, the viability of cells was measured using the MTT Assay.
3. Results
3.1. Expression of CtBP1 is increased in MDR cancer cells
We observed that the expression of CtBP1 was increased in P-gp-overexpressing MDR cancer cell lines, NCI/ADR-RES and A2780/DX, as compared to their drug sensitive counterparts (Figure 1a). Densitometry analysis of the immunoblot in Figure 1a shows that CtBP1 expression was ~ 4 -fold higher in MDR cell lines relative to their respective sensitive lines, MCF-7 and A2780 (Figure 1b).
Figure 1. Expression of CtBP1 in sensitive and MDR cancer cells.
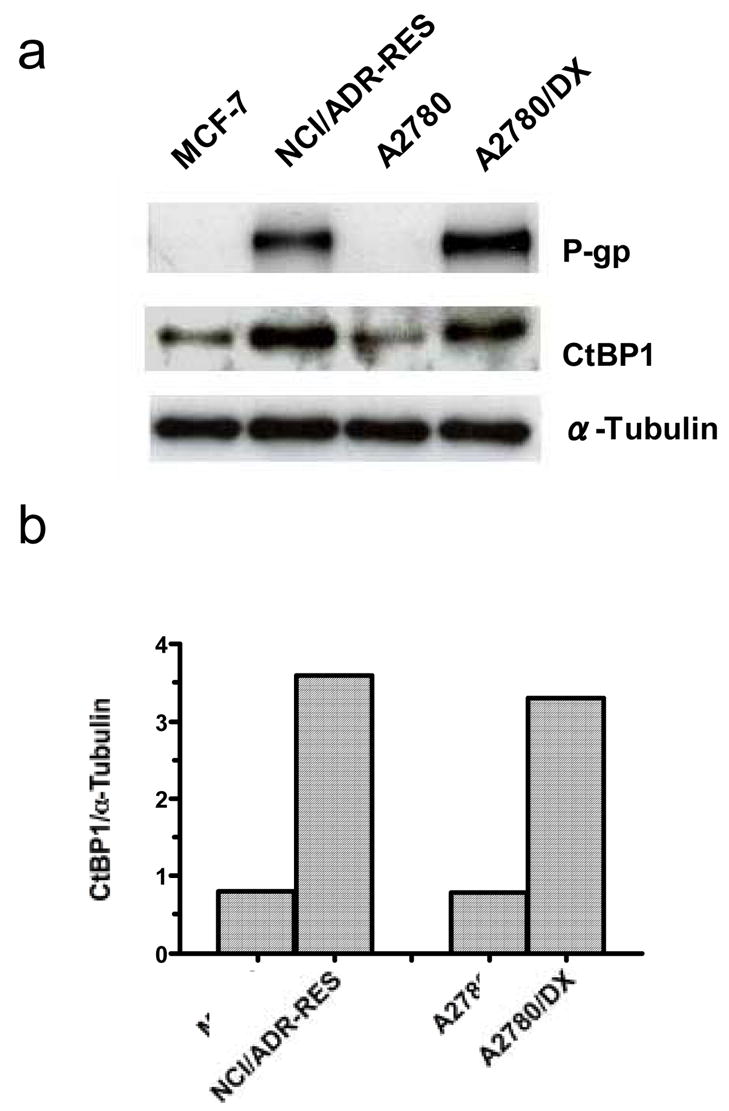
a. Cell lysates were prepared from sensitive and MDR cancer cells using RIPA buffer, and equal amounts (50 μg) of proteins were resolved by 6% SDS-PAGE. Proteins were transferred to nitrocellulose membrane, and CtBP1 and P-gp were detected by immunoblotting with monoclonal anti-CtBP1 or anti-P-gp antibodies, respectively. α–tubulin was used as a loading control. b. CtBP1 protein expression in sensitive and MDR cells was quantified by densitometry using Scion Image software (Scion Corporation, Frederick, Maryland). Results are the representative of three similar experiments.
3.2. Knockdown of CtBP1 decreases the expression of endogenous MDR1 mRNA and P-gp in MDR cancer cells
To determine whether or not CtBP1 plays a role in the regulation of MDR1 gene expression, we knocked down the expression of CtBP1 with a gene – specific siRNA, and measured the expression of MDR1 mRNA and P-gp. Figure 2a shows that silencing of CtBP1 expression by the targeting siRNA remarkably decreased the amount of MDR1 mRNA in MDR cell lines, NCI/ADR-RES and A2780/DX, as determined by RT-PCR. Non-targeting siRNA had no effect on MDR1 mRNA expression. Expression of the MDR1 gene product, P-gp, was also reduced with the knockdown of CtBP1, as analyzed by Western blot (Figure 2b). To control for potential off-target effects of siRNA, we tested multiple CtBP1 – targeted siRNA sequences. Figure 2C demonstrates the similar effects of another CtBP1-siRNA sequence on expression of MDR1 mRNA, suggesting that the effect of silencing CtBP1 on MDR1 expression is specific. In human colon carcinoma cell line HCT-15, which intrinsically expresses MDR1, treatment with the CtBP1 - targeted siRNA also inhibited the expression of MDR1 mRNA and P-gp (Figure 2d). These results indicate a role for CtBP1 in the activation of the MDR1 expression.
Figure 2. Effect of CtBP1-siRNA on expression of MDR1 mRNA and P-gp.

a. MDR cell lines, NCI/ADR-RES and A2780DX, were treated with 100 nM of a CtBP1-targeted siRNA (CtBP1siRNA), or non-targeting RNA, or mock, which only consisted of oligofectamine and OPTI-MEM® I reduced serum medium. Seventy-two hours later, total RNA was extracted from the cells and the RT reaction was performed. MDR1 and CtBP1 cDNA were amplified by PCR using the MDR1 primer (5′-ATATCAGCAGCCCACATCAT-3′; 5′-GAAGCACTGGGATGTCCGGT-3′) and CtBP1 primer (5′-TACCACACCATCACTCTCAC-3′; 5′-CTCTGGACTCGTGTGCCCTC-3′), respectively. GAPDH was used as an internal control. Aliquots of PCR products were electrophoresed on 1.5% agarose gels, and PCR fragments were visualized by ethidium bromide staining. b. NCI/ADR-RES and A2780DX cells were treated with 100 nM of a CtBP1 siRNA (CtBP1siRNA), or non-targeting RNA, or mock, and cell lysates were prepared from the treated cells. Equal amounts (50 μg proteins) of cell lysates were separated by 6% SDS-polyacrylamide gel electrophoresis, and then transferred onto nitrocellulose membrane. The membranes were immunoblotted with monoclonal anti-P-gp, anti-CtBP1, or anti-tubulin antibodies. Detections of proteins were performed using enzyme-linked chemiluminescence. Protein bands were quantified by the Molecular Analyst software. Results are the representative of three similar experiments. c. NCI/ADR-RES and A2780DX cells were treated with 100 nM of CtBP1siRNA-2, and the effect of the siRNA on MDR1 mRNA expression was determined as described above. d. HCT-15 colon carcinoma cells were treated with 100 nM of a CtBP1 siRNA (CtBP1siRNA) or non-targeting RNA, and the effects of the siRNA on MDR1 mRNA and P-gp expression were determined as described above. Results are the representative of two similar experiments.
3.3. CtBP1 activates MDR1 gene expression though direct interaction with its promoter
To further analyze the role of CtBP1 in MDR1 transcription, we measured and compared the activity of the MDR1 promoter (luciferase activity) in A2780 and MCF-7 cells transiently co-transfected with MDR1 promoter-luciferase constructs and a CtBP1 expression vector or empty control vector. As shown in Figure 3, co-transfection with a CtBP1 expression vector led to a ~2–4 fold induction of MDR1 promoter activity, as reflected in the increase of luciferase activity of pMDR1 -1202, -221, -136 and -75. CtBP1 did not affect the activity of pMDR1 -4 (Figure 3), suggesting that -75/-4 is the region required for the CtBP1-induced effect. To gain insight into the mechanism underlying the effect of CtBP1 on MDR1 transcription, we performed chromatin immunoprecipitation (ChIP; Figure 4a) and electromobility shift assays (EMSA; Figure 4b). As demonstrated in Figure 4a, immunoprecipitation of the cross-linked chromatin from MDR cell lines NCI/ADR-RES and A2780/DX with the antibody specific for CtBP1 followed by PCR amplification of the eluted DNA using the MDR1 promoter-specific primer revealed that CtBP1 bound to the MDR1 promoter in vivo. The physical interaction of CtBP1 with the MDR1 promoter was also observed in EMSA assays in which nuclear extracts from MCF-7 cells transfected with the CtBP1 plasmid were incubated with a biotin-labeled MDR1 promoter fragment. Figure 4b demonstrates that MDR1 promoter DNA formed a specific complex with CtBP1 protein.
Figure 3. Activation of the MDR1 promoter by CtBP1.

A2780 (a) and MCF-7 (b) cells were plated in 6-well tissue culture plates, then co-transfected with 1 μg of MDR1 promoter deletion constructs (pMDR1) and 0.5 μg of pcDNA3.0-CtBP1 expression vector or pcDNA3.0 control vector using Lipofectamine. Renilla luciferase reporter construct pRL-TK (20 ng) was used as an internal control for transfection efficiency. Forty hours after transfection, luciferase activity was measured with equivalent amounts of protein extracts. Luciferase activity of the MDR1 promoter was normalized to the activity of a co-transfected Renilla luciferase expression vector and protein content. Activity in the presence of pcDNA3.0 was arbitrarily set at 1. Each value represents the mean ± SD of triplicate determinations from one of three identical experiments.
Figure 4. Binding of CtBP1 to the MDR1 promoter.
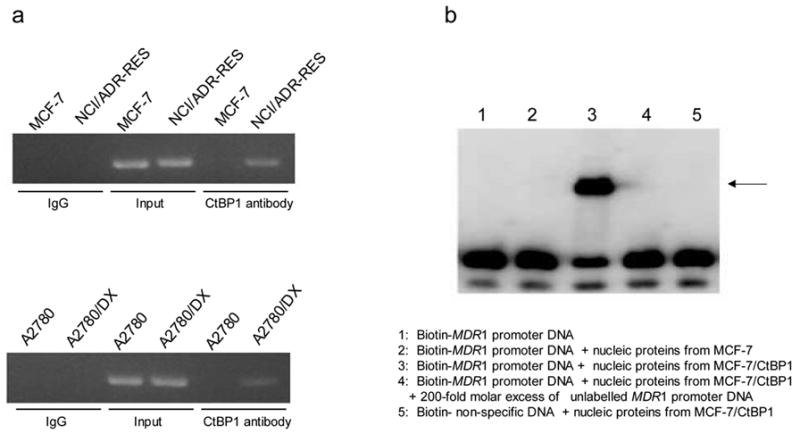
a. ChIP: Nucleic extracts were prepared from sensitive cell lines, MCF7 and A2780, or MDR cell lines, MCF7/ADR and A2780/DX. ChIP assays were performed using the chromatin immunoprecipitation kits from Active motif and antibody against CtBP1, as described in “Materials and Methods”. The primer corresponding to the MDR1 promoter region -164 and -11 upstream of the transcription start site was used for PCR to detect the presence of the MDR1 promoter DNA. DNA amount used for PCR was adjusted and normalized according to the MDR1 gene copy number in sensitive and MDR cell lines. Input control: sonicated, pre-cleared genomic DNA without the ChIP but amplified with the same MDR1 primer. Results are the representative of two similar experiments. b. An autoradiograph of EMSA: Nuclear extracts of MCF-7 cells were incubated with the biotin-labeled oligonucleotides corresponding to the promoter region (-75 and -50 of the MDR1 gene: TGGCTGGGCAGGAACAGCGCCGGGGC) in the absence (lane 3) or presence of 200-fold molar excess of unlabeled competitor DNAs (lane 4). Lane 1 contained no nuclear extract, and Lane 5 contained an unrelated DNA sequence. The samples were separated by a 4% nondenaturing polyacrylamide gel electrophoresis. The arrow indicates the specific CtBP1-DNA complex. Results are the representative of three similar experiments.
CtBP1 was previously reported to regulate the transcription of certain genes through altering the status of methylation and acetylation at lysine 9 of histone H3 at the promoters of those genes (23). To detect the possible role of histone modifications in CtBP1-induced activation of MDR1 transcription, ChIP assays were utilized to compare the mono-methylation, di-methylation, and acetylation of histone H3 at the MDR1 promoter in NCI/ADR-RES cells treated with the CtBP1 siRNA, non-targeting siRNA or mock. We found that mono-methylation, di-methylation and acetylation at lysine 9 of histone H3 at the MDR1 promoter were not affected by the knockdown of CtBP1 (Figure 5), suggesting that the effect of CtBP1 on MDR1 activation does not involve histone modification.
Figure 5. CtBP1 does not affect histone modification at the endogenous MDR1 promoter.
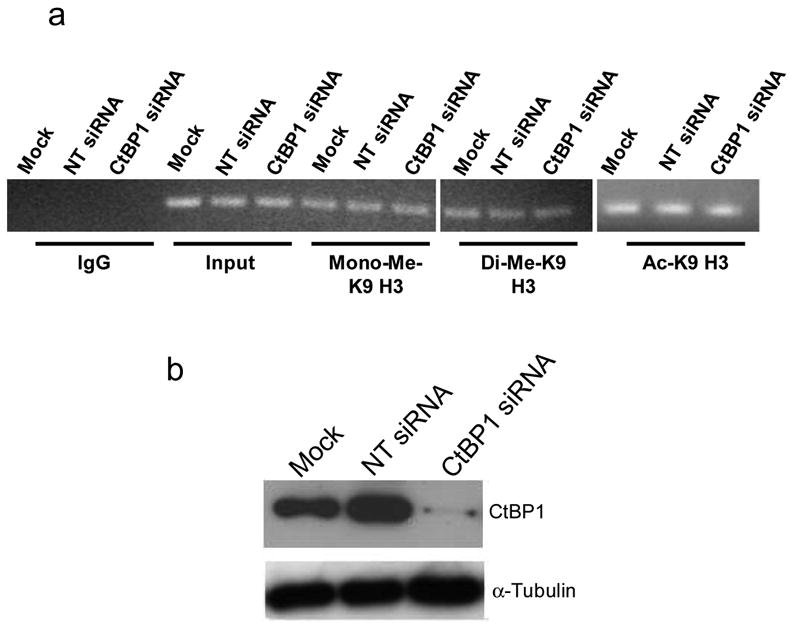
a. ChIP analysis of mono-methylation, di-methylation and acetylation at lysine 9 of histone H3 on the MDR1 promoter in NCI/ADR-RES cells treated with mock, non-targeting siRNA or CtBP1 siRNA. Anti-monomethyl-Histone H3 (Lys9), anti-dimethyl-Histone H3 (Lys9) and anti-acetyl-Histone H3 (Lys9) antibodies were used in ChIP. b. Western blot analysis of CtBP1 in NCI/ADR-RES cells treated with mock, non-targeting siRNA or CtBP1 siRNA. Results are the representative of two similar experiments.
3.4. Silencing of CtBP1 expression can modulate MDR phenotype
We next tested whether knockdown of CtBP1 expression could increase sensitivity of MDR cancer cells to cytotoxic drugs that are transported by P-gp. MDR cell lines, NCI/ADR-RES and A2780DX, were treated with the CtBP1 siRNA or non-targeting siRNA for 48 hours, and then were seeded in 96-well plates and incubated in the presence of various concentrations of doxorubicin, vinblastine or hydroxyurea for 60 h. As shown in Table 1, compared with the treatment of non-targeting RNA, the sensitivity of MDR cells to doxorubicin and vinblastine was increased 2.7~4.0- and 2.2~3.0 fold respectively by the treatment with the CtBP1 siRNA. The sensitivity to non-P-gp transportable drug, hydroxyurea, was not affected by silencing of CtBP1 expression (Table 1). Silencing of CtBP1 also increased intracellular accumulation of the drugs that are substrates for P-gp. As show in Figure 6, the intracellular accumulation of doxorubicin was increased in CtBP1-siRNA-treated NCI/ADR-RES cells, as compard with that in non-targeting siRNA-treated cells.
Table 1.
Effect of silencing of CtBP1 expression on sensitivity of MDR cancer cells to chemotherapeutic drugs.
| IC50 | ||||
|---|---|---|---|---|
| Doxorubicin (μM) | Vinblastine (nM) | Hydroxyurea (μM) | ||
| NCI/ADR-RES | NT siRNA | 17 ± 2 | 293 ± 15 | 125 ± 15 |
| CtBP1 siRNA | 6.3 ± 0.3* | 95 ± 6** | 130 ± 9 | |
| A2780DX | NT siRNA | 1.2 ± 0.1 | 55 ± 2 | |
| CtBP1 siRNA | 0.3 ± 0.1** | 25 ± 5* | ||
IC50 is the concentration of a drug that inhibited 50% cell viability compared to vehicle-treated controls in MTT assay. Results are the mean ± standard error of three separate experiments.
p < 0.05 versus NT siRNA treatment;
p < 0.01 versus NT siRNA treatment.
Figure 6. Effect of silencing of CtBP1 expression on doxorubicin accumulation in NCI/ADR-RES cells.
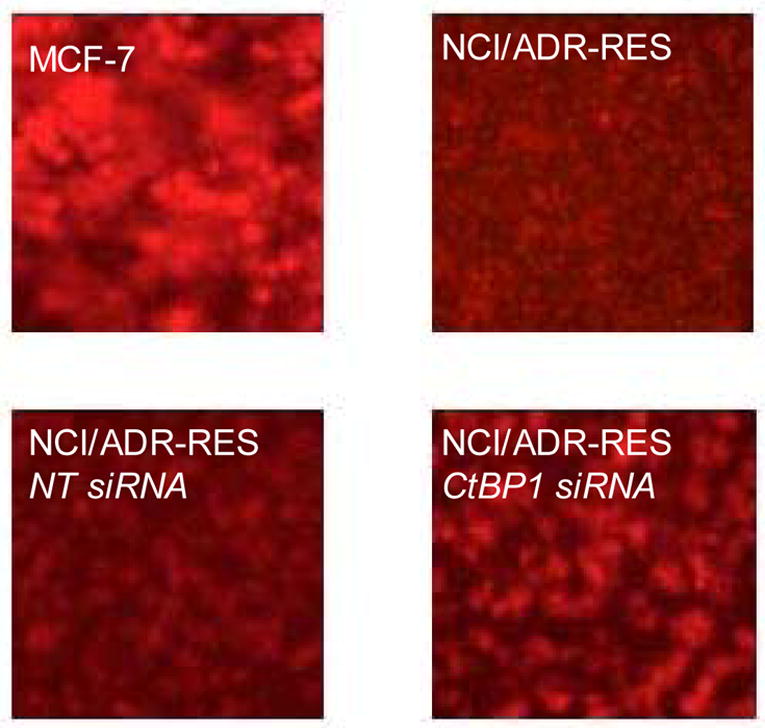
NCI/ADR-RES cells grown in 100-mm culture dishes were treated with CtBP1-targeted siRNA (100 nM) or non-targeting siRNA. Seventy-two hours later, cells were incubated with 25 μM of doxorubicin for 2h, followed by washing three times with PBS. Doxorubicin accumulation was observed under a fluorescence microscope with 100X lens. Results are representative of three similar experiments.
4. Discussion
In this study, we demonstrate that CtBP1, a “conventional” transcriptional co-repressor, plays a role in transcriptional activation of the MDR1 gene. We found that the expression of CtBP1 is increased in human MDR ovarian cancer cell lines, NCI/ADR-RES and A2780/DX (Figure 1), and knockdown of CtBP1 by RNA interference results in reduction of MDR1 mRNA and P-gp (Figure 2) and leads to sensitization of MDR cells to chemotherapeutic drugs that are substrates of this transporter protein (Table 1). We further show that CtBP1 directly binds to the MDR1 promoter both in in vitro (Figure 4b) and in vivo (Figure 4a) assays and enhances the promoter activity of this gene (Figure 3). These results reveal, for the first time, the role for CtBP1 as an “activator” of MDR1 transcription.
CtBP1 has long been known as a transcriptional co-repressor. Although the exact mechanism of its repressive effect on gene transcription has not been fully defined, it is generally appreciated that through association with transcription factors that contain PXDLS motif, CtBP1 can be recruited to DNA, thereby repressing gene transcription through epigenetic events such as chromatin remodeling. For example, CtBP1-mediated repression of transcription has been demonstrated to result from the histone modifications caused by the CtBP complex, which contains histone-modifying enzymes such as histone deacetylases and histone methyltransferases (23). The NADH activity of CtBP also contributes to its co-repressor function (24). However, we found in the current study that the effect of CtBP1 on MDR1 gene transcription was stimulatory rather than repressive, because knockdown of CtBP1 expression caused a reduction in the amount of MDR1 mRNA and P-gp (Figure 2 and 3). Since CtBP1 expression was increased in the drug resistant cells derived by step-wise selection with chemotherapeutic drugs as compared to that in sensitive cells that were not exposed to any drug (Figure 1), it is likely that the up-regulation of CtBP1 by drug treatment is an important contributing factor in the induction of MDR1 expression. In the cells with silencing of CtBP1 expression, inhibition of MDR1 mRNA expression (Figure 2a) was greater than that of P-gp expression (Figure 2b). This is likely due to the high content of P-gp in these cells (25–27) and the relatively long half-life (14–17 h) of the protein (28). The role for CtBP1 in the activation of MDR1 expression was demonstrated not only in the MDR cancer cell lines whose expression of MDR1/P-gp is induced by drug treatments (Figure 2a and b), but also in the cancer cells that intrinsically express MDR1/P-gp (Figure 2d).
Using MDR1 promoter deletion constructs we have identified the sequence between −75 to −4 to be the CtBP1 responsive region (Figure 3). This conclusion is supported by the results of ChIP and EMSA, which respectively show that CtBP1 binds to the MDR1 promoter sequence (Figure 4a) and that the binding occurs to between −75 and −50 within the promoter (Figure 4b). Since these sequences contain the MDR1 enhancesome, a promoter region that includes binding sites for some transcription factors such as NF-Y and Sp1 and responds to a variety of stressful stimuli (9, 10), it is likely that CtBP1 acts on the MDR1 enhancesome. These results suggest that in addition to acting as a transcription co-repressor, CtBP1 can also activate gene transcription through its direct effect on promoter region of the genes. Recently, the function of CtBP as a transcriptional activator has also been reported for Wnt target genes. It was demonstrated that CtBP direct activates Wnt transcriptional targets through its interaction with the Wnt-regulated enhancer of the genes (20). Therefore, it appears that CtBP1 is a “dual” regulator, i.e., activator and repressor of transcription, and inhibiting or activating gene transcription by CtBP appears to be context-dependent. The “dual” function of CtBP1 might account for its broad effects on various biological and path-physiologic processes such as development, tumorigenesis, and cell survival.
Transcriptional regulation of the MDR1 gene has also been known to involve epigenetic mechanisms. For instance, it has been shown that the activation of MDR1 expression by chemotherapeutic drugs is correlated with methylation and hyperacetylation of histone H3 as well as methylation status of the MDR1 promoter (21, 22, 29, 30). These histone modifications are brought about by histone-modifying enzymes such as histone acetyltransferase and histone deacetylase, which are known to be required in the transcriptional regulation of the MDR1 gene (21). Although CtBP1 has been shown to modulate the transcription of certain genes through altering histone modifications, we did not observe any detectable changes in mono-methylation, di-methylation, or acetylation of histone H3 at MDR1 promoter when CtBP1 was silenced (Figure 5), suggesting that CtBP1-induced activation of MDR1 transcription is not mediated by changes in histone modification.
Recently, several studies have reported the role of CtBP in supporting cell survival. It was demonstrated that CtBP repressed expression of proapoptotic genes and caused inhibition of apoptosis (31, 32). Here we show that CtBP1 can act as an activator of MDR1 gene expression (Figure 3), and inhibition of CtBP1 by RNA-mediated interference leads to a down-regulation of MDR1 mRNA and P-gp (Figure 2), an increased sensitivity to chemotherapeutic drugs that are the substrates of this transporter (Table 1), and an enhanced intracellular drug accumulation (Figure 6). In addition, the expression of CtBP1 in MDR cancer cells is found to be higher than that in sensitive cells (Figure 1). These studies concur to support the role of CtBP as a surviving-promoting factor. The results that CtBP1 induced a moderate (~2–4 fold) increase in MDR1 promoter activity (Figure 3) as well as knockdown of CtBP1 caused a moderate (~2–4 fold) reversal of drug resistance (Table 1) might reflect that CtBP1 is only one of the multiple collaborative components that participate in activating MDR1 gene expression.
In summary, we demonstrate a novel function of CtBP1 in activating MDR1 gene expression, and this function appears to be mediated through its direct interaction with the enhancesome region of the MDR1 promoter. The finding of this study implicates CtBP1 in drug resistance, and may contribute to elucidation of the mechanism of induction of MDR1 gene and to exploration of new effective means to modulating MDR phenotype.
Acknowledgments
This study was supported by US Public Health Service grants NCI CA 66077 and CA 72720. We are thankful to Dr. Yang Shi, Harvard Medical School, for his generous gift of CtBP1 plasmid.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Gottesman MM, Ling V. The molecular basis of multidrug resistance in cancer: the early years of P-glycoprotein research. FEBS Lett. 2006;580:998–1009. doi: 10.1016/j.febslet.2005.12.060. [DOI] [PubMed] [Google Scholar]
- 2.Abolhoda A, Wilson AE, Ross H, Danenberg PV, Burt M, Scotto KW. Rapid activation of MDR1 gene expression in human metastatic sarcoma after in vivo exposure to doxorubicin. Clin Cancer Res. 1999;5:3352–3356. [PubMed] [Google Scholar]
- 3.Chaudhary PM, Roninson IB. Induction of multidrug resistance in human cells by transient exposure to different chemotherapeutic drugs. J Natl Cancer Inst. 1993;85:632–639. doi: 10.1093/jnci/85.8.632. [DOI] [PubMed] [Google Scholar]
- 4.Chin KV, Chauhan SS, Pastan I, Gottesman MM. Regulation of mdr RNA levels in response to cytotoxic drugs in rodent cells. Cell Growth Differ. 1990;1:361–365. [PubMed] [Google Scholar]
- 5.Chin KV, Tanaka S, Darlington G, Pastan I, Gottesman MM. Heat shock and arsenite increase expression of the multidrug resistance (MDR1) gene in human renal carcinoma cells. J Biol Chem. 1990;265:221–226. [PubMed] [Google Scholar]
- 6.Hu Z, Jin S, Scotto KW. Transcriptional activation of the MDR1 gene by UV irradiation. Role of NF-Y and Sp1. J Biol Chem. 2000;275:2979–2985. doi: 10.1074/jbc.275.4.2979. [DOI] [PubMed] [Google Scholar]
- 7.Uchiumi T, Kohno K, Tanimura H, Matsuo K, Sato S, Uchida Y, Kuwano M. Enhanced expression of the human multidrug resistance 1 gene in response to UV light irradiation. Cell Growth Differ. 1993;4:147–157. [PubMed] [Google Scholar]
- 8.Yang JM, Vassil AD, Hait WN. Activation of phospholipase C induces the expression of the multidrug resistance (MDR1) gene through the Raf-MAPK pathway. Mol Pharmacol. 2001;60:674–680. [PubMed] [Google Scholar]
- 9.Scotto KW, Johnson RA. Transcription of the multidrug resistance gene MDR1: a therapeutic target. Mol Interv. 2001;1:117–125. [PubMed] [Google Scholar]
- 10.Scotto KW. Transcriptional regulation of ABC drug transporters. Oncogene. 2003;22:7496–7511. doi: 10.1038/sj.onc.1206950. [DOI] [PubMed] [Google Scholar]
- 11.Bergman LM, Blaydes JP. C-terminal binding proteins: emerging roles in cell survival and tumorigenesis. Apoptosis. 2006;11:879–888. doi: 10.1007/s10495-006-6651-4. [DOI] [PubMed] [Google Scholar]
- 12.Boyd JM, Subramanian T, Schaeper U, La Regina M, Bayley S, Chinnadurai G. A region in the C-terminus of adenovirus 2/5 E1a protein is required for association with a cellular phosphoprotein and important for the negative modulation of T24-ras mediated transformation, tumorigenesis and metastasis. Embo J. 1993;12:469–478. doi: 10.1002/j.1460-2075.1993.tb05679.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chinnadurai G. CtBP, an unconventional transcriptional corepressor in development and oncogenesis. Mol Cell. 2002;9:213–224. doi: 10.1016/s1097-2765(02)00443-4. [DOI] [PubMed] [Google Scholar]
- 14.Tripathi MK, Misra S, Khedkar SV, Hamilton N, Irvin-Wilson C, Sharan C, Sealy L, Chaudhuri G. Regulation of BRCA2 gene expression by the SLUG repressor protein in human breast cells. J Biol Chem. 2005;280:17163–17171. doi: 10.1074/jbc.M501375200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nibu Y, Zhang H, Bajor E, Barolo S, Small S, Levine M. dCtBP mediates transcriptional repression by Knirps, Kruppel and Snail in the Drosophila embryo. Embo J. 1998;17:7009–7020. doi: 10.1093/emboj/17.23.7009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Postigo AA, Dean DC. ZEB represses transcription through interaction with the corepressor CtBP. Proc Natl Acad Sci U S A. 1999;96:6683–6688. doi: 10.1073/pnas.96.12.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sundqvist A, Sollerbrant K, Svensson C. The carboxy-terminal region of adenovirus E1A activates transcription through targeting of a C-terminal binding protein-histone deacetylase complex. FEBS Lett. 1998;429:183–188. doi: 10.1016/s0014-5793(98)00588-2. [DOI] [PubMed] [Google Scholar]
- 18.Zhang CL, McKinsey TA, Lu JR, Olson EN. Association of COOH-terminal-binding protein (CtBP) and MEF2-interacting transcription repressor (MITR) contributes to transcriptional repression of the MEF2 transcription factor. J Biol Chem. 2001;276:35–39. doi: 10.1074/jbc.M007364200. [DOI] [PubMed] [Google Scholar]
- 19.Koipally J, Georgopoulos K. Ikaros interactions with CtBP reveal a repression mechanism that is independent of histone deacetylase activity. J Biol Chem. 2000;275:19594–19602. doi: 10.1074/jbc.M000254200. [DOI] [PubMed] [Google Scholar]
- 20.Fang M, Li J, Blauwkamp T, Bhambhani C, Campbell N, Cadigan KM. C-terminal-binding protein directly activates and represses Wnt transcriptional targets in Drosophila. Embo J. 2006;25:2735–2745. doi: 10.1038/sj.emboj.7601153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jin S, Scotto KW. Transcriptional regulation of the MDR1 gene by histone acetyltransferase and deacetylase is mediated by NF-Y. Mol Cell Biol. 1998;18:4377–4384. doi: 10.1128/mcb.18.7.4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.David GL, Yegnasubramanian S, Kumar A, Marchi VL, De Marzo AM, Lin X, Nelson WG. MDR1 promoter hypermethylation in MCF-7 human breast cancer cells: changes in chromatin structure induced by treatment with 5-Aza-cytidine. Cancer Biol Ther. 2004;3:540–548. doi: 10.4161/cbt.3.6.845. [DOI] [PubMed] [Google Scholar]
- 23.Shi Y, Sawada J, Sui G, Affar el B, Whetstine JR, Lan F, Ogawa H, Luke MP, Nakatani Y, Shi Y. Coordinated histone modifications mediated by a CtBP co-repressor complex. Nature. 2003;422:735–738. doi: 10.1038/nature01550. [DOI] [PubMed] [Google Scholar]
- 24.Kumar V, Carlson JE, Ohgi KA, Edwards TA, Rose DW, Escalante CR, Rosenfeld MG, Aggarwal AK. Transcription corepressor CtBP is an NAD(+)-regulated dehydrogenase. Mol Cell. 2002;10:857–869. doi: 10.1016/s1097-2765(02)00650-0. [DOI] [PubMed] [Google Scholar]
- 25.Cohen JS, Lyon RC, Chen C, Faustino PJ, Batist G, Shoemaker M, Rubalcaba E, Cowan KH. Differences in phosphate metabolite levels in drug-sensitive and -resistant human breast cancer cell lines determined by 31P magnetic resonance spectroscopy. Cancer Res. 1986;46:4087–4090. [PubMed] [Google Scholar]
- 26.Yu G, Ahmad S, Aquino A, Fairchild CR, Trepel JB, Ohno S, Suzuki K, Tsuruo T, Cowan KH, Glazer RI. Transfection with protein kinase C alpha confers increased multidrug resistance to MCF-7 cells expressing P-glycoprotein. Cancer Commun. 1991;3:181–189. doi: 10.3727/095535491820873263. [DOI] [PubMed] [Google Scholar]
- 27.Alaoui Jamali MA, Yin MB, Mazzoni A, Bankusli I, Rustum YM. Relationship between cytotoxicity, drug accumulation, DNA damage and repair of human ovarian cancer cells treated with doxorubicin: modulation by the tiapamil analog RO11-2933. Cancer Chemother Pharmacol. 1989;25:77–83. doi: 10.1007/BF00692343. [DOI] [PubMed] [Google Scholar]
- 28.Muller C, Laurent G, Ling V. P-glycoprotein stability is affected by serum deprivation and high cell density in multidrug-resistant cells. J Cell Physiol. 1995;163:538–544. doi: 10.1002/jcp.1041630314. [DOI] [PubMed] [Google Scholar]
- 29.Baker EK, Johnstone RW, Zalcberg JR, El-Osta A. Epigenetic changes to the MDR1 locus in response to chemotherapeutic drugs. Oncogene. 2005;24:8061–8075. doi: 10.1038/sj.onc.1208955. [DOI] [PubMed] [Google Scholar]
- 30.Chen KG, Wang YC, Schaner ME, Francisco B, Duran GE, Juric D, Huff LM, Padilla-Nash H, Ried T, Fojo T, Sikic BI. Genetic and epigenetic modeling of the origins of multidrug-resistant cells in a human sarcoma cell line. Cancer Res. 2005;65:9388–9397. doi: 10.1158/0008-5472.CAN-04-4133. [DOI] [PubMed] [Google Scholar]
- 31.Grooteclaes M, Deveraux Q, Hildebrand J, Zhang Q, Goodman RH, Frisch SM. C-terminal-binding protein corepresses epithelial and proapoptotic gene expression programs. Proc Natl Acad Sci U S A. 2003;100:4568–4573. doi: 10.1073/pnas.0830998100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Q, Yoshimatsu Y, Hildebrand J, Frisch SM, Goodman RH. Homeodomain interacting protein kinase 2 promotes apoptosis by downregulating the transcriptional corepressor CtBP. Cell. 2003;115:177–186. doi: 10.1016/s0092-8674(03)00802-x. [DOI] [PubMed] [Google Scholar]


