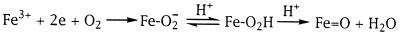Abstract
P450 cytochromes (P450) catalyze many types of oxidative reactions, including the conversion of olefinic substrates to epoxides by oxygen insertion. In some instances epoxidation leads to the formation of products of physiological importance from naturally occurring substrates, such as arachidonic acid, and to the toxicity, carcinogenicity, or teratogenicity of foreign compounds, including drugs. In the present mechanistic study, the rates of oxidation of model olefins were determined with N-terminal-truncated P450s 2B4 and 2E1 and their respective mutants in which the threonine believed to facilitate proton delivery to the active site was replaced by alanine. Styrene epoxidation, cyclohexene epoxidation and hydroxylation to give 1-cyclohexene-3-ol, and cis- or trans-butene epoxidation (without isomerization) and hydroxylation to give 2-butene-1-ol were all significantly decreased by the 2B4 T302A mutation. Reduced proton delivery in this mutant is believed to interfere with the activation of dioxygen to the oxenoid species, as shown earlier by decreased hydroxylation of several substrates and enhanced aldehyde deformylation via a presumed peroxo intermediate. Of particular interest, however, the T303A mutation of P450 2E1 resulted in enhanced epoxidation of all of the model olefins along with decreased allylic hydroxylation of cyclohexene and butene. These results and a comparison of the ratios of the rates of epoxidation and hydroxylation support the concept that two different species with electrophilic properties, hydroperoxo-iron (FeO2H)3+ and oxenoid-iron (FeO)3+, can effect olefin epoxidation. The ability of cytochrome P450 to use several different active oxidants generated from molecular oxygen may help account for the broad reaction specificity and variety of products formed by this versatile catalyst.
The P450 cytochromes (P450s) constitute a very large and versatile family of thiolate-ligated heme proteins that use reducing equivalents derived from NADPH or NADH and molecular oxygen to catalyze a variety of oxidative reactions. These include hydrocarbon hydroxylation, olefin epoxidation, heteroatom oxygenation, dealkylation of ethers, thioethers, and substituted amines, desaturation at isolated carbon—carbon bonds, and aldehyde deformylation (1, 2). Some of these reactions, such as hydrocarbon hydroxylation and desaturation, are energetically less favorable than others—for example, olefin epoxidation. A hypervalent iron-oxene species is generally believed to be the oxidant in these reactions, with the protein serving as a template for binding and orientation of the substrate (3, 4). This concept of the oxidant has been derived largely from the well-elucidated chemical properties of peroxidases and of porphyrin biomimetic models, as well as from the requirement for a species capable of oxygen insertion into unactivated carbon–hydrogen bonds (2, 3). Efforts by various laboratories to identify the putative iron-oxene in P450-catalyzed reactions by spectroscopic means have had partial success (5, 6), and recent developments in transient state crystallographic techniques appear to have identified such a species in the reaction cycle of P450cam (7).
In the demethylation of androgens to estrogens by P450arom, a nucleophilic iron-peroxo species was proposed as the oxidant to account for the isotopic labeling patterns observed in the reaction products (8). Subsequently, our laboratory showed that purified isoforms of liver microsomal P450 catalyze a comparable reaction, the oxidative deformylation of a variety of xenobiotic aldehydes to olefins and formate (9–11). Consistent with an iron-peroxo species as the active oxidant in these reactions was the demonstration that, of several agents known to support many P450-catalyzed reactions in the absence of O2 and NADPH, H2O2 supports olefin formation in the oxidative deformylation of cyclohexane carboxyaldehyde to yield cyclohexene, but iodosobenzene, m-chloroperbenzoic acid, and cumyl hydroperoxide do not (9).
As shown in Fig. 1, the activation by P450 of molecular oxygen to the putative oxenoid iron species requires the uptake of two protons. Two electron reduction of O2 gives peroxo-iron, which upon protonation yields hydroperoxo-iron. Uptake of a second proton would then lead irreversibly to the oxene species and water or, alternatively, to the ferric enzyme and free hydrogen peroxide. It is not completely clear whether proton uptake occurs in this sequential manner or in a concerted reaction. Solvent isotope inventory studies on the hydroxylation of camphor by P450cam indicate the participation of two protons in the activation of molecular oxygen (12). The extent of the first proton uptake is apparently determined by the pKa of the hydroperoxo-iron complex, and, depending on whether the second proton uptake occurs at the oxygen atom proximal or distal to the heme iron, either H2O2 is released from the enzyme or heterolytic cleavage of the O—O bond occurs to yield the putative oxene species. The route of proton delivery to the active site and the mechanism of O—O bond cleavage are now partly understood. The crystal structures of four bacterial P450s, namely, cam (13), BM-3 (14), terp (15), and EryF (16), and sequence alignments (17, 18) reveal a highly conserved threonine in the I helix at the proposed oxygen-binding pocket, and within hydrogen bonding distance of the peroxo-iron complex that has been implicated in a proton delivery network (13, 19, 20). Consistent with such a role for the conserved threonine, camphor hydroxylation by P450cam (19, 20) and fatty acid hydroxylation by P450 BM-3 (21) were significantly diminished on the mutation of this amino acid residue to alanine. More recently, replacement of the conserved threonine of P450cam by unnatural amino acids has indicated a likely role for the oxygen atom of the threonine hydroxyl group in hydrogen bonding with the water that serves as the proton donor to the peroxo heme-iron complex (22).
Figure 1.
Scheme showing steps in oxygen activation by P450 involving electron and proton uptake, where Fe represents the heme iron atom.
This laboratory recently reported evidence for a functional role of a peroxo species in substrate oxygenation by a mammalian P450 (23). Mutation of the conserved Thr-302 in ΔP450 2B4 (recombinant 2B4 with N-terminal amino acids 2–27 deleted) to give the Ala variant resulted in a greatly diminished rate of reactions expected to require an oxenoid oxidant: cyclohexane hydroxylation, benzphetamine N-demethylation, and 1-phenylethanol oxidation. In contrast, the deformylation of cyclohexane carboxyaldehyde to cyclohexene, presumably by the action of a nucleophilic peroxo oxidant to yield an intermediate peroxyhemiacetal, was enhanced about 10-fold. The peroxo- and hydroperoxo-iron complexes shown in Fig. 1 are nucleophilic oxidants; however, the hydroperoxo complex also has electrophilic properties. To determine whether the hydroperoxo species can function as an electrophilic oxidant in some P450-catalyzed reactions, we have now examined the effect of the mutation of the conserved threonine in ΔP450s 2B4 and 2E1 (recombinant 2E1 with N-terminal amino acids 3–29 deleted) on the epoxidation and allylic hydroxylation of alkenes. These reactions have been selected because they represent two categories of electrophilic oxidations that can be effected by different oxidant species. Electrophilic peroxo-metal complexes (24) and high-valent oxenoid-metal complexes (25) can effect epoxidation of unfunctionalized olefins, whereas an oxidant such as an oxenoid species that is capable of hydrogen atom abstraction is essential for allylic hydroxylation (2, 3).
The findings in this paper on the epoxidation and allylic hydroxylation of unfunctionalized olefins, in conjunction with our earlier studies on the oxidative deformylation of aldehydes, lead us to propose a broader concept for oxidative reactions catalyzed by the P450 family of heme proteins. Thus, in addition to the generally accepted oxenoid-heme species, the peroxo- and hydroperoxo-heme complexes are also competent oxidants and, depending on the particular substrate or P450 isozyme involved, any of the three species derived from the reduction of molecular oxygen may serve as the primary oxidant. This versatility with respect to the active oxidant may help account for the remarkably broad substrate specificity of the P450 cytochromes.
Epoxidation of alkenes and arenes is a widespread reaction catalyzed by P450 cytochromes. Some examples in which the formation of an epoxide has been implicated are the conversion of arachidonic acid to a vasoactive metabolite (26), of benzo[a]pyrene to the ultimate carcinogenic diol-epoxide (27, 28), and of urethane and vinyl carbamate to a carcinogenic product (29, 30), as well as drug metabolism leading to the hepatotoxicity of furosemide (31) and the teratogenicity of phenytoin (32) and thalidomide (33). For this study, however, model olefin substrates were selected for the ease with which the products could be quantitated, the availability of cis and trans isomers in one case, and the choice of compounds that undergo hydroxylation as well as epoxidation.
MATERIALS AND METHODS
Materials.
The secretion–expression vector pIN-III-ompA3 described by Shen et al. (34) was used for expression of rat liver NADPH–P450 reductase in Escherichia coli strain C-1A. The specific activity of the reductase purified from this source according to Hanna et al. (35) was 49.0 units (micromoles of cytochrome c reduced per min) per milligram of protein at 30°C, and the specific content was 12.8 nmol/mg of protein. Δ2B4 and Δ2B4 T302A (truncated P450 with threonine mutated to alanine) were expressed and purified as reported previously to a specific content of 4–11 nmol/mg of protein (23). Peter Hlavica (Ludwig-Maximilians-Universität, Munich) has informed us that P450 2B4 cDNA without the sequence-encoding amino acids 2–27, prepared in this laboratory (36), and derived from the full-length cDNA given us by Gasser et al. (37), encodes for Ser in position 221 rather than the expected Pro (38). This finding was corroborated by us, and we also found that the codon for Ser-221 was present in the original 2B4 cDNA (designated clone B0) given to us. Kostas P. Vatsis (this laboratory) has recently cloned, expressed, and purified the truncated protein containing Pro-221 and shown that it is identical to the truncated protein containing Ser-221 in a typical reaction with benzphetamine as substrate.
The Δ2E1 clone used for heterologous expression was constructed as described by Larson et al. (39), and the T303A amino acid change was effected by site-directed mutagenesis and PCR. The sense (5′ → 3′) and antisense (3′ → 5′) amplification primers encompassed Δ2E1 nucleotides 764–833 and 1,205–1,246, respectively, and the 5′ primer was made with an A to G substitution at position 828 to produce the desired mutation; PCR was carried out as detailed (40). The PCR-generated fragment (483 bp) was cleaved with BsrGI and MluI and cloned into similarly digested Δ2E1 to give rise to Δ2E1 T303A (truncated P450 with threonine mutated to alanine). The identity of the mutant was established by DNA sequencing of the PCR-generated segment (nucleotides 764–1,246), as well as of the 5′ end of the clone (nucleotides −30 to 115) to verify the truncation. The truncated protein and its T303A mutant were expressed and purified as described (41) to give preparations with a specific content of 14–17. The results to be described were independent of the specific content of the various P450 cytochrome preparations examined in this study.
Analytical grade reagents were obtained commercially and used as such unless otherwise stated. The purity of styrene, cyclohexene, and cis- and trans-butene, obtained from Aldrich, was established by gas chromatography. Each of the butenes contained no more than 1% of the other isomer by this analysis.
Enzyme Reaction Mixtures.
The enzyme system was reconstituted by mixing the concentrated P450 and reductase preparations with a freshly sonicated solution of dilauroylglyceryl-3-phosphorylcholine and allowing the mixture to stand on ice for 15–60 min before addition of the other components. A typical reaction mixture contained 0.2 nmol of Δ2B4 or Δ2B4 T302A (or 0.1 nmol of Δ2E1 or Δ2E1 T303A), the reductase in a 2:1 molar ratio with P450, 30 μg of phospholipid, 100 units of bovine liver catalase (Sigma), 5.0 μg of bovine erythrocyte superoxide dismutase (Sigma), and 200 μmol of potassium phosphate buffer (pH 7.4). Such mixtures were incubated at 37°C for 5 min before addition of 5.0 μl of a 400-mM methanolic solution of styrene or cyclohexene and 800 nmol of NADPH as the final component. When butene was the substrate, NADPH was added and then 5.0 μl of 400-mM substrate in methanolic solution as the final component, and each reaction vial was promptly sealed with a rubber septum. The final volume of the reaction mixtures was 2.0 ml in all cases. After incubation for an appropriate time at 37°C, the reactions were quenched by the addition of 500 μl of 2.0 N NaOH along with a methanolic solution of the appropriate internal standard (15 nmol).
Analytical Procedures.
Except for analysis of the butane-2,3-epoxides, which were quantitated by head space gas analysis, an epoxide and an alcohol served as standards for determination of the extent of product recovery in the extraction process. For epoxidation of styrene and cyclohexene, the standards were 4-chlorostyrene oxide and cyclopentene oxide, respectively. For the allylic hydroxylation products of 2-butenes and of cyclohexene, the internal standards were 2-methyl-2-butene-1-ol and cyclohexanol, respectively. Each experiment was carried out in triplicate, and the results are given as the mean with the SD from the mean.
A Hewlett–Packard Model 6890 gas chromatograph, with flame ionization detector, attached to a Model 7694 head space gas sampler with a 3-ml sampling loop was used for determination of cis- and trans-butane-2,3-epoxides. The 3-ft column (2-mm inner diameter) contained 4% Supelco Carbowax 20 M with 0.8% KOH on 60/80 Carbopak B, and the carrier gas was nitrogen at a flow rate of 5 ml/min. Reaction mixtures were heated at 75°C for 5 min before sampling, and the column was maintained at 75°C for 5 min and then raised to 130°C at a rate of 5 degrees per min. Under these conditions the cis and trans isomers of butane-2,3-epoxide were eluted at 14.8 and 15.5 min, respectively. The column was purged at 220°C for 5 min prior to injection of the next sample. Standard curves for quantitation of the two epoxides were obtained under the same conditions, except that the enzymes were omitted; a linear response was obtained in the range from 50 to 1,200 pmol.
Styrene oxide and 4-chlorostyrene oxide were analyzed by GC on a 30-m 0.25-micron DB5 silica capillary column. To a quenched reaction mixture the appropriate internal standard was added, and the mixture was extracted with 2 ml of CH2Cl2 by vigorous mixing, followed by centrifugation for clear separation of the aqueous and organic phases. The organic layer was removed, dried over anhydrous sodium sulfate, and concentrated to approximately 0.2 ml in a vacuum centrifuge attached to a water aspirator. Care was taken to prevent evaporation to 0.1 ml or less as the epoxides are somewhat volatile and could be partly lost. A 1.0-μl aliquot was injected onto the column by a Hewlett–Packard Model 6890 series auto liquid sampler. The carrier gas was nitrogen at a flow rate of 2 ml/min; the initial temperature was maintained at 50°C for 5 min and then raised to 220°C at a rate of 10 degrees per min. The column was purged for 5 min at 220°C before injection of the next sample. The retention times of styrene oxide and the 4-chloro derivative were 10.2 and 13.2 min, respectively.
Cyclohexene oxide, cyclopentene oxide, cyclohexanol, 2-cyclohexene-1-ol, 2-butene-1-ol, and 2-methyl-2-butene-1-ol were analyzed on a 30-m 0.32-micron Supelcowax-10 capillary column; the retention times were 7.2, 4.3, 14.2, 15.9, 9.1, and 12.1 min, respectively, under the following conditions. The carrier gas was nitrogen at a flow rate of 2 ml/min, and the initial temperature was 50°C for 5 min, which was then raised at 5 degrees per min to 125°C. The column was purged at 275°C for 5 min before injection of the next sample. For 2-butene-1-ol determination, reaction mixtures that had been analyzed by the head space method for the butane-2,3-epoxides were extracted with 2.5 ml of methylene chloride, and the organic extract was dried and concentrated in a vacuum centrifuge to approximately 50 μl. A 1.0-μl aliquot was then injected onto the capillary column.
RESULTS AND DISCUSSION
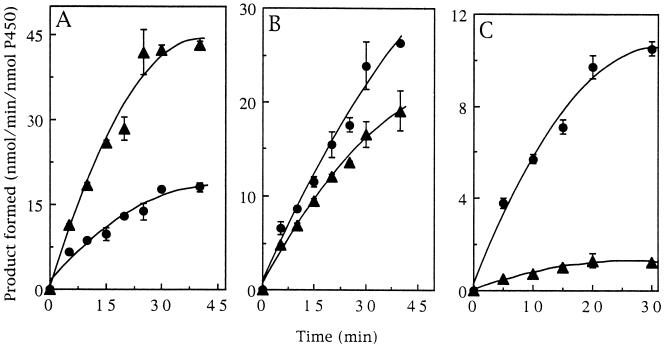
With the three model substrates examined, namely, styrene, cyclohexene, and 2-butene, the formation of epoxides and of hydroxylation products was linear with time over only a short interval. This result, which was seen with ΔP450s 2B4 and 2E1, as well as their respective mutants with a threonine residue replaced by alanine, appears to be a consequence of enzyme inactivation. Some typical kinetic results are shown in Fig. 2. In experiments not presented, the lack of linearity was found not to be attributable to depletion of NADPH, O2, or substrate, and was not prevented by the presence of catalase and superoxide dismutase in the reaction mixtures. Initial rates were determined at 5 or 10 min, when loss of activity was minimal, and are given without correction.
Figure 2.
Product formation as a function of time. (A) Conversion of styrene to the oxide by Δ2E1 (•) and Δ2E1 T303A (▴). (B) Conversion of cyclohexene to 1-cyclohexene-3-ol by Δ2E1 (•) and Δ2E1 T303A (▴). (C) Conversion of trans-2-butene to the trans-2-oxide by Δ2B4 (•) and Δ2B4 T302A (▴).
The rates of oxidation of the various substrates with the two cytochromes and the mutants are summarized in Table 1. With styrene, a particularly interesting difference was seen between the two P450 isozymes. Replacement of the threonine residue in Δ2B4 by alanine resulted in a 3-fold decrease in epoxide formation, a result that would be predicted from our previous study on other substrate oxidations with this mutant (23). In sharp contrast, replacement of the corresponding threonine residue in Δ2E1 gave an increase of more than 2-fold in epoxidation. A possible explanation of this finding is that in Δ2E1 T303A an electrophilic oxygenating species other than oxenoid-iron, possibly hydroperoxo-iron, contributes to styrene epoxidation. The next substrate to be examined was cyclohexene, which was found to undergo both epoxidation and hydroxylation by all of the P450s. As shown in the Table 1, the T302A mutation of Δ2B4 gave a large decrease in both products. Again, in contrast, the T303A mutation of Δ2E1 resulted in a significant increase in the formation of cyclohexene oxide (1.7-fold), but a decrease in allylic hydroxylation to 1-cyclohexene-3-ol. In line with the proposed explanation of the results with styrene, the threonine to alanine mutation in Δ2E1 may cause a partial block in proton delivery to the active site; this would favor the hydroperoxo-iron species as an epoxidizing agent, but would decrease the level of the oxenoid-iron species that participates in cyclohexene hydroxylation. An additional model substrate, 2-butene, was examined because of the availability of the cis and trans isomers and the additional advantage that each was found to undergo epoxidation and allylic hydroxylation. As indicated in the table, the epoxidation of cis-butene by the four enzymes was consistently faster than that of the trans isomer. Product formation was highly stereospecific, with no epimerization detected in the epoxides, which is consistent with the results previously reported by Wistuba et al. (42) with a number of alkenes in microsomal suspensions. Replacement of the threonine residue in Δ2B4 caused a significant decrease in epoxide formation with both the cis and trans substrates, whereas replacement of this residue in Δ2E1 gave a large increase in the epoxidation reaction, 2.7-fold for the cis-butene and 5- to 8-fold for the trans. In contrast to these results for epoxidation of the olefinic bond, allylic hydroxylation of both isomers of butene was significantly reduced by the mutation of ΔP450 2E1 as well as of ΔP450 2B4.
Table 1.
Rates of oxidation of olefinic substrates by P450 cytochromes
| Substrate | Product determined | Reaction rate, nmol/min per nmol of P450
|
|||
|---|---|---|---|---|---|
| Δ2B4 | Δ2B4 T302A | Δ2E1 | Δ2E1 T303A | ||
| Styrene | Styrene oxide | 47.8 ± 0.5 | 15.9 ± 0.8 | 14.5 ± 0.6 | 33.0 ± 0.4 |
| Cyclohexene | Cyclohexene oxide | 48.7 ± 4.4 | 13.0 ± 0.5 | 6.7 ± 0.1 | 11.4 ± 0.2 |
| Cyclohexene | 1-cyclohexene-3-ol | 29.3 ± 1.7 | 7.8 ± 1.1 | 6.6 ± 0.7 | 4.8 ± 0.1 |
| cis-2-butene | cis-2-butene oxide | 6.4 ± 0.6 | 3.8 ± 0.1 | 36.0 ± 2.0 | 97.0 ± 3 |
| cis-2-butene | 2-butene-1-ol | 0.15 ± 0.02 | <0.05* | 0.40 ± 0.03 | 0.21 ± 0.06 |
| trans-2-butene | trans-2-butene oxide | 3.8 ± 0.2 | 0.48 ± 0.03 | 4.8 ± 0.1 | 27.8 ± 0.5 |
| trans-2-butene | 2-butene-1-ol | 0.11 ± 0.02 | <0.05* | 1.2 ± 0.1 | 0.5 ± 0.1 |
The results are given as the mean with the SD for triplicate determinations from a typical experiment.
Only a trace amount of the alcohol was formed by the mutant Δ2B4 enzyme.
In cyclohexene and the isomers of 2-butene the allylic carbon—hydrogen bond and the olefinic bond, which represent distinct electrophilic reaction centers in the same molecule, are within a 3-Å spherical radius. The small size and symmetric nature of these molecules obviate consideration of steric constraints within the P450 active site as an explanation of the results presented above. In this connection, Rietjens et al. (43) have shown that aromatic hydroxylation of fluorobenzenes in vivo and in liver microsomes is limited only by the electronic parameters of the aromatic ring and not by steric factors. Hence, the distribution of products observed in the oxidation of cyclohexene and of cis- and trans-2-butene reflects the chemical steps of the reaction. When a single olefinic substrate gives two oxidation products, an epoxide, P1, and an alcohol, P2, from a common reactive oxidant, say P450 (Fe=O), the rates of formation are given by kepox[(Fe=O)(olefin)] and khydrox[(Fe=O)(olefin)], respectively, and the ratio of products formed, P1/P2, is equal to the ratio of the rate constants, kepox/khydrox. If mutation of a residue in the enzyme perturbs only proton delivery, the concentration of the active complex and the absolute rates of product formation could change. However, kepox/khydrox would remain unchanged and, consequently, the ratio of products formed would be expected to remain constant. Conversely, if the two products are derived from different oxidant species, say Fe—OOH for epoxidation and Fe=O for hydroxylation, then the rates of formation of P1 and P2 are given by kepox[(Fe—OOH)(olefin)] and khydrox[(Fe=O)(olefin)], respectively. In this case, the ratio of products is dependent on the concentration of each oxidant species; if a change in proton delivery alters the steady-state concentrations of the reactive complexes, the ratio of products would be expected to be altered. Thus, the ratio of products obtained from a single substrate with the parent and mutant enzymes may distinguish whether the products are derived from a common oxidant or from distinct oxidants. As shown in Table 2, mutation of the conserved threonine in ΔP450 2B4 caused no change in the ratio of epoxide to allyl alcohol formed from cyclohexene, but with Δ2E1 the ratio was increased from 1.0 to 2.4, a result consistent with involvement of different oxidants in formation of the different products. Similarly, with cis- and trans-butene the product ratios with Δ2E1 were increased from 90 to 460 and from 4.0 to 56, respectively, but the ratios could not be determined for the Δ2B4 mutation because only a trace of the alcohol was formed.
Table 2.
Effect of Thr → Ala mutation on the ratio of the rates of epoxidation and hydroxylation of olefins
| Substrate | Ratio of initial rates, epoxidation/hydroxylation
|
|||
|---|---|---|---|---|
| Δ2B4 | Δ2B4 T302A | Δ2E1 | Δ2E1 T303A | |
| Cyclohexene | 1.7 | 1.7 | 1.0 | 2.4 |
| cis-butene | 43 | ND* | 90 | 460 |
| trans-butene | 35 | ND* | 4.0 | 56 |
ND, not determined (only a trace of the alcohol was formed by this enzyme).
The epoxidation of an unactivated olefin is an electrophilic reaction that can be effected by peroxo-metal complexes as well as by high valent metal oxo complexes (24, 25), whereas allylic hydroxylation requires an oxidant capable of hydrogen atom abstraction (2). The enhancement in epoxidation and suppression in allylic hydroxylation observed on replacement of T303 in ΔP450 2E1 is consistent with the hydroperoxo-heme complex functioning as the active electrophilic oxidant in epoxidation. Mutation of the conserved threonine in Δ2E1 may possibly shift the protonation equilibrium in favor of the hydroperoxo-heme complex. The hydroperoxo species is also capable of nucleophilic oxidations, consistent with a large enhancement in oxidative deformylation observed with this mutant (44). In contrast to the results with Δ2E1 T303A, both epoxidation and allylic hydroxylation are suppressed by the T302A mutation in ΔP450 2B4. In the latter cytochrome, removal of the conserved threonine may shift the protonation equilibrium to the peroxo-iron complex, causing it to be the predominant oxidant. Because this species is nucleophilic, the oxidative deformylation reaction is enhanced, as described (23). An alternative explanation for the findings in this study might be that the mutation results in alteration of the active site architecture, which is then responsible for different rates in the oxidation of the substrate to the epoxide and the allylic alcohol. However, the Ks and Km values for the binding and epoxidation of styrene by 2E1 and 2B4 are the same as for the respective mutants and, additionally, the ratio of hydroxylation at the primary and secondary positions of 2-methylbutane is unaffected by the mutations (data not shown). These findings argue against a structural basis for the observed results. Another possible explanation for our results that might be considered is that the rate-limiting steps leading to the epoxide and the allylic alcohol from the same substrate could be affected differently by the T303A mutation of ΔP450 2E1.
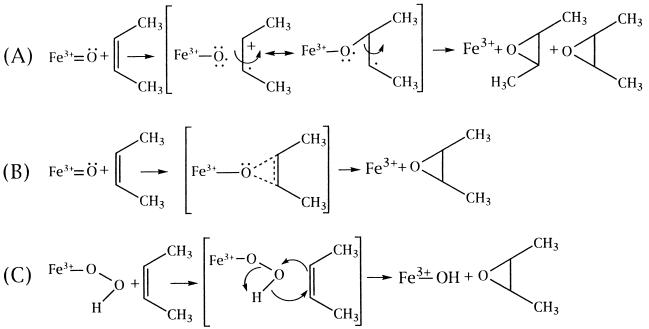
The epoxidation of olefins catalyzed by P450 has been proposed to occur by an intermediate charge-transfer complex between the olefin and the oxenoid-iron as shown in Fig. 3, reaction A (25). By this mechanism the epoxidation of cis- or trans-2-butene would be expected to result in epimeric mixtures of the butane-2,3-epoxides, because the rotational energy barrier about the C2—C3 bond in butane is only 3.5 kcal/mol (45). However, as reported in this study with purified P450s and previously with microsomal suspensions (42), epimerization was not observed in the epoxidation of either isomer of 2-butene. Studies on the P450cam-catalyzed epoxidation of trans-1-phenyl-2-vinylcyclopropane, a very sensitive radical clock probe, provided no evidence for the involvement of radicals or cations in the reaction (46). Collectively, the stereochemical and radical clock results may be interpreted to reflect an oxygen insertion by an oxenoid-heme species (reaction B), reminiscent of the insertion of carbenes into olefinic bonds (47, 48). Alternatively, the radical clock studies, in conjunction with the stereochemical and mutagenesis findings presented in the present paper, could be explained by a mechanism (reaction C) in which the hydroperoxo-heme complex is the oxidant, resulting in epoxidation of the olefin by a concerted mechanism, similar to peracid epoxidation of olefins.
Figure 3.
Mechanisms of olefin epoxidation (A) by oxenoid-iron involving a charge-transfer complex and leading to epimerization, (B) by oxenoid-iron leading to a concerted insertion of oxygen, and (C) by hydroperoxo-iron in a concerted reaction.
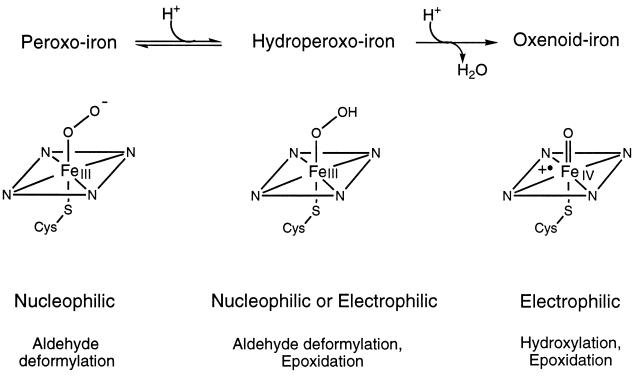
In summary, the results presented suggest that the hydroperoxo complex of P450 is an active oxidant capable of epoxidation of unactivated olefinic bonds. The extent to which such an oxidant may participate in diverse oxidative reactions catalyzed by P450 cytochromes remains to be established. As shown in Fig. 4, we propose that the versatility in oxidative reactions may, in part, be attributed to the ability of P450 to use the peroxo-, hydroperoxo-, or oxenoid-iron species as the active oxidant depending on the substrate and the type of reaction effected.
Figure 4.
Proposed versatility in P450 oxygenating species. The nucleophilic or electrophilic properties and typical reactions catalyzed are indicated under the structures of the peroxo, hydroperoxo, and oxenoid species.
Acknowledgments
We thank Dr. Norman M. Olken for constructing the Δ2E1 T303A plasmid and Dr. Kostas P. Vatsis for sequence confirmation. This investigation was supported by National Institutes of Health Grants DK-10339 and AA-06221.
ABBREVIATIONS
- P450
cytochrome P450
- 2B4 and 2E1
P450 2B4 (rabbit liver) and P450 2E1 (rabbit liver), respectively
- Δ2B4 and Δ2E1
recombinant 2B4 and 2E1 with N-terminal amino acids 2–27 and 3–29 deleted, respectively
- Δ2B4 T302A and Δ2E1 T303A
truncated P450s with threonine mutated to alanine
References
- 1.Coon M J, Ding X, Pernecky S J, Vaz A D N. FASEB J. 1992;6:669–673. doi: 10.1096/fasebj.6.2.1537454. [DOI] [PubMed] [Google Scholar]
- 2.Sono M, Roach M P, Coulter E D, Dawson J H. Chem Rev. 1996;96:2841–2887. doi: 10.1021/cr9500500. [DOI] [PubMed] [Google Scholar]
- 3.White R E, Coon M J. Annu Rev Biochem. 1980;49:315–356. doi: 10.1146/annurev.bi.49.070180.001531. [DOI] [PubMed] [Google Scholar]
- 4.Guengerich F P, Macdonald T L. FASEB J. 1990;4:2453–2459. doi: 10.1096/fasebj.4.8.2185971. [DOI] [PubMed] [Google Scholar]
- 5.Blake R C, II, Coon M J. J Biol Chem. 1989;264:3694–3701. [PubMed] [Google Scholar]
- 6.Egawa T, Shimada H, Ishimura Y. Biochem Biophys Res Commun. 1994;201:1464–1479. doi: 10.1006/bbrc.1994.1868. [DOI] [PubMed] [Google Scholar]
- 7.Schlichting I, Berendzen J, Chu K, Stock A M, Sweet R M, Ringe D, Petsko G A, Davies M, Mueller E J, Benson D, Sligar S. FASEB J. 1997;11:A769. (abstr.). [Google Scholar]
- 8.Akhtar M, Calder M R, Corina D L, Wright J N. Biochem J. 1982;201:569–580. doi: 10.1042/bj2010569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vaz A D N, Roberts E S, Coon M J. J Am Chem Soc. 1991;113:5886–5887. [Google Scholar]
- 10.Roberts E S, Vaz A D N, Coon M J. Proc Natl Acad Sci USA. 1991;88:8963–8966. doi: 10.1073/pnas.88.20.8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vaz A D N, Kessell K S, Coon M J. Biochemistry. 1994;33:13651–13661. doi: 10.1021/bi00250a015. [DOI] [PubMed] [Google Scholar]
- 12.Aikens J, Sligar S G. J Am Chem Soc. 1994;116:1143–1144. [Google Scholar]
- 13.Poulos T L, Finzel B C, Howard A J. J Mol Biol. 1987;195:687–700. doi: 10.1016/0022-2836(87)90190-2. [DOI] [PubMed] [Google Scholar]
- 14.Ravichandran K G, Boddupalli S S, Hasemann C A, Peterson J A, Deisenhofer J. Science. 1993;261:731–736. doi: 10.1126/science.8342039. [DOI] [PubMed] [Google Scholar]
- 15.Hasemann C A, Ravichandran K G, Peterson J A, Deisenhofer J. J Mol Biol. 1994;236:1169–1185. doi: 10.1016/0022-2836(94)90019-1. [DOI] [PubMed] [Google Scholar]
- 16.Cupp-Vickery J R, Poulos T L. Nat Struct Biol. 1995;2:144–153. doi: 10.1038/nsb0295-144. [DOI] [PubMed] [Google Scholar]
- 17.Nelson D R, Strobel H W. Biochemistry. 1989;28:656–660. doi: 10.1021/bi00428a036. [DOI] [PubMed] [Google Scholar]
- 18.Gotoh O, Fujii-Kuriyama Y. In: Frontiers in Biotransformation. Ruckpaul K, Rein H, editors. Vol. 1. Berlin: Akademie; 1989. pp. 195–243. [Google Scholar]
- 19.Imai M, Shimada H, Watanabe Y, Matsushima-Hibiya Y, Makino R, Koga H, Horiuchi T, Ishimura Y. Proc Natl Acad Sci USA. 1989;86:7823–7827. doi: 10.1073/pnas.86.20.7823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinis S A, Atkins W M, Stayton P S, Sligar S G. J Am Chem Soc. 1989;111:9252–9253. [Google Scholar]
- 21.Yeom H, Sligar S G, Li H, Poulos T L, Fulco A J. Biochemistry. 1995;34:14733–14740. doi: 10.1021/bi00045a014. [DOI] [PubMed] [Google Scholar]
- 22.Kimata Y, Shimada H, Hirose T, Ishimura T. Biochem Biophys Res Commun. 1995;208:96–102. doi: 10.1006/bbrc.1995.1310. [DOI] [PubMed] [Google Scholar]
- 23.Vaz A D N, Pernecky S J, Raner G M, Coon M J. Proc Natl Acad Sci USA. 1996;93:4644–4648. doi: 10.1073/pnas.93.10.4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Michaelson R C, Palermo R E, Sharpless K B. J Am Chem Soc. 1977;99:1990–1992. [Google Scholar]
- 25.Groves J T, Gross Z. NATO ASI Ser Ser C. 1995;459:39–47. [Google Scholar]
- 26.Carroll M A, Schwartzman M, Capdevila J, Falok J R, McGiff J C. Eur J Pharmacol. 1987;138:281–283. doi: 10.1016/0014-2999(87)90445-6. [DOI] [PubMed] [Google Scholar]
- 27.Yang S K, McCourt D W, Roller P P, Gelboin H V. Proc Natl Acad Sci USA. 1976;73:2594–2598. doi: 10.1073/pnas.73.8.2594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deutsch J, Leutz J C, Yang S K, Gelboin H V, Chiang Y L, Vatsis K P, Coon M J. Proc Natl Acad Sci USA. 1978;75:3123–3127. doi: 10.1073/pnas.75.7.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dahl G A, Miller J, Miller E C. Cancer Res. 1978;38:3793–3804. [PubMed] [Google Scholar]
- 30.Guengerich F P, Kim D-H. Chem Res Toxicol. 1991;4:413–421. doi: 10.1021/tx00022a003. [DOI] [PubMed] [Google Scholar]
- 31.Mitchell J R, Nelson W L, Potter W Z, Sasame H A, Jollow D L. J Pharmacol Exp Ther. 1976;199:41–52. [PubMed] [Google Scholar]
- 32.Martz F, Faillinger C, Blake D A. J Pharmacol Exp Ther. 1977;203:231–239. [PubMed] [Google Scholar]
- 33.Gordon G B, Spielberg S P, Blake D A, Balasubramanian V. Proc Natl Acad Sci USA. 1981;78:2545–2548. doi: 10.1073/pnas.78.4.2545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen A L, Porter T D, Wilson T F, Kasper C B. J Biol Chem. 1989;264:7584–7589. [PubMed] [Google Scholar]
- 35.Hanna I H, Teiber J F, Kokones K L, Hollenberg P F. Arch Biochem Biophys. 1998;350:324–332. doi: 10.1006/abbi.1997.0534. [DOI] [PubMed] [Google Scholar]
- 36.Pernecky S J, Olken N M, Bestervelt L L, Coon M J. Arch Biochem Biophys. 1995;318:446–456. doi: 10.1006/abbi.1995.1253. [DOI] [PubMed] [Google Scholar]
- 37.Gasser R, Negishi M, Philpot R M. Mol Pharmacol. 1988;33:22–30. [PubMed] [Google Scholar]
- 38.Tarr G E, Black S D, Fujita V S, Coon M J. Proc Natl Acad Sci USA. 1983;80:6552–6556. doi: 10.1073/pnas.80.21.6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Larson J R, Coon M J, Porter T D. J Biol Chem. 1991;266:7321–7324. [PubMed] [Google Scholar]
- 40.Pernecky S J, Larson J R, Philpot R M, Coon M J. Proc Natl Acad Sci USA. 1993;90:2651–2655. doi: 10.1073/pnas.90.7.2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Larson J R, Coon M J, Porter T D. Proc Natl Acad Sci USA. 1991;88:9141–9145. doi: 10.1073/pnas.88.20.9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wistuba D, Nowotny H-P, Trager O, Schurig V. Chirality. 1989;1:127–136. doi: 10.1002/chir.530010206. [DOI] [PubMed] [Google Scholar]
- 43.Rietjens I M C M, Soffers A E M F, Veeger C, Vervoort J. Biochemistry. 1993;32:4801–4812. doi: 10.1021/bi00069a015. [DOI] [PubMed] [Google Scholar]
- 44.McGinnity D F, Vaz A D N, Coon M J. FASEB J. 1997;11:A807. (abstr.). [Google Scholar]
- 45.Kingsbury C A. J Chem Educ. 1979;56:431–437. [Google Scholar]
- 46.Miller V P, Fruetel J A, Ortiz de Montellano P R. Arch Biochem Biophys. 1992;298:697–702. doi: 10.1016/0003-9861(92)90468-c. [DOI] [PubMed] [Google Scholar]
- 47.Skell P S, Woodworth R C. J Am Chem Soc. 1956;78:4496–4497. [Google Scholar]
- 48.Hoffman R. J Am Chem Soc. 1968;90:1475–1485. [Google Scholar]