Abstract
Background
Data on pH regulation of the cardiac potassium current, IK1, suggest species dependent differences in the molecular composition of the underlying Kir2 channel proteins.
Objective
We have tested the hypothesis that the presence of the Kir2.3 isoform in hetero-tetrameric channels modifies channel sensitivity to pH.
Methods
Voltage clamp was performed on HEK293 cells stably expressing guinea pig Kir2.1 and/or Kir2.3 isoforms and on sheep cardiac ventricular myocytes at varying extracellular pH (pHo) and in the presence of CO2 to determine the sensitivity of macroscopic currents to pH. Single channel activity was also recorded from the HEK293 stables to determine the mechanisms of the changes in current.
Results
Biophysical characteristics of whole-cell and single-channel currents in Kir2.1/Kir2.3 double stables displayed properties attributable to isoform heteromerization. Whole-cell Kir2.1/Kir2.3 currents rectified in a manner reminiscent of Kir2.1 but were significantly inhibited by extracellular acidification in the physiological range (pKa ~ 7.4). Whole cell currents were more sensitive to a combined extra- and intracellular acidification produced by CO2. At pHo = 6.0, unitary conductances of heteromeric channels were reduced. Ovine cardiac ventricular cell IK1 was pHo- and CO2- sensitive, consistent with the expression of Kir2.1 and Kir2.3 in this species.
Conclusions
Kir2.1 and Kir2.3 isoforms form heteromeric channels in HEK293. The presence of Kir2.3 subunit(s) in heteromeric channels confers pH sensitivity to the channels. The single and double stable cells presented in this study are useful models for studying physiological regulation of heteromeric Kir2 channels in mammalian cells.
Keywords: pH sensitivity, Kir2.1, Kir2.3, IK1, heteromerization
INTRODUCTION
The classical inward rectifier current (IK1) is responsible for stabilizing the resting membrane potential, for determining excitation threshold, and for initiating the final repolarization phase of the cardiac action potential [1–3]. Kir2.1-Kir2.4 (Kir2.x) inwardly rectifying potassium subfamily of channel proteins are the molecular correlates of IK1 [3–5], with very distinct isoform-specific properties [3, 4]. Important differences exist, for example, in the sensitivity of Kir2.x isoforms to phosphorylating agents [4, 6], protons [7–9] and to barium ions [10]. Given the increasing evidence that Kir2.x subunits form heteromeric channels when heterologously expressed and in native cardiac cells [10–13], it is conceivable that such a heteromerization process enhances the regulatory potential of IK1.
In humans, although Kir2.1, Kir2.2 and Kir2.3 are all expressed in the myocardium, with Kir2.1≫Kir2.2>Kir2.3 [14], much attention has been given to the Kir2.1 isoform since genetic mutations in the KCNJ2 gene that encodes Kir2.1 have been linked to ion channel malfunctions (channelopathies) associated with cardiac rhythm disturbances [12, 15, 16]. In addition, when Kir2.1 is co-expressed with other isoforms, the rectification profile and extracellular potassium ([K+]o) sensitivity of the Kir2.1 isoform were shown to be dominant in the heteromeric Kir2.1/Kir2.3 complex [17]. In contrast, the contributions of the other isoforms to the properties of IK1 remain fairly unexplored. There have been conflicting reports in the literature on proton effects on IK1 [18–20]. It is possible that these discordant observations reflect differences in species- and tissue-dependent expression patterns of Kir2.x proteins. In this study, we were interested in testing the hypothesis that the presence of the pH sensitive Kir2.3 isoform in the heteromeric Kir2 channels will confer pH sensitivity to the channel complex.
Studying physiological regulation of heteromeric channels requires suitable conditions such as a mammalian cell line that stably expresses individual or combinations of Kir2.x isoforms. We have generated HEK293 cells stably expressing single (Kir2.1 or Kir2.3) or double (Kir2.1/Kir2.3) isoforms to examine proton effects on whole-cell and unitary current properties of homo- and heteromeric Kir2.1, Kir2.3 channels.
METHODS
Cell culture
HEK293 cells (ATCC, CRL1573) were transfected using Effectene (Qiagen, Valencia, CA). Stable cell lines were generated using equal amounts of plasmids containing the DNA sequences of guinea pig origin of either Kir2.1 (pcEP4 vector) or Kir2.3 (pcDNA3 vector). Antibiotics (hygromycin or neomycin respectively) were added to the growth media and colonies that were stably expressing the resistance marker were selected. Double stables (Kir2.1/Kir2.3) were generated by transfecting Kir2.1 into the Kir2.3 stable HEK293 cells followed by selection with both antibiotics.
Western Blot
Western Immunoblots were performed as described by [17]. Anti-Kir2.1 and anti-Kir2.3 primary antibodies (Alomone Labs) were diluted to 1:2000 in blocking buffer (5% non-fat dry milk in PBS-T). Incubation in primary antibodies was done overnight at 4°C. Incubation in secondary antibody horseradish peroxidase-conjugated anti-Rabbit IgG (Sigma) (1:10000 dilution) was done in blocking solution for 30 minutes at room temperature. Immunoreactive bands were visualized with chemiluminescent ECL Western Blotting Detection Reagents (Amersham Biosciences).
Cardiac myocytes
Sheep ventricular myocytes were isolated using the Langendorff retrograde perfusion method as previously described [17]. The protocol followed conforms with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH) Publication No. 85-23, revised 1996. Briefly, sheep (15–17 kg) were anesthetized with sodium pentobarbital (30 mg/kg I.V). Following thoracotomy, the heart was immediately removed, transported form the operation room in a cardioplegic solution, cannulated and retrogradely perfused (160 mL/min) with Tyrode’s solution containing (in mM): NaCl 148, KCl 5.4, MgCl2 1.0, CaCl2 1.8, NaH2PO4 0.4, Glucose 5.5, HEPES 15; pH 7.4 (NaOH) at 37°C until the effluent was clear of blood. Then, a Ca2+-free solution containing (in mM): NaCl 148, KCl 5.4, MgCl2 1.0, NaH2PO4 0.4, Glucose 5.5, HEPES 15, Albumin 1 mg/mL; pH 7.2 (NaOH) was perfused for 10 minutes. Collagenase 200 units/mL, Worthington, type II (Lakewood, NJ) was added to the Ca2+-free solution and perfused for 40 minutes. KB storage solution (in mM) KCl 80, MgSO4 5, KH2PO4 30, Glucose 20, EGTA 0.25, Creatine 5, β-Hydroxybutyric acid 5, Taurine 20, Pyruvic acid 5, ATP 5; pH 7.4 (KOH) was then perfused for 10 minutes. Cells were obtained by mechanical dissociation of sections of the left ventricle. The isolated cells were kept at 37°C in KB solution for another 30 minutes before Ca2+ reintroduction [17]. Cells were kept at room temperature until use.
Electrophysiology
Whole-cell recordings were carried out as described previously [17, 21, 22] using Axopatch 1D and 200B amplifiers (Axon Instruments, Union City, CA). Pipette DC resistance was 2–3 MΩ for whole-cell IK1 recordings and 8–12 MΩ for cell-attached single-channel recordings. The patch pipette series resistance after establishing whole cell configuration was ~12 to 15 MΩ and was electronically compensated by approximately 80%. Experiments were performed at 37 ± 0.5°C. Tip potential corrections were made as described previously [23].
Voltage-clamp ramps were applied from −100 to 0 mV while superfusing with Tyrode’s solution: (in mM) NaCl 140, KCl 5.4, CaCl2 1.8, NaH2PO4 0.33, HEPES 5.0; pH 7.4 (NaOH). The pipette filling solution utilized contained (in mM): KCl 20, K-aspartate 90, KH2PO4 10, EDTA 5.0, K2ATP 1.9, HEPES 5.0 and Mg2+ 7.9; pH 7.2 (KOH) (with the above concentration of EDTA, Mg2+ concentration is expected to be 1.1 mM [17]). IK1 was recorded in sheep myocytes using 100 ms voltage steps to −100 mV (holding potential = −50 mV) while superfusing with Tyrode’s solution. The pipette filling solution contained (in mM): KCl 148, MgCl2 1, EGTA 5, HEPES 5, Creatine 2, ATP 5, Phosphocreatine 5; pH 7.2 (KOH).
All unitary currents were measured in cell-attached patches. In these experiments, the bath solution contained (in mM): KCl 140, CaCl2 1.8, HEPES 5, NaH2PO4 0.33; pH 7.4 (KOH) at a voltage of −120 mV, sampled at 10 kHz and filtered at 200 Hz. The pipette solution contained (in mM) KCl 140, CaCl2 1, HEPES 5; pH 7.4 (KOH). Based on acquisition filter settings, only events longer than 20 ms were considered for analysis. To construct events histograms in the double stables, approximately equal number of events (n = 150 – 200) were taken from each patch, thus ensuring that events from a particular patch did not skew the distribution of the overall events histogram [17].
Acidification
Changes in pHo for whole-cell recording and the pipette solution for cell attached recording were achieved as previously described [21], using (in mM) MES, 5 (pH = 5.0 – 6.5), HEPES, 5 (pH = 7.0 – 8.0), or Tris, 5 (pH = 8.5 – 9.0). The pH values were adjusted using HCl or NaOH just before experiments. Acidification of both the extracellular and intracellular spaces was achieved by bubbling 100% CO2 to the respective bath solution. The pH of this solution after 5 minutes of bubbling was measured to be equal to 5.0 which would result in an intracellular pH reduction to ~ 6.8 [9].
Data analysis
Data were analyzed and presented using the pCLAMP 9 suite of programs (Axon Instruments, Union City, CA) and Origin® 7.0 software (OriginLab Corporation, Northampton, MA). All experimental results were presented as mean ± SEM. The significance of differences between the means was evaluated by one-way ANOVA or student’s t-test as appropriate. A value of P ≤ 0.05 was used as the criterion for significance.
RESULTS
Extracellular acidification effects on whole-cell Kir2.1/Kir2.3 currents
HEK293 cells
Isoform-specific proton effects on Kir2.x have been amply demonstrated in a variety of studies in which channels were heterologously expressed primarily in Xenopus oocytes [7, 8, 24]. Nevertheless, because the key electrophysiological experiments on heteromerization in the present study were carried out on stably expressed channels in HEK293 cells, it was imperative to first of all determine proton effects on stably expressed homomeric channels in HEK293 cells. These data served as control for comparison with currents recorded from the heteromeric channels.
Figure 1 illustrates pHo effects on whole-cell currents in HEK293 cells expressing Kir2.1 or Kir2.3 homomeric channels. Panel A shows superimposed sample whole-cell Kir2.1 current traces in control (pHo = 7.4) and at pHo = 5.0. In Figure 1B, peak inward (filled squares) and peak outward currents (open squares), normalized to current values at pHo = 7.4, are plotted as a function of pHo. The data show that between pHo = 5.0 and pHo = 9.0, there is no significant change in inward or outward currents recorded from homomeric Kir2.1 channels (n = 6–10 at any pH value). In contrast, a similar change in proton concentration significantly modulated current through Kir2.3 channels. Figure 1C shows sample Kir2.3 current traces in control and at pHo = 5.0. Two points are noteworthy in this panel: 1) rectification of Kir2.3 current has a distinct profile compared with the rectification profile of Kir2.1 current, as previously published [17], 2) pHo reversibly reduced inward and outward components of Kir2.3 currents. Figure 1D summarizes the pHo effect on the Kir2.3 isoform. Data (n = 6 –10) were obtained for peak inward (filled circles) and peak outward (open circles) current normalized to control pHo in each case. Acidification of the extracellular space reduced whole-cell inward and outward Kir2.3 currents, with similar pKa values (~7.35). Note that at pHo = 6.0 the amount of current was reduced by ~60%. The curve was fitted to the inward current data. Thus, the proton effects obtained with the HEK293 single stables faithfully reproduced what was reported for homomeric Kir2.1 and Kir2.3 channels expressed in oocytes [7, 8, 24].
Figure 1. pHo effect on homomeric Kir2.1 and Kir2.3 channels in HEK293 cells.

A. Acidification to pHo = 5.0 from control (pHo = 7.4) has no significant effect on whole-cell Kir2.1 current B. Peak inward (filled squares) and peak outward (open squares) Kir2.1 whole-cell currents normalized to current values at pHo = 7.4 and plotted as a function of pHo (n = 6–10). C. Acidification to pHo = 5.0 from control (pHo = 7.4) significantly inhibits whole-cell Kir2.3 current D. Peak inward (filled circles) and peak outward (open circles) Kir2.3 whole-cell currents normalized to current values at pHo = 7.4 and plotted as a function of pHo (n = 7–11). Increases in pHo blocked whole-cell inward and outward currents. See text for details.
To test pHo effects on heteromeric Kir2.x channels, we used the HEK293 double stable cell line which simultaneously expresses Kir2.1 and Kir2.3 isoforms. In Figure 2A a Western Blot shows the presence of Kir2.1 and Kir2.3 channel subunits in these cells. In order to confirm that Kir2.1 and Kir2.3 heteromerize when expressed in HEK293 cells, coimmunoprecipitation experiments were performed in HEK293 cells concomitantly transfected with tagged Kir2.1 and Kir2.3 constructs (Flag and GluGlu tags respectively). The use of anti-tag antibodies ensures the specificity required for this immunoassay. Cell lysates were incubated with anti-Flag beads which specifically immobilized Kir2.1. The beads were washed and bound Kir2.3 was detected with an anti-GluGlu antibody in the immunoblot (data not shown). Figure 2B shows the average data (n = 4) of ramp-generated current-voltage (I-V) relationship for the double stables. Barium (1 mM)-sensitive currents are plotted as a function of command potential. Note that the I-V relationship obtained in the double stables has a rectification profile similar to that of Kir2.1 channels. As for the rectification profile described for the Kir2.1 I-V relationship [17], the plot in figure 2B could be fitted with a single Boltzmann function:
Figure 2. Properties of Kir2.1/Kir2.3 double stables in HEK293 cells.

A. Western Blot showing the presence of Kir2.1 and Kir2.3 channel proteins in the double stables. B. Ramp-generated, Ba2+ (1 mM)-sensitive currents in the double stables. Whole-cell currents are plotted as a function of command potential (n = 4).
where the relative chord conductance, Gc, was calculated at different membrane voltages, Vm, where V1/2 is the voltage at midpoint of rectification and dx = RT/zF, where z stands for the steepness of rectification; F, for Faraday’s constant; R, for gas constant and T, for absolute temperature. The z value for the double stables was z = 1.85 ± 0.12.
Figure 3 demonstrates that whole-cell currents through heteromeric channels are pH-sensitive. Sample currents in control (pHo = 7.4) and at pHo = 5.0 are superimposed in Figure 3A. Once more, note the parallel in the rectification profile of the current traces with that shown in Figure 1A, but the difference in proton effect. Even more importantly, note the similarity in rectification between the control trace and the trace at pHo = 5.0, which indicates that protons affected the same subset of channel population (a single Boltzmann function was also sufficient to fit rectification of current in control and at pHo = 5.0; data not shown). Moreover, the subtracted current (inset) clearly shows that the pH sensitive current has the profile of Kir2.1, further suggestive of heteromerization. Figure 3B is a titration curve for the pHo effect on the double stables. Data (n = 7 –11) were obtained for peak inward (filled triangles) and peak outward (open triangles) current normalized to control pHo in each case. Acidification of the extracellular space reduced whole-cell inward and outward currents, with pKas of 7.45 ± 0.09 and 7.35 ± 0.06 respectively (p = NS). The curve was fitted to the inward current data. Note that at pHo = 6.0, the amount of current is reduced by ~ 40%. Figure 3C shows superimposed traces in control (pHo = 7.4) and after 5 minutes of bubbling 100% CO2. Figure 3D shows in a histogram of normalized peak inward currents (n = 5) that under such conditions the amount of current is reduced by an additional 20% for a total of 60% inhibition.
Figure 3. pH-sensitivity of heteromeric Kir2.1/Kir2.3 currents in HEK293 cells.

A. Acidification to pHo = 5.0 from control (pHo = 7.4) significantly inhibits whole-cell Kir2.1/Kir2.3 current. Inset: Subtracted (pH sensitive) current. B. Peak inward (filled triangles) and peak outward (open triangles) whole-cell Kir2.3 currents normalized to current values at pHo = 7.4 and plotted as a function of pHo (n = 6–10). pHo blocked whole-cell currents, with pKa of 7.5 ± 0.09 and 7.4 ± 0.06 (p = NS), respectively for inward and outward currents. C. Effects of acidification by CO2 on whole-cell Kir2.1/Kir2.3 currents. D. Average data of peak inward whole-cell Kir2.1/Kir2.3 currents normalized to current values at pHo = 7.4 (n = 5).
In order to further demonstrate the effect of intra- and extracellular acidification in channels necessarily heteromeric, an additional set of CO2 experiments was carried out in HEK293 cells expressing concatemeric constructs of Kir2.1-Kir2.3. Expression of Kir2.1-Kir2.3 fusion proteins forces the channel stoichiometry to 1:1, i.e, two subunits of Kir2.1 and two subunits of Kir2.3 per channel. Figure 4 presents the results obtained following acidification with CO2. Panel A shows superimposed sample current traces during control and CO2 conditions. Panel B shows normalized peak inward currents (n = 5). Note that when all the channels have a 1:1 forced stoichiometry, acidification by CO2 causes current inhibition by approximately 75%.
Figure 4. pH-sensitivity of Kir2.1-Kir2.3 concatamers channels in HEK293 cells.

A. Control (pHo = 7.4) current trace and a trace during acidification by CO2 in the same cell. B. Average data of peak inward currents normalized to control current values at (n = 5).
Sheep ventricular myocytes
Kir2.1 and Kir2.3 are the molecular correlates of IK1 in ovine ventricular myocytes [17]. Given the results from heterologously expressed heteromeric channels in Figure 3, we examined pH- sensitivity of IK1 in sheep ventricular myocytes. Peak inward currents were measured at −100 mV steps. Figure 5A shows superimposed current traces at pHo = 7.4 and pHo = 5.0 from the same myocyte. The plot in Figure 5B is the average of data of pHo effects on sheep myocytes (n = 3–5 at each pH value) and has a pKa of 7.40 ± 0.14. Figure 5C shows superimposed currents at pHo = 7.4 and after 5 minutes of superfusion with CO2 bubbled Tyrode’s solution. Figure 5D is a histogram of normalized peak inward current at −100 mV during control and after the CO2 challenge (n = 5). The data show that acidification by CO2 reduced IK1 in sheep ventricular myocytes in a manner similar to the reduction of current through heteromeric channels in the double stables (Figure 3).
Figure 5. pHo-sensitivity of IK1 in sheep ventricular myocytes.

A. Superimposed current traces at −100 mV at pHo = 7.4 and pHo = 5.0. B. Average data obtained from three animals (n = 3–5 for each data point). Currents were normalized to the value at pHo = 8. Protons inhibited IK1 with a pKa of 7.40 ± 0.14. Inset shows voltage protocol. C. Superimposed current traces at −100 mV during control and during acidification by CO2. D. Histogram showing average peak inward current at −100 mV during acidification by CO2, normalized to control values (n = 5).
Extracellular acidification effects on unitary heteromeric Kir2.1/Kir2.3 currents
A test that is critically important to our overall hypothesis is the demonstration of proton sensitivity of unitary current flux in heteromeric channels. We have shown that these channels are pHo- and pHi-sensitive. For convenience we have chosen to carry out single channel experiments by modifying only the pHo. We began by generating an events histogram of unitary currents in the single stable HEK293 cells expressing individual (Kir2.1 or Kir2.3) isoforms, which, again, would serve as control for the data obtained from heteromeric channels in the double stables. Figure 6A shows representative cell-attached patch recordings from cells expressing only Kir2.1 (top) and only Kir2.3 (bottom) channels. Events histograms in panel B were plotted for Kir2.1 (n = 270: 4 patches) and for Kir2.3 (n = 250: 3 patches). Note that the data for the two isoforms were plotted on the same abscissa and that there is a clear separation in the unitary conductance (γ) values between the two isoforms. For Kir2.3 unitary events, γ ranged between 10 – 20 pS, and between 26 – 42 pS for Kir2.1. The non-bell shaped Kir2.1 histogram is suggestive of the presence of substates consistent with previous reports [11]. For this reason, only patches that contained one channel were considered for analysis in the recordings from the double stables.
Figure 6. Properties of unitary Kir2.1 and Kir2.3 channels in HEK293 cells.

A. Representative cell-attached patch recordings in cells expressing Kir2.1 (top) and Kir2.3 (bottom). The solid line represents channel resting state B. Events histograms for unitary events in patches containing Kir2.1 or Kir2.3 channels.
In order to examine the pHo-sensitivity of the heteromeric channels at low pHo (pHo = 6.0), we first obtained the events histogram for the pH-sensitive Kir2.3 isoform at pHo = 6.0. Our data in HEK293 cells show that acidification of the extracellular space reduced unitary current amplitude through Kir2.3 as previously demonstrated in Xenopus oocytes [24]. The representative cell-attached patch recordings under control (pHo = 7.4) and at pHo = 6.0 (Figure 7A) clearly reveal smaller γ values at the lower pH. Consistent with this reduction in γ, the distribution of unitary events at pHo = 6.0 is left-shifted (Figure 7B, n = 242; 3 patches). To emphasize this shift, the events histogram under control conditions for Kir2.3 (Figure 6B) is shown in grey and labeled as pHo = 7.4. We did not investigate pHo sensitivity of Kir2.1 channels. We assumed that homomeric Kir2.1 single channels are insensitive to pH challenges in the range we have studied (5.0 to 9.0). This assumption is supported by previous studies that demonstrate insensitivity to pH of single channels at this same range of values [25].
Figure 7. pHo-sensitivity of homomeric Kir2.3 channels in HEK293 cells.

A. Representative cell-attached patch recordings under control (pHo = 7.4) and at pHo = 6.0. The solid line represents channel resting state. B. Events histogram of Kir2.3 channels at pHo = 6.0 compared with histogram at control pHo = 7.4. The histogram labeled pHo = 7.4 is the same as shown in Figure 6.
Data on the unitary events from cell-attached patches in the double stables are illustrated in Figure 8. A representative cell-attached patch recording (Figure 8A) shows transitions ~24 pS. An events histogram (Figure 8B) (n = 722; 4 patches) gives an overall distribution of unitary channel conductances from the double stables. The data show a broad distribution of γ values, (~ 10 pS to ~ 40 pS; Figure 8B). Importantly, there is a relatively high number of channel events in which γ values are between 20 – 30 pS, with a major peak centered at ~ 20 pS. The dashed lines are the histograms for the individual Kir2.1 and Kir2.3 isoforms from Figure 6B. The superimposition enables the identification of a range of γ values (~ 21 – 26 pS) not present in the control histograms, reflecting the unitary events of heteromeric Kir2.1 and Kir2.3 channels.
Figure 8. Unitary conductance properties of heteromeric channels in HEK293 double stables expressing Kir2.1 and Kir2.3 channels.
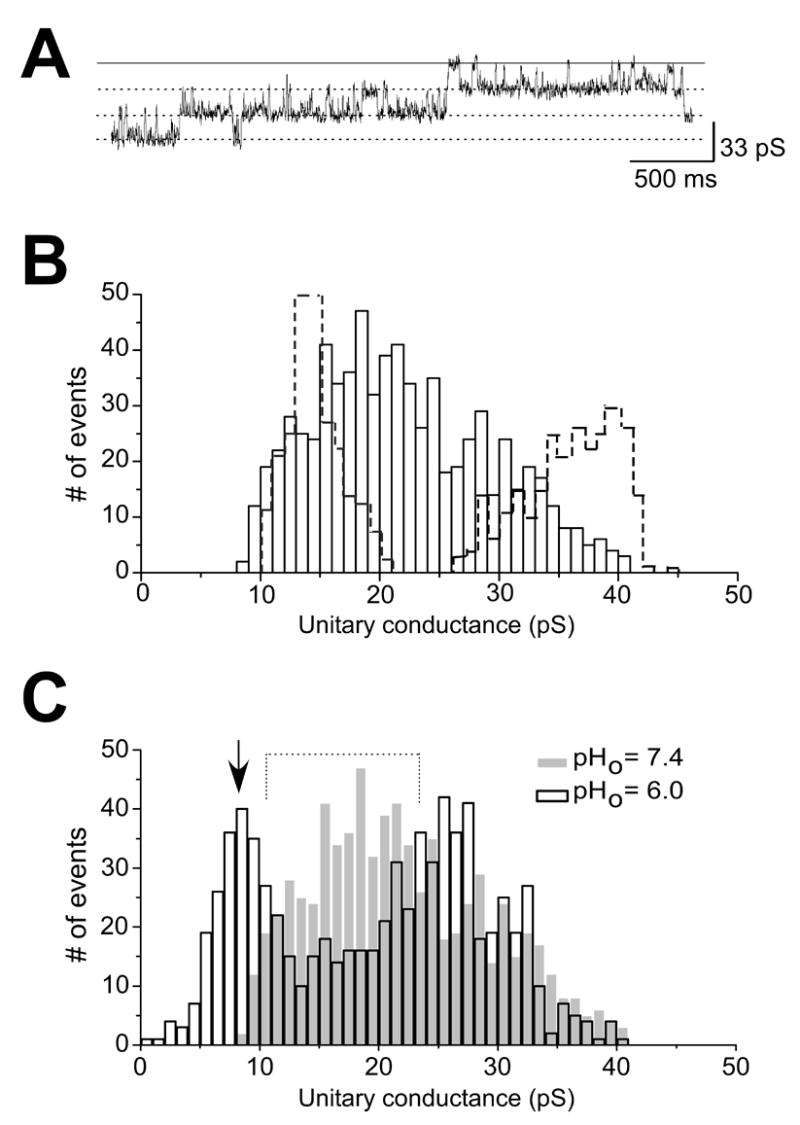
A. Cell-attached patch recording in the double stables showing transitions of ~ 24 pS. The solid line represents channel resting state. B. Events histogram depicting the distribution of unitary channel conductances. Note the relatively high number of channel events with γ in the range between 20 – 30 pS. Histograms with dashed lines are same as shown in Figure 6B. C. Events histogram obtained from cell-attached patches in the double stable cell line. Control histogram (pHo = 7.4; same data as shown in Figure 7B) is superimposed. Dotted line shows the reduction in events between 10 – 20 pS. Arrow indicates the appearance of a major peak at ~ 10 pS.
Figure 8C gives a summary of the data for pHo effects on the heteromeric channels in the double stables (n = 744; 4 patches). The data are superimposed on the control histogram (i.e., at pHo = 7.4) for the channels as shown in Figure 8B. A number of points are immediately noteworthy when the distribution of the histograms are considered in relation to each other: 1) a reduction of events between ~ 10 – 20 pS (dotted lines), 2) the appearance of a major peak at ~ 10 pS (arrow), 3) a reduction of number of channel events at ~ 30–35 pS. These observations are consistent with proton inhibition of homomeric Kir2.3 channels as well as heteromeric (Kir2.1/Kir2.3) channels, which would all have a reduction in unitary conductance values with the pHo challenge. It would appear as though that the majority of channels that populate the ~ 10 pS peak originated from heteromeric channels with γ of ~ 20 pS, however, this may not necessarily be the case, as elaborated upon in the discussion of this paper. Finally, cell attached recordings in cells transfected with Kir2.1-Kir2.3 concatemers showed that net control unitary conductance (28 pS) was reduced to ~ 12 pS at pHo = 6 (150 events; 2 patches each) (data not shown). Thus, the data form the concatemers also support our hypothesis that Kir2.3 subunit in a heteromeric channel confers pH sensitivity to the channel.
DISCUSSION
We have used HEK293 cells stably expressing single or double Kir2.x isoforms (Kir2.1 and/or Kir2.3) to study proton dependent regulation of heteromeric Kir2.x channels in a mammalian expression system. Whole cell and unitary current analysis show that the heteromeric channels are pH-sensitive. Our study also shows that IK1 in sheep ventricular myocytes is pH sensitive. The demonstration of pH-sensitivity of IK1 in sheep ventricular myocytes is consistent with Kir2.1 and Kir2.3 isoforms underlying inward rectification in the species. Overall, the results imply that the pH-sensitivity of heteromeric Kir2.1/Kir2.3 channels can be attributed to the Kir2.3 subunit component of the channels.
pH effects on macroscopic currents
HEK293 cells
The biophysical characteristics, e.g., rectification profile as well as proton effects in whole-cell currents recorded in the double stables strongly suggest heteromerization of the Kir2.1 and Kir2.3 channels in the HEK293 cells. Because a single Boltzmann function was sufficient to fit rectification in the double stables, the profile of rectification in heteromeric channels is similar to the rectification of homomeric Kir2.1 channels. We had demonstrated previously that rectification of Kir2.1 could be fitted with a single Boltzmann function [17], while a double Boltzmann function was the minimum required to fit the rectification of Kir2.3 currents. These results imply that Kir2.1 is dominant in determining the profile of rectification in the double stables. The observation that currents in the double stables were inhibited by acidification, suggests that the channel hetero-tetramers contain one or more Kir2.3 subunits. The fact that acidification (pHo) challenge did not alter the rectification profile of current is informative: it suggests that the acidification affected the same population of channels. In comparison to whole-cell Kir2.3 current where inhibition by protons (pHo = 5.0) was 60 %, in the double stables there was ~ 40% inhibition of current. The difference in pH-sensitivity between the homomeric (Kir2.3) and heteromeric (Kir2.1/Kir2.3) currents can be attributed to homomeric Kir2.1 (and perhaps a sub-population of heteromeric) channels in the double stables which have reduced (or no) sensitivity to the pHo challenge. Our experiments following combined intracellular and extracellular acidification using CO2 showed very significant inhibition of current compared to acidification of the extracellular space alone. This is consistent with previous reports in the literature of the greater sensitivity to intracellular acidification of Kir2.3 channels [9].
Cardiac ventricular myocytes
An earlier study from our laboratory used molecular and biophysical approaches to show that Kir2.1 and Kir2.3 were the isoforms expressed in the sheep ventricles. [17]. The results of the experiments in Figure 5 clearly show that IK1 in the sheep ventricular myocyte is pH-sensitive, and in a manner similar to heterologously expressed Kir2.1/Kir2.3 channels. Considering species-dependent differential expressions of Kir2.x channels, this idea can explain the apparent inconsistencies reported for proton effects of IK1 in the different studies [18–20].
pHo effects on heteromeric unitary currents
Unitary current events recorded under control conditions in the double stables displayed a broad spectrum of unitary conductances (Figure 8). There were unitary events that did not follow the distribution of homomeric channels from either Kir2.1 or Kir2.3 channels, thus providing strong evidence for heteromerization. A significant number of events were distributed within the range of 18 pS to 30 pS. It is possible that our approach for the coexpression which allowed for a free stoichiometric association, as well as subunit arrangements within the tetrameric channel, resulted in channels with a spectrum of values of γ, but a majority of which were in the 18 pS to 30 pS range. In this regard, it is interesting to note that the concatenated constructs of Kir2.1 and Kir2.3 [12] resulted in channels with an average γ of ~ 26 pS.
A key observation from our experiments in the double stables is the proton-induced shift of the events histogram (Figure 8C), consistent with the role of Kir2.3 isoform in proton sensing in the heteromeric channels. It is conceivable that the redistribution of the population of events between 18–30 pS indicates that majority of events with γ in this range, represent the most pHo-sensitive heteromeric channels. Nevertheless, given the possibility of free association of subunits discussed above, heteromeric channels may have γ values that span the entire range of conductances. It is possible that isoform stoichiometry and the order of isoform arrangement in the tetrameric channel determine the value of γ and pHo-sensitivity of the channel. Thus, some heteromeric channels may have conductances similar to homomeric Kir2.1 or Kir2.3 channels and, depending on their pH-sensitivity, would be redistributed or shifted by varying degrees in the histogram.
The current hypothesis on extracellular proton-induced gating of Kir2.3 suggests that a histidine residue (H117) in the M1-to-H5 linker that influences one of two titratable cysteine residues located in the M1-to-Pore and Pore-to-M2 linkers [24]. Perhaps similar molecular interactions in the constituent Kir2.3 subunits in the heteromeric channels are responsible for the pHo-sensitivity demonstrated in our investigation. The molecular mechanism of intracellular pH sensitivity of Kir2.3 is unknown but would very likely depend on differences in channel pore properties. Electrophysiological and crystallographic studies have recently examined cytoplasmic domain structures potentially involved in modulating pore properties of Kir channels [26]. A flexible pore-facing loop, dubbed the G-loop, has a glycine residue in addition to other small or hydrophobic amino acids. The loop forms a girdle which is capable of constricting the pore size to ~ 3Å. The G-loop and a cluster of aspartic acid residues are thought to function as regulatory elements of the channel from the cytoplasm. It is interesting that whereas Kir2.1 and Kir2.3 have the same residues in the G-loop, the neighboring residues are remarkably different in the two isoforms. The consequence of these differences in channel regulation remains to be explored.
Physiological significance
A number of channel proteins of the Kir family of have been shown to play a role in proton regulation in a variety of cells. For example, heteromerization of Kir4 and Kir5 channels are thought to be involved in proton regulation in the renal tubular epithelia [27] and in the central nervous system [28]. Kir2.3 is expressed in a variety of systems, including the cardiovascular and the central nervous systems [4], where pH regulation is an important aspect of normal and abnormal function. With the demonstrated overlapping cardiac expression of Kir2.1 and Kir2.3 isoforms [11, 14, 17] and the possibility for heteromerization within the Kir2.x family, results from our experiments imply that heteromeric Kir2.1/Kir2.3 channels will be modulated by pH changes as well as by other factors such as phosphorylating agents. Our study showed proton regulation for both inward and outward current components. Outward components of inward rectifier currents are key participants in excitability and repolarization of the cardiac impulse. The proton sensitivity of ovine IK1 (pKa = 7.40 ± 0.14) in the present study would suggest a physiologically relevant modulation of the channel. Results of our experiments using CO2 are consistent with previously reported data in literature, and are relevant to the understanding of proton dependent regulation of IK1 under pathological conditions such as ischemia. It has been demonstrated for example, that under ischemia, there is initially a decline in intracellular pH which is followed by a decline in extracellular pH. These parallel changes in pH are thought to be protective to the myocardium [29].
Potential limitations
We recognize some potential limitations in our experiments in that that our analyses of pH effects on heteromeric channels did not consider issues concerning channel assembly e.g., the order or sequence of Kir2.1 and Kir2.3 isoforms in the tetrameric channels. Moreover, some of our experiments used concatamers to force Kir2.1 and Kir2.3 stoichiometry to 1:1. The experiments may be limited in that the impact of concatamerization on channel fundamental properties is unknown. Furthermore, in the parallel study of the pH effect on IK1 using the ovine isolated cardiac myocyte model, stoichiometric combinations of the Kir2.1 and Kir2.3 subunits are unknown. Because factors such as channel-associated proteins and the lipid environment in the two cell systems may be significantly different, they may indeed influence the nature of hetero-tetramerization as well as proton sensitivity. Interestingly, though, the titration curves in the two systems were similar. Nevertheless, our major objective, i.e., to demonstrate that the presence of one or more subunits of the Kir2.3 isoform in the hetero-tetrameric channels will modify the channel sensitivity to protons, was achieved. Finally, endogenous outward currents have been reported in HEK293 cells [30–32]. We found rare occurrences of unitary events with conductances smaller than 4 pS during single channel recordings. These most likely correspond to endogenous channels. Such events were not considered in any of the events histograms presented in this study.
Acknowledgments
We would like to thank Fang Lu and Jiang Jiang for their technical support. We are very grateful to Drs. Jalife, Delmar and Vikstrom for their critiques.
Footnotes
Financial Support by NIH Grant 5RO1HL070074
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Anumonwo JMB, Freeman LC, Kwok WM, Kass RS. Potassium channels in the heart: Electrophysiology and Pharmacological Regulation. Cardiovascular Drug Reviews. 1991;9:299–316. [Google Scholar]
- 2.Shimoni Y, Clark RB, Giles WR. Role of an inwardly rectifying potassium current in rabbit ventricular action potential. J Physiol. 1992;448:709–727. doi: 10.1113/jphysiol.1992.sp019066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopatin AN, Nichols CG. Inward rectifiers in the heart: an update on I(K1) J Mol Cell Cardiol. 2001;33:625–638. doi: 10.1006/jmcc.2001.1344. [DOI] [PubMed] [Google Scholar]
- 4.Stanfield PR, Nakajima S, Nakajima Y. Constitutively active and G-protein coupled inward rectifier K+ channels: Kir2.0 and Kir3.0. Rev Physiol Biochem Pharmacol. 2002;145:47–179. doi: 10.1007/BFb0116431. [DOI] [PubMed] [Google Scholar]
- 5.Zaritsky JJ, Redell JB, Tempel BL, Schwarz TL. The consequences of disrupting cardiac inwardly rectifying K(+) current (I(K1)) as revealed by the targeted deletion of the murine Kir2.1 and Kir2.2 genes. J Physiol. 2001;533:697–710. doi: 10.1111/j.1469-7793.2001.t01-1-00697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen NA, Brenman JE, Snyder SH, Bredt DS. Binding of the inward rectifier K+ channel Kir 2.3 to PSD-95 is regulated by protein kinase A phosphorylation. Neuron. 1996;17:759–767. doi: 10.1016/s0896-6273(00)80207-x. [DOI] [PubMed] [Google Scholar]
- 7.Jiang C, Qu Z, Xu H. Gating of Inward Rectifier K+ Channels by Proton-Mediated Interactions of Intracellular Protein Domains. Trends in Cardiovascular Medicine. 2002;12:5–13. doi: 10.1016/s1050-1738(01)00132-3. [DOI] [PubMed] [Google Scholar]
- 8.Qu Z, Yang Z, Cui N, Zhu G, Liu C, Xu H, Chanchevalap S, Shen W, Wu J, Li Y, Jiang C. Gating of Inward Rectifier K+ Channels by Proton-mediated Interactions of N- and C-terminal Domains. J Biol Chem. 2000;275:31573–31580. doi: 10.1074/jbc.M003473200. [DOI] [PubMed] [Google Scholar]
- 9.Zhu G, Chanchevalap S, Cui N, Jiang C. Effects of intra- and extracellular acidifications on single channel Kir2.3 currents. J Physiol (Lond) 1999;516:699–710. doi: 10.1111/j.1469-7793.1999.0699u.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schram G, Pourrier M, Wang Z, White M, Nattel S. Barium block of Kir2 and human cardiac inward rectifier currents: evidence for subunit-heteromeric contribution to native currents. Cardiovascular Research. 2003;59:328–338. doi: 10.1016/s0008-6363(03)00366-3. [DOI] [PubMed] [Google Scholar]
- 11.Liu GX, Derst C, Schlichthorl G, Heinen S, Seebohm G, Bruggemann A, Kummer W, Veh RW, Daut J, Preisig-Muller R. Comparison of cloned Kir2 channels with native inward rectifier K+ channels from guinea-pig cardiomyocytes. J Physiol. 2001;532:115–126. doi: 10.1111/j.1469-7793.2001.0115g.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Preisig-Muller R, Schlichthorl G, Goerge T, Heinen S, Bruggemann A, Rajan S, Derst C, Veh RW, Daut J. Heteromerization of Kir2.x potassium channels contributes to the phenotype of Andersen’s syndrome. PNAS. 2002;99:7774–7779. doi: 10.1073/pnas.102609499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schram G, Melnyk P, Pourrier M, Wang Z, Nattel S. Kir2.4 and Kir2.1 K(+) channel subunits co-assemble: a potential new contributor to inward rectifier current heterogeneity. J Physiol. 2002;544:337–349. doi: 10.1113/jphysiol.2002.026047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Z, Yue L, White M, Pelletier G, Nattel S. Differential distribution of inward rectifier potassium channel transcripts in human atrium versus ventricle. Circulation. 1998;98:2422–2428. doi: 10.1161/01.cir.98.22.2422. [DOI] [PubMed] [Google Scholar]
- 15.Plaster NM, Tawil R, Tristani-Firouzi M, Canun S, Bendahhou S, Tsunoda A, Donaldson MR, Iannaccone ST, Brunt E, Barohn R, Clark J, Deymeer F, George AL, Jr, Fish FA, Hahn A, Nitu A, Ozdemir C, Serdaroglu P, Subramony SH, Wolfe G, Fu YH, Ptacek LJ. Mutations in Kir2.1 cause the developmental and episodic electrical phenotypes of Andersen’s syndrome. Cell. 2001;105:511–519. doi: 10.1016/s0092-8674(01)00342-7. [DOI] [PubMed] [Google Scholar]
- 16.Priori SG, Pandit SV, Rivolta I, Berenfeld O, Ronchetti E, Dhamoon A, Napolitano C, Anumonwo J, di Barletta MR, Gudapakkam S, Bosi G, Stramba-Badiale M, Jalife J. A Novel Form of Short QT Syndrome (SQT3) Is Caused by a Mutation in the KCNJ2 Gene. Circ Res. 2005;96:800–807. doi: 10.1161/01.RES.0000162101.76263.8c. [DOI] [PubMed] [Google Scholar]
- 17.Dhamoon AS, Pandit SV, Sarmast F, Parisian KR, Guha P, Li Y, Bagwe S, Taffet SM, Anumonwo JMB. Unique Kir2.x Properties Determine Regional and Species Differences in the Cardiac Inward Rectifier K+ Current. Circ Res. 2004;94:1332–1339. doi: 10.1161/01.RES.0000128408.66946.67. [DOI] [PubMed] [Google Scholar]
- 18.Xu Z, Patel KP, Rozanski GJ. Intracellular protons inhibit transient outward K+ current in ventricular myocytes from diabetic rats. Am J Physiol Heart Circ Physiol. 1996;271:H2154–2161. doi: 10.1152/ajpheart.1996.271.5.H2154. [DOI] [PubMed] [Google Scholar]
- 19.Komukai K, Brette F, Orchard CH. Electrophysiological response of rat atrial myocytes to acidosis. Am J Physiol Heart Circ Physiol. 2002;283:H715–724. doi: 10.1152/ajpheart.01000.2001. [DOI] [PubMed] [Google Scholar]
- 20.Yan D-H, Nishimura K, Yoshida K, Nakahira K, Ehara T, Igarashi K, Ishihara K. Different intracellular polyamine concentrations underlie the difference in the inward rectifier K+ currents in atria and ventricles of the guinea-pig heart. J Physiol (Lond) 2005;563:713–724. doi: 10.1113/jphysiol.2004.077677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Anumonwo JM, Horta J, Delmar M, Taffet SM, Jalife J. Proton and zinc effects on HERG currents. Biophys J. 1999;77:282–298. doi: 10.1016/S0006-3495(99)76889-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Samie FH, Berenfeld O, Anumonwo J, Mironov SF, Udassi S, Beaumont J, Taffet S, Pertsov AM, Jalife J. Rectification of the background potassium current: a determinant of rotor dynamics in ventricular fibrillation. Circ Res. 2001;89:1216–1223. doi: 10.1161/hh2401.100818. [DOI] [PubMed] [Google Scholar]
- 23.Delmar M, Michaels DC, Jalife J. Slow recovery of excitability and the Wenckebach phenomenon in the single guinea pig ventricular myocyte. Circ Res. 1989;65:761–774. doi: 10.1161/01.res.65.3.761. [DOI] [PubMed] [Google Scholar]
- 24.Coulter KL, Perier F, Radeke CM, Vandenberg CA. Identification and molecular localization of a pH-sensing domain for the inward rectifier potassium channel HIR. Neuron. 1995;15:1157–1168. doi: 10.1016/0896-6273(95)90103-5. [DOI] [PubMed] [Google Scholar]
- 25.Shieh RC, Chang JC, Kuo CC. K+ binding sites and interactions between permeating K+ ions at the external pore mouth of an inward rectifier K+ channel (Kir2.1) J Biol Chem. 1999;274:17424–17430. doi: 10.1074/jbc.274.25.17424. [DOI] [PubMed] [Google Scholar]
- 26.Pegan S, Arrabit C, Zhou W, Kwiatkowski W, Collins A, Slesinger PA, Choe S. Cytoplasmic domain structures of Kir2.1 and Kir3.1 show sites for modulating gating and rectification. Nat Neurosci. 2005;8:279–287. doi: 10.1038/nn1411. [DOI] [PubMed] [Google Scholar]
- 27.Tanemoto M, Kittaka N, Inanobe A, Kurachi Y. In vivo formation of a proton-sensitive K+ channel by heteromeric subunit assembly of Kir5.1 with Kir4.1. J Physiol (Lond) 2000;525:587–592. doi: 10.1111/j.1469-7793.2000.00587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang Z, Xu H, Cui N, Qu Z, Chanchevalap S, Shen W, Jiang C. Biophysical and Molecular Mechanisms Underlying the Modulation of Heteromeric Kir4.1-Kir5.1 Channels by CO2 and pH. J Gen Physiol. 2000;116:33–46. doi: 10.1085/jgp.116.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Post JA, Wang SY, Langer GA. pHe, [Ca2+]e, and cell death during metabolic inhibition: role of phospholipase A2 and sarcolemmal phospholipids. Am J Physiol. 1998;274:H18–26. doi: 10.1152/ajpheart.1998.274.1.H18. [DOI] [PubMed] [Google Scholar]
- 30.Avila G, Sandoval A, Felix R. Intramembrane charge movement associated with endogenous K+ channel activity in HEK-293 cells. Cell Mol Neurobiol. 2004;24:317–330. doi: 10.1023/B:CEMN.0000022765.52109.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu SP, Kerchner GA. Endogenous voltage-gated potassium channels in human embryonic kidney (HEK293) cells. J Neurosci Res. 1998;52:612–617. doi: 10.1002/(SICI)1097-4547(19980601)52:5<612::AID-JNR13>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 32.Zhu G, Zhang Y, Xu H, Jiang C. Identification of endogenous outward currents in the human embryonic kidney (HEK 293) cell line. J Neurosci Methods. 1998;81:73–83. doi: 10.1016/s0165-0270(98)00019-3. [DOI] [PubMed] [Google Scholar]


