Abstract
Investigations using selective lesion techniques suggest that the septohippocampal cholinergic system may not be critical for spatial orientation. These studies employ spatial tasks that provide the animal with access to both environmental and self-movement cues; therefore, intact performance may reflect spared spatial orientation or compensatory mechanisms associated with one class of spatial cues. The present study investigated the contribution of the septohippocampal cholinergic system to spatial behavior by examining performance in foraging tasks in which cue availability was manipulated. Thirteen female Long–Evans rats received selective lesions of the medial septum/vertical band with 192 IgG saporin, and 11 received sham surgeries. Rats were trained to forage for hazelnuts in an environment with access to both environmental and self-movement cues (cued condition). Manipulations include altering availability of environmental cues associated with the refuge (uncued probe), removing all visual environmental cues (dark probe), and placing environmental and self-movement cues into conflict (reversal probe). Medial septum lesions disrupted homeward segment topography only under conditions in which self-movement cues were critical for organizing food hoarding behavior (dark and reversal). These results are consistent with medial septum lesions producing a selective impairment in self-movement cue processing and suggest that these rats were able to compensate for deficits in self-movement cue processing when provided access to environmental cues.
Keywords: Medial septum, Dead reckoning, Spatial mapping, Path integration, Long–Evans species
1. Introduction
Studies investigating the role of the septohippocampal system in spatial orientation have reported conflicting results. Those that used nonselective medial septum lesion techniques have consistently observed impaired performance on a variety of spatial tasks [49,20,19,25,52,35,1, for a review see 15]. In contrast, studies that used 192 IgG-Saporin to target medial septum cholinergic cells have observed impaired and spared performance on similar spatial tasks [6,4,34,7,28,47,29,8,23,16 for a review see 45]. Although selectivity and extent of the lesion technique was a likely factor influencing these mixed results, the inability of the spatial tasks to dissociate specific cue use may have also contributed to the performance observed across studies.
Spatial orientation can be derived from multiple sources of information to guide performance on spatial tasks [18,51]. One source of information depends on cues present in the environment. Rats can either use a single environmental cue or the relationships among multiple environmental cues to guide navigation [53,9]. Another source of information can be derived from recently generated self-movement cues (proprioceptive, vestibular, optic flow, efferent copies). These self-movement cues can be used to calculate the current position of the rat relative to the point of origin through dead reckoning [10,41,2,36,48,14,50,32]. Although the use of multiple sources of information affords rats the ability to maintain spatial orientation under novel or changing environmental conditions, it also presents challenges for examining the neural basis of spatial orientation. For example, lesions that disrupt systems involved in processing self-movement cues may spare systems related to processing environmental cues. This would provide rats with a potential compensatory mechanism that may be used to guide performance on certain spatial tasks.
A recent study has examined the role of the septohippocampal system in the processing of both classes of cues [33]. Rat exploratory trip organization has been shown to depend on the processing of self-movement cues [60,61,59]. Electrolytic medial septum lesions disrupted specific components of the exploratory trip, consistent with impaired processing of self-movement cues. The hidden platform version of the water maze task was included to examine the ability of the rats to use environmental cues to guide movements. Although performance improved across training days, the lesions produced a consistent attenuation of learning the location of the hidden platform. The authors concluded that the improved performance reflected a sparing of environmental cue use, whereas the attenuated performance was consistent with previous studies reporting a role for self-movement cue processing in the water maze [67,63]. One limitation of the Martin et al. study involves the use of a non-selective lesion technique. Damage to non-cholinergic cells may have contributed to the impairments observed on both tasks. A second limitation in the foregoing study reflects the assessment of environmental and self-movement cue use on tasks that varied in a number of dimensions (motivation, motor demands, temporal pacing of trips versus trials). Therefore, the effects of selective cholinergic lesions on spatial orientation remain to be determined.
Rats' natural proclivity to carry food items to a refuge for consumption has been shown to be a robust technique to dissociate the use of environmental and self-movement cues [68,32]. After searching for a randomly located food item, the rat can either use environmental cues to pilot or self-movement cues to dead reckon to return to the refuge. The current study investigated the effects of injecting the immunotoxin 192 IgG-Saporin into the medial septum on foraging behavior. Subsequent to immunotoxin or sham lesions, rats were trained to carry hazelnuts to a visible refuge. Cue use was examined during a series of probes that manipulated rats' access to environmental cues and placed them in conflict with self-movement cues. Each food hoarding trip was divided into a searching and homeward segment. Maximum speeds observed on each segment were used to evaluate group differences related to bradykinesia or hyperkinesia. Searching segment path circuity was used to investigate group differences in locating the hazelnut, whereas homeward segment path circuity reflected accuracy in returning to the refuge. The results of this investigation will contribute to understanding the role of the septohippocampal cholinergic system in spatial orientation.
2. Material and methods
2.1. Animals
Previous studies using food hoarding behavior to investigate the neural basis of spatial orientation have traditionally used female rats [68,65,32,66,58]. Building on this work, subjects were 24 female Long–Evans rats bred at Northern Illinois University from stock purchased from Harlan Sprague–Dawley. Although food hoarding proclivity has been observed to vary across the estrous cycle [13], food hoarding is a ubiquitous behavior of food deprived female rats. Both sham and lesion rats were run in mixed squads thereby minimizing any potential effect of the estrous cycle mediating group differences. At the beginning of the experiment, rats weighed approximately 250 g. Rats were housed in groups of two in plastic cages in the colony room with the temperature maintained at 20–21 °C with a 12 h light/dark cycle. All experimental procedures in this study were approved by the local Institutional Animal Care and Use Committee (IACUC), which follows the standards set by the National Institutes of Health.
2.2. Surgery
Rats were deeply anesthetized with a mixture of isoflurane and oxygen during surgery. Rats received either medial septum lesions (MS) or sham surgeries (Sham). Medial septum lesions were produced by microinjections of 192 IgG-Saporin (Lot #: 41–105; Advanced Targeting Systems, San Diego, CA) into the medial septum-diagonal band of Broca. 192 IgG-Saporin (0.50 μg/μL) was injected at two sites per hemisphere, coordinates relative to bregma and the surface of the dura: AP: +0.30, ML: ±0.20, DV1: −7.5 [0.20 μL each site], DV2: −6.5 [0.15 μL each site]. These procedures have been previously shown to produce significant decreases in ChAT-immunopositive neurons in the medial septum, decrease AChE fiber staining restricted to the hippocampus, and have no effects on immunoreactivity for parvalbumin-positive neurons in the medial septum [4,12,22,29,8]. Injections were made at the rate of 0.10 μL/min. The cannula was left in place for 3 min to prevent diffusion of 192 IgG-Saporin up the needle tract. Animals receiving sham surgeries were treated identically except the cannula was lowered +2.0 above the most dorsal coordinates (DV −4.5) and no toxin was administered.
2.3. Feeding
After rats had recovered, they were maintained at 85% of their ad libitum weight during the course of the experiment. Throughout the experiment, rats searched for randomly located hazelnuts (Duck Soup Co-Op, DeKalb, IL). Rats have been found to reliably carry food items of sufficient size to a refuge [62]. After testing each day, rats were supplementally fed LabDiet Laboratory Rodent Pellets in their plastic cages.
2.4. Apparatus
The apparatus was a large circular table (200 cm in diameter) located 75 cm above the floor. The table was located in a large room with many cues, including a door, chair, and posters attached to the walls. An infrared camera was positioned perpendicular to the surface of the table. The testing room was lightproof such that, when all of the lights were turned off, no light was present in the room. The experimenter used infrared goggles to observe the rat during dark testing. Infrared is a wavelength that rats are not able to detect [42].
Rats were provided with a refuge that was always located at the periphery of the table. The “cued” refuge was a small dark box (20 cm × 29 cm × 22 cm) located on the surface of the table with a circular hole (11.5 cm in diameter) in one of the sides, thereby providing the rat with direct access to the table. The “hidden” refuge was a similar dark box with an open top and small ramp. The hidden refuge was positioned just below the surface of the table such that the rat could use the ramp to climb onto the table.
2.5. Procedure
Each rat was randomly assigned a release location at the periphery of the table that remained stable across daily training sessions. Rats were trained to leave the cued refuge and search for a randomly located hazelnut under normal light conditions. After locating the hazelnut, the rat carried it to the refuge for consumption. While the rat was still in the refuge, a second hazelnut was placed on the table. Initially, hazelnuts were placed close to the refuge; however, over days, the distance between the refuge and the hazelnut was gradually increased. This procedure was repeated until the rat was able to successfully retrieve five hazelnuts, four that were at least halfway across the table (used for analyses) and one on the first half of the table (to motivate the rat to search the entire table). The table was rotated and wiped down after each rat's testing session. Daily training continued until the rat retrieved all five hazelnuts for four consecutive days (approximately 10 days of training). On the fourth day, the cued session was videotaped. Subsequently, cued sessions were alternated with probe sessions. Modifications of the apparatus and/or testing room were done prior to transporting the animal to the testing room.
2.5.1. Uncued probe
The apparatus was modified such that the refuge was located below the surface of the table. Rats were given four trials to locate the hazelnut and return to the hidden refuge. Use of a hidden refuge removes proximal visual cues, restricting the rats to using environmental and/or self-movement cues for the uncued probe.
2.5.2. Dark probe
The apparatus was modified such that the refuge was located below the surface of the table and the room lights were turned off. Rats were given four trials to locate the hazelnut and return to the hidden refuge under dark conditions. This probe eliminates all visual cues, thereby restricting the rat to using self-movement cues.
2.5.3. Reversal probe
The apparatus was modified such that the hidden refuge was located 180° different from that previously experienced. Rats were given four trials to locate the hazelnut and return to the hidden refuge under standard light conditions. The reversal probe produces a conflict between environmental cues learned during cued training (and uncued probe) and self-movement information generated on the outward segment of a trip.
2.6. Analysis of food hoarding behavior
The four trips extending at least halfway across the table were used for analyses. Each trip was converted from analog recording to a digital computer file using the Peak Performance system (Peak Performance Technologies, Inc. Englewood, CO 80112, USA) at a sampling rate of 30 Hz. Rat movements were tracked by selecting one pixel every five frames that corresponded to the midpoint between the animal's forelimbs. The resulting x- and y-coordinates from rats were scaled to real world units and used to calculate moment-to-moment speeds.
Each food hoarding trip was segmented into searching and homeward segments. The searching segment was defined as all movements that displaced the rat from the refuge until locating the hazelnut. The homeward segment was defined as all movements occurring after locating the hazelnut until the rat returned to the refuge. Maximum speed and path circuity values were used to evaluate the kinematic and topographic characteristics of both segments. Maximum speed (m/s) reflected the highest linear speed obtained on a segment. Path circuity, a measure of a segment's directness, was calculated by dividing the shortest distance between start and end points by distance actually traveled for each searching and homeward segment. Relatively direct segments are associated with values of 1.0–0.9. Segments restricted to the periphery of the table are associated with values of 0.7–0.6. As the segment becomes progressively more circuitous, values decrease.
2.7. Histology
Subsequent to behavioral testing, animals were deeply anesthetized and perfused with phosphate buffered saline followed by 4% paraformaldehyde in 0.1 M phosphate buffered picric acid. Brains were removed and stored in the same solution for 2 days at 4 °C. Brains were cut into 40 μm sections using a vibrotome, and every third section was mounted on chromalum subbed slides. Slides were stained for acetylcholinesterase [24].
Coronal brain sections at the level of the dorsal hippocampus (approximately 3.3 mm posterior to bregma) from each rat were photographed using a digital camera (Penguin 600 CL, Pixera Corporation, USA) attached to a microscope (Olympus BH2-RFCA, Olympus America Inc., USA). Digital photographic files were converted to grey scale and opened with Scion Image for Windows (Scion Corporation, USA; freely available on the Internet at http://www.scioncorp.com). Optical density values were obtained from a rectangular area (100 × 300 pixels) in the hippocampus (including regions CA1, CA3, and DG) and cortex (including somatosensory and motor). Optical density values were expressed as a function of the gray scale value (white: 0.0; black: 255).
3. Results
3.1. Histology
Photographs of acetylcholinesterase stained brain sections (Fig. 1) from representative Sham (top panel) and MS (middle panel) rats demonstrate a reduction in AChE specific to the hippocampus in the MS lesioned rat. Average optical density values obtained from both groups supported these observations (bottom panel of Fig. 1). The ANOVA conducted on cortical and dorsal hippocampal optical density values revealed significant effects of group [F(1, 22) = 9.582, p = .005], brain area [F(1, 22) = 53.796, p = .000], and Group × Brain Area interaction [F(1, 22) = 13.083, p = .002]. Simple effect analyses revealed that cortical levels of AChE were similar between groups but that the MS group had a significantly lower optical density value for the hippocampus. Infusion of 192 IgG-saporin into the medial septum produced a decrease in acetylcholinesterase restricted to the hippocampus. Although the current study did not examine ChAT-immunopositive or parvalbumin-positive neurons in the medial septum, previous work has demonstrated that this lesion technique produces significant decreases in the former and no effect on the latter [4,12,22,29,8].
Fig. 1.
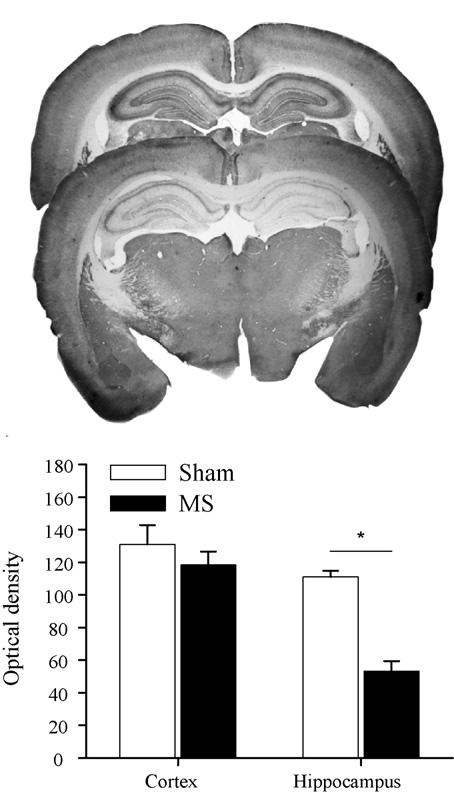
Photographs of coronal brain sections stained for acetylcholinesterase at the level of the dorsal hippocampus from representative sham (top panel) and MS (middle panel) rats. Mean hippocampal and cortical optical density values (+S.E.) are plotted for sham and MS rats (bottom panel). Note: Reductions in acetylcholinesterase staining associated with MS lesions are restricted to the hippocampus (p < .05).
3.2. Food hoarding behavior
All rats searched for the randomly located hazelnuts and carried them to the refuge for consumption. Although no group differences were observed in searching segment movement characteristics, accuracy of the homeward segment varied between groups as a function of testing condition.
3.2.1. Cued testing
During cued testing, both groups' searching segments covered large portions of the open field (left-hand panels of Fig. 2). Upon locating the hazelnut, both groups carried it directly to the refuge (right-hand panels of Fig. 2). Kinematic and topographic characteristics of searching segments did not differ between groups. The ANOVA conducted on searching segment maximum speed failed to find significant effects of group [F(1, 22) = 0.280, p = .602], trip [F(3, 66) = 0.948, p = .423], or Group × Trip interaction [F(3, 66) = 0.091, p = .965]. The ANOVA conducted on searching segment path circuity failed to find significant effects of group [F(1, 22) = 1.671, p = .210], trip [F(3, 66) = 0.683, p = .566], or Group × Trip interaction [F(3, 66) = 0.781, p = .509].
Fig. 2.
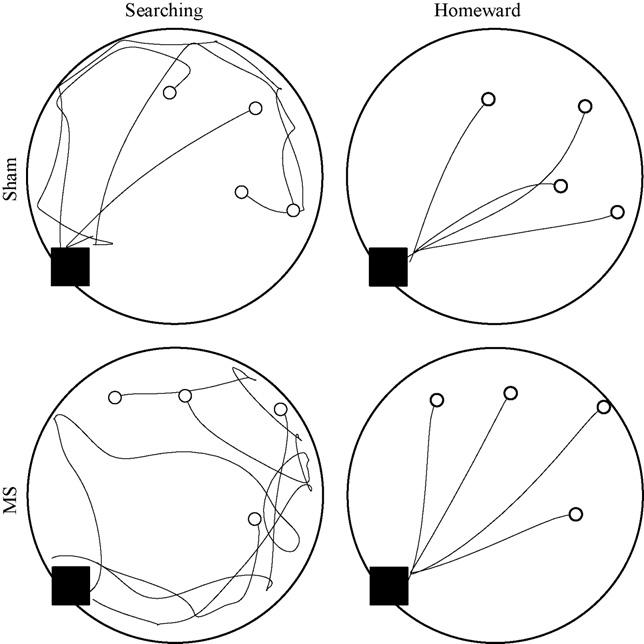
Four trips are plotted from representative sham (top panels) and MS (bottom panels) rats during cued testing. Searching and homeward segments are plotted separately in the left and right panels, respectively. The black square indicates the location of the cued refuge and the open circles indicate the locations of the four hazelnuts.
Homeward segment kinematic and topographic characteristics were also found to be consistent across groups. The ANOVA conducted on homeward segment maximum speed failed to find any effect of group [F(1, 22) = 0.038, p = .848], trip [F(3, 66) = 0.307, p = .820], or Group × Trip interaction [F(3, 66) = 2.073, p = .112]. The ANOVA conducted on homeward segment path circuity failed to reveal a significant effect of group [F(1, 22) = 0.002, p = .962], trip [F(3, 66) = 0.305, p = .822], or Group × Trip interaction [F(3, 66) = 2.606, p = .059]. These results are consistent with both groups having a spared ability in use of either environmental or self-movement cues to organize food hoarding behavior.
3.2.2. Uncued probe
During the uncued probe, both groups searched for the hazelnut (left-hand panels of Fig. 3) and, upon locating it, carried it directly to the hidden refuge (right-hand panels of Fig. 3). Kinematic and topographic characteristics of searching segments did not differ between groups. The ANOVA conducted on searching segment maximum speed failed to find significant effects of group [F(1, 22) = 1.169, p = .291], trip [F(3, 66) = 1.940, p = .132], or Group × Trip interaction [F(3, 66) = 0.865, p = .464]. The ANOVA conducted on searching segment path circuity failed to find significant effects of group [F(1, 22) = 0.036, p = .851], trip [F(3, 66) = 0.634, p = .596], or Group × Trip interaction [F(3, 66) = 0.023, p = .995].
Fig. 3.
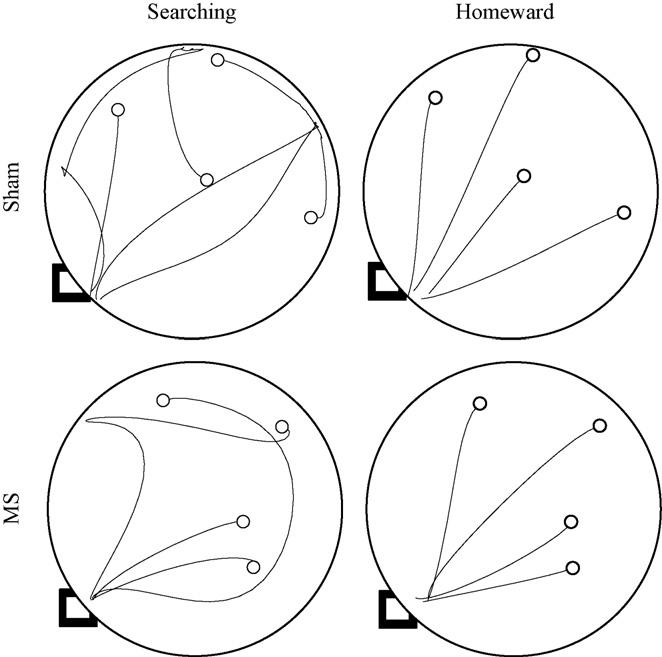
Four trips are plotted from representative sham (top panels) and MS (bottom panels) rats during the hidden probe. Searching and homeward segments are plotted separately in the left and right panels, respectively. The black square indicates the location of the hidden refuge and the open circles indicate the locations of the four hazelnuts.
Homeward segment kinematic and topographic characteristics were also found to be consistent across groups. The ANOVA conducted on homeward segment maximum speed failed to find significant effects of group [F(1, 22) = 2.422, p = .134], trip [F(3, 66) = 1.575, p = .204], or Group × Trip interaction [F(3, 66) = 0.488, p = .692]. The ANOVA conducted on homeward segment path circuity failed to reveal significant effects of group [F(1, 22) = 2.765, p = .111], trip [F(3, 66) = 0.458, p = .712], or Group × Trip interaction [F(3, 66) = 0.642, p = .591]. These results are consistent with both groups having a spared ability to use either environmental cues not associated with the home base or self-movement cues to organize food hoarding behavior.
3.2.3. Dark probe
During the dark probe, both groups traveled along circuitous paths while searching for the hazelnut (see left-hand panels of Fig. 4). Upon finding the hazelnut, MS rats returned to the hidden refuge along more circuitous paths, relative to sham rats (see right-hand panels of Fig. 4). Kinematic and topographic characteristics of searching segments did not differ between groups (see left-hand panels of Fig. 5). The ANOVA conducted on search segment maximum speed failed to reveal significant effects of group [F(1, 22) = 0.049, p = .827], trip [F(3, 66) = 1.147, p = .337], or Group × Trip interaction [F(3, 66) = 0.235, p = .872]. The ANOVA conducted on searching segment path circuity failed to reveal significant effects of group [F(1, 22) = 2.869, p = .104], trip [F(3, 66) = 2.612, p = .059], or Group × Trip interaction [F(3, 66) = 2.503, p = .067].
Fig. 4.
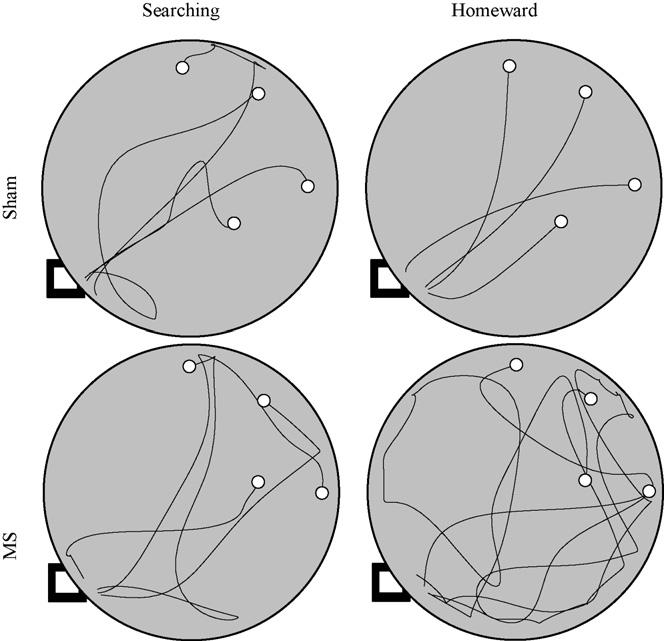
Four trips are plotted from representative sham (top panels) and MS (bottom panels) rats during the dark probe. Searching and homeward segments are plotted separately in the left and right panels, respectively. The black square indicates the location of the hidden refuge and the open circles indicate the locations of the four hazelnuts.
Fig. 5.
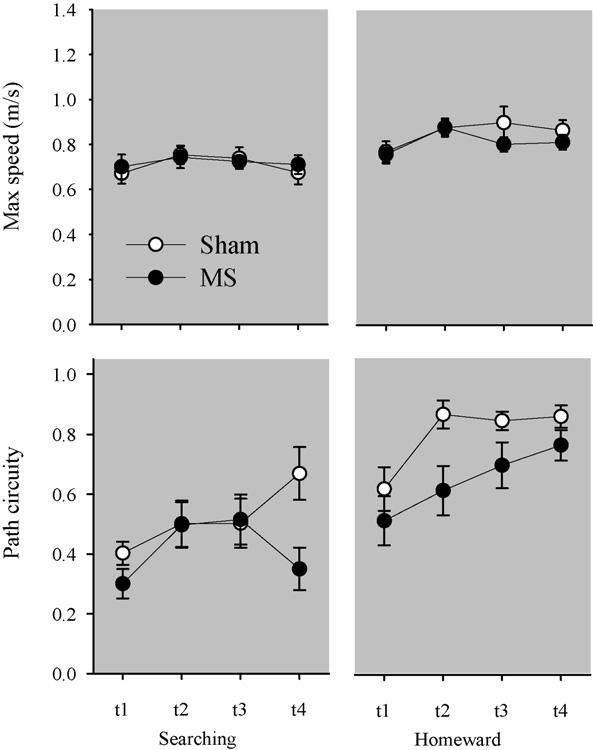
Top panels plot the mean maximum speed (±S.E.) associated with searching (left panel) and homeward (right panel) segments across the four trips during the dark probe. Bottom panels plot the mean path circuity (±S.E.) associated with searching and homeward segments across the four trips during the dark probe.
Although homeward segment kinematics did not vary between groups, differences in homeward segment topography were observed between groups (see right-hand panel of Fig. 5). The ANOVA conducted on homeward segment maximum speed revealed a significant effect of trip [F(3, 66) = 3.105, p = .032]; post hocs revealed that both groups exhibited slower maximum speeds on the first homeward segment than on subsequent trips (Tukey LSD; p < .05). The main effect of group [F(1, 22) = 1.062, p = .314] and the Group × Trip interaction [F(3, 66) = 0.628, p = .600] were not found to be significant. The ANOVA conducted on homeward segment path circuity revealed significant main effects of group [F(1, 22) = 8.507, p = .008] and trip [F(3, 66) = 5.999, p = .001]. The Group × Trip interaction [F(3, 66) = 0.649, p = .587] was not found to be significant. A post hoc comparison of group means revealed that the MS group's homeward segments were significantly more circuitous than the homeward segments observed in the sham group (Tukey LSD; p < .05). Additional post hoc analyses revealed that both groups' first trips were significantly more circuitous than subsequent trips (Tukey LSD; p < .05). These results are consistent with MS lesions impairing rats' use of self-movements to organize food hoarding behavior.
3.2.4. Reversal probe
During the reversal probe, rats were released from a refuge that was 180° different from that previously experienced. On the first trip, all rats left the novel refuge, searched for the hazelnut, and carried it to the former location of the refuge (see left-hand panel of Fig. 6). Upon observing the absence of the refuge, sham rats returned to the novel location of the refuge. In contrast, MS rats perseverated at the former location of the refuge or began a random search. On the second trip, sham rats carried the hazelnut directly to the new refuge location, whereas MS rats would frequently display visits to the former refuge location prior to returning to the new refuge location (see right-hand panels of Fig. 6). Kinematic and topographic characteristics of searching segments did not differ between groups (see left-hand panels of Fig. 7). The ANOVA conducted on searching segment maximum speeds revealed a significant effect of trip [F(3, 66) = 3.274, p = .026], and post hoc analyses revealed that the slowest maximum speeds were observed on the first trip (Tukey LSD; p < .05). The main effect of group [F(1, 22) = 1.096, p = .306] and Group × Trip interaction [F(3, 66) = 1.842, p = .148] were not significant. The ANOVA conducted on searching segment path circuity failed to reveal significant effects of group [F(1, 22) = 2.245, p = .148], trip [F(3, 66) = 0.892, p = .450], or Group × Trip interaction [F(3, 66) = 2.342, p = .081].
Fig. 6.
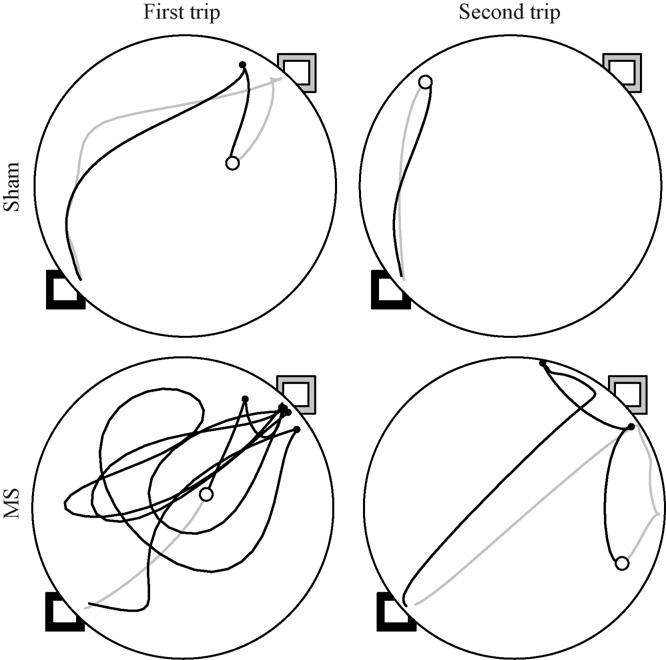
First and second trips are plotted from representative sham (top panels) and MS (bottom panels) rats during the reversal probe. Searching and homeward segments are represented by gray and black lines, respectively. The black square indicates the new location of the refuge; whereas the gray square indicates the former location of the refuge. The open circle represents the location of hazelnut. Solid circles represent stops after finding the hazelnut.
Fig. 7.
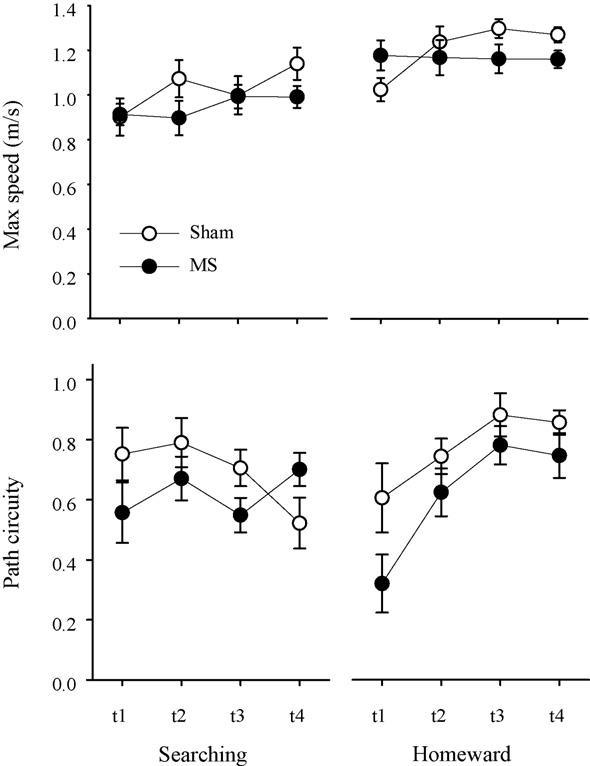
Mean maximum speed (±S.E.) associated with searching (top left panel) and homeward (top right panel) segments are plotted for each group during the four trips of the reversal probe. Mean path circuity (±S.E.) associated with searching (bottom left panel) and homeward (bottom right panel) segments are plotted for each group during the four trips of the reversal probe.
Under reversal conditions, both kinematic and topographic characteristics of homeward segments differed between groups (see right-hand panel of Fig. 7). The ANOVA conducted on homeward segment maximum speed revealed a significant Group × Trip interaction [F(3, 66) = 3.310, p = .025], while the effects of group [F(1, 22) = 0.540, p = .470] and trip [F(3, 66) = 2.617, p = .058] were not significant. Post hoc analyses revealed that the MS rats exhibited faster homeward maximum speeds on the first trip; however, sham rats' maximum speeds were the fastest on the third and fourth trips (Tukey LSD; p < .05). The ANOVA conducted on homeward segment path circuity revealed significant effects of group [F(1, 22) = 5.215, p = .032] and trip [F(3, 66) = 10.520, p = .000], whereas the Group × Trip interaction [F(3, 66) = 0.719, p = .544] was not significant. Post hoc analyses revealed that the MS rats had more circuitous homeward segments relative to sham rats; however, both groups' homeward segments became more direct across trips. These results are consistent with MS lesions sparing the rats' use of environmental cues while impairing the use of self-movement cues involved in organizing food hoarding behavior.
4. Discussion
The current study examined the effects of selective cholinergic medial septum lesions on rats' use of environmental and self-movement cues during a food hoarding task. MS and sham groups were initially trained to leave a cued refuge to search for randomly located hazelnuts. Upon finding a hazelnut, both groups carried it directly to the cued refuge. Access to environmental cues was manipulated during a series of probes. Both groups exhibited similar searching segment characteristics during cued training and across all of the probes; however, group differences were observed on homeward segment characteristics during the dark and reversal probes. These results demonstrate that selective cholinergic medial septum lesions differentially influenced the use of environmental and self-movement cues.
4.1. Medial septum lesions spare environmental cue use
Multiple lines of evidence within the present study suggest that selective medial septum lesions spared rats' use of environmental cues. First, during cued training, rats had access to environmental cues associated with the refuge and testing room as well as access to self-movement cues. The absence of group differences during this cued training suggests that the MS group was able to use at least one of these cues to accurately return to the refuge location. Second, although environmental cues associated with the refuge were absent under the hidden probe, both environmental cues associated with the testing room and self-movement cues were still available to the rats. Again, the absence of group differences suggests that the MS group was able to use either source of information to guide navigation even with a hidden refuge. Third, the reversal probe placed the environmental cues experienced during cued training and the hidden probe in conflict with self-movement cues. Upon locating the hazelnut during the first trial of the reversal probe, both groups immediately returned to the former location of the refuge. This demonstrates that both groups were using environmental cues to guide navigation. Group differences observed during the remainder of the first trip will be discussed in the next section. Finally, both groups displayed a significant increase in homeward segment accuracy across trips during the reversal probe. This observation is consistent with a majority of studies reporting that selective medial septum lesions spare matching-to-place performance in the water maze [5,23,16], working memory performance in the radial arm maze [55], and reward alternation in the T-maze [26]. Therefore, the improved performance during the reversal probe may be related to rats' access to environmental cues, in which both groups encoded the new location of the refuge relative to these cues. All of these results demonstrate that rats with selective medial septum lesions used environmental cues to guide navigation during cued training, hidden probe, and reversal probe.
4.2. Medial septum lesions impair self-movement cue processing
Two aspects of performance demonstrated that selective medial septum lesions impaired processing of self-movement cues. First, the dark probe restricted rats to using self-movement cues to guide navigation. Observing that selective medial septum lesions significantly increased homeward segment path circuity during the dark probe is consistent with impaired processing of self-movement cues. In addition, self-movement cues have been posited to contribute to aspects of performance during the reversal probe. Specifically, subsequent to visiting the former refuge location, rats may use self-movement cues to derive the new location of the refuge. Although rats in the sham group carried the hazelnut to the new location of the refuge immediately after visiting the former refuge location, rats in the medial septum group continued to perseverate at the former location of the refuge typically ending in a random search for the new location of the refuge. These results demonstrate that selective medial septum lesions impaired use of self-movement cues.
Interestingly, both groups displayed improvements in homeward segment accuracy across trips during the dark probe. One possible explanation for the improved performance involves a systematic modification of searching path organization across trips. It is easier to derive the direction and distance of the homeward segment if the searching segment is less circuitous. Observing that path circuity did not vary across trips conflicted with this account of improved performance. A second possible explanation for the improved performance reflects rats' use of olfactory cues to guide movements on the homeward segment. Rats may be tracking olfactory cues deposited on the surface of the table or associated with the refuge [57]. Observing that rats do not follow the paths outlined by previous searching segments conflicts with an odor tracking account of improved performance. In addition, navigation via odor tracking is relatively slower than what is observed during dead reckoning based navigation [61]; therefore, the increase in maximum speeds observed across trips in the current study conflicts with an odor tracking account of improved performance. Another possible explanation suggests a more selective role for the medial septum in dead reckoning based navigation. Previous work has demonstrated that rats organize their exploratory trips into tour and homeward segments [64]. The homeward segment is a non-circuitous path, directed to the refuge, and is associated with consistent temporal pacing of linear speeds. Observing these characteristics independent of environmental cue availability or familiarity is consistent with the rats' ability to use self-movement cues generated on the tour segment to estimate the direction and distance to the refuge [60,59]. Cell specific lesions of the hippocampus have been shown to disrupt both components of the homeward segment [61]. Electrolytic medial septum lesions have been shown to disrupt temporal pacing of homeward segment linear speeds, consistent with an impaired ability to derive distance estimates from self-movement cues [33]. In addition, the medial septum lesions significantly increased homeward segment path circuity relative to sham rats; however, both groups had fairly direct homeward path segments (i.e., path circuity values above 0.9). These observations supported at least a moderate sparing of the ability to derive estimates of direction from self-movement cues. Therefore, improved performance observed in the current study may be related to a selective sparing of self-movement cue processing related to estimating direction.
4.3. Role of septohippocampal cholinergic system in spatial orientation
Controversy has surrounded the role of the septohippocampal cholinergic system in spatial orientation. Initial studies using electrolytic lesion techniques reported impaired performance in a variety of spatial tasks [49,20,19,25,52]. This lesion technique damages non-cholinergic cells in the medial septum as well as fibers of passage, thereby limiting the inferences that can be drawn from the observed impairments in performance. The development of 192 IgG-Saporin eliminated both of these problems [69]. Initial studies using this lesion technique resulted in impaired performance in the water maze [27] and radial arm maze [70]; however, these studies used intracerebroventricular injections of 192 IgG-Saporin, a procedure associated with Purkinje cell loss in the cerebellum [56]. Subsequent studies injecting 192 IgG-Saporin directly into the medial septum have typically failed to result in impaired performance in the water maze [5,23,16] or radial arm maze [55]. The decreased effectiveness of more selective lesion techniques to disrupt performance on spatial tasks has challenged the importance of the septohippocampal cholinergic system in spatial orientation.
In general, the assessment of spatial behavioral use in these studies was based on the cognitive map theory of hippocampal function [44]. The cognitive map theory posits that the hippocampus mediates encoding the relationships between environmental cues. Both the observation of place cells in the hippocampus [43,39,40] and impaired spatial learning subsequent to hippocampal lesions [37,38] have supported the cognitive map theory of hippocampal function. However, a series of studies employing detailed behavioral analyses has challenged the cognitive map view of hippocampal function. For example, animals with hippocampal lesions have been shown to use environmental cues to guide performance [67,63,31,17]. In addition, hippocampal lesions have been shown to impair self-movement cue processing [31,61,21]. The results of the current study add to a growing literature supporting a role for the septohippocampal system in dead reckoning based navigation.
Loss of cholinergic function in the limbic system has been shown to correlate with the magnitude of cognitive deficits observed in Alzheimer's Disease [46,3, however see 11]. During the progression of Dementia of the Alzheimer's type (DAT), patients frequently engage in wandering behavior or become lost in a familiar setting, such as one's home [30]. Studies investigating the cognitive deficits related to wandering behavior observed in DAT patients have reported specific impairments in processing optic flow information [54]. Optic flow is generated as visual information passes across the retina. Radial optic flow reflects a specific pattern of movement across the retina generated as an organism moves through an environment. This type of self-movement cue can be used to estimate distance traveled. Although patients suffering from DAT have exhibited similar thresholds for detecting horizontal optic flow as age matched controls, they had significantly elevated thresholds for detecting radial optic flow [54]. Therefore, topographic disorientation associated with DAT may depend in part on impaired processing of self-movement cues, such as optic flow. These results parallel the deficits in self-movement cue processing associated with selective hippocampal cholinergic deafferentation observed in the present study.
5. Conclusions
The current study examined the role of the septohippocampal cholinergic system in spatial orientation. Rats with selective medial septum lesions used environmental cues to guide navigation. When restricted to using self-movement cues, specific impairments in performance were observed. These results demonstrate that rats use multiple sources of information to maintain spatial orientation, and they illustrate the importance of restricting cue availability when investigating the neural basis of spatial orientation.
Acknowledgements
We would like to thank Patricia S. Wallace for help with manuscript preparation and students Lynniece Carter, Shane Knapp, and Kelly Kusman for analyses related to this project. This work was supported by the National Institute of Neurological Disorders and Stroke Grant NS051218 to D. Wallace.
Footnotes
Publisher's Disclaimer: This article was published in an Elsevier journal. The attached copy is furnished to the author for non-commercial research and education use, including for instruction at the author's institution, sharing with colleagues and providing to institution administration.
References
- 1.Bannerman DM, Matthews P, Deacon RM, Rawlins JN. Medial septal lesions mimic effects of both selective dorsal and ventral hippocampal lesions. Behav Neurosci. 2004;118(5):1033–41. doi: 10.1037/0735-7044.118.5.1033. [DOI] [PubMed] [Google Scholar]
- 2.Barlow JS. Inertial navigation as a basis for animal navigation. J Theor Biol. 1964;6:76–117. doi: 10.1016/0022-5193(64)90067-0. [DOI] [PubMed] [Google Scholar]
- 3.Bartus RT, Dean RL, III, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217(4558):408–14. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- 4.Baxter MG, Bucci DJ, Gorman LK, Wiley RG, Gallagher M. Selective immunotoxic lesions of basal forebrain cholingeric cells: effects on learning and memory in rats. Behav Neurosci. 1995;109(4):714–22. doi: 10.1037//0735-7044.109.4.714. [DOI] [PubMed] [Google Scholar]
- 5.Baxter MG, Gallagher M. Intact spatial learning in both young and aged rats following selective removal of hippocampal cholinergic input. Behav Neurosci. 1996;110(3):460–7. doi: 10.1037//0735-7044.110.3.460. [DOI] [PubMed] [Google Scholar]
- 6.Berger-Sweeney J, Heckers S, Mesulam MM, Wiley RG, Lappi DA, Sharma M. Differential effects on spatial navigation of immunotoxin-induced cholinergic lesions of the medial septal area and nucleus basalis magnocellularis. J Neurosci. 1994;14(7):4507–19. doi: 10.1523/JNEUROSCI.14-07-04507.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cahill JF, Baxter MG. Cholinergic and noncholinergic septal neurons modulate strategy selection in spatial learning. Eur J Neurosci. 2001;14(11):1856–64. doi: 10.1046/j.0953-816x.2001.01807.x. [DOI] [PubMed] [Google Scholar]
- 8.Chang Q, Gold PE. Impaired and spared cholinergic functions in the hippocampus after lesions of the medial septum/vertical limb of the diagonal band with 192 IgG-saporin. Hippocampus. 2004;14:170–9. doi: 10.1002/hipo.10160. [DOI] [PubMed] [Google Scholar]
- 9.Cheng K. A purely geometric module in the rat's spatial representation. Cognition. 1986;23:149–78. doi: 10.1016/0010-0277(86)90041-7. [DOI] [PubMed] [Google Scholar]
- 10.Darwin C. Origin of certain insects. Nature. 1873;7:417–8. [Google Scholar]
- 11.Davis KL, Mohs RC, Marin DB, Purohit DP, Perl DP, Lantz M, et al. Neuropeptide abnormalities in patients with early Alzheimer disease. Arch Gen Psychiatry. 1999;56(11):981–7. doi: 10.1001/archpsyc.56.11.981. [DOI] [PubMed] [Google Scholar]
- 12.Dornan WA, McCampbell AR, Tinkler GP, Hickman LJ, Bannon AW, Decker MW, et al. Comparison of site-specific injections into the basal forebrain on water maze and radial arm maze performance in the male rat after immunolesioning with 192 IgG saporin. Behav Brain Res. 1996;82(1):93–101. doi: 10.1016/s0166-4328(97)81112-6. [DOI] [PubMed] [Google Scholar]
- 13.Estep DQ, Lanier DL, Dewsbury DA. Variation of food hoarding with the estrous cycle of Syrian Golden hamsters (Mesocricetus auratus) Horm Behav. 1978;11:259–63. doi: 10.1016/0018-506x(78)90029-6. [DOI] [PubMed] [Google Scholar]
- 14.Etienne AS, Maurer R, Saucy F, Teroni E. Short-distance homing in the golden hamster after a passive outward journey. Anim Behav. 1986;34:696–715. [Google Scholar]
- 15.Everitt BJ, Robbins TW. Central cholinergic systems and cognition. Annu Rev Psychol. 1997;48:649–84. doi: 10.1146/annurev.psych.48.1.649. [DOI] [PubMed] [Google Scholar]
- 16.Frielingsdorf H, Thal LJ, Pizzo DP. The septohippocampal cholinergic system and spatial working memory in the Morris water maze. Behav Brain Res. 2006;168:37–46. doi: 10.1016/j.bbr.2005.10.008. [DOI] [PubMed] [Google Scholar]
- 17.Gaffan EA, Bannerman DM, Healey AN. Rats with hippocampal lesions learn about allocentric place cues in a non-navigational task. Behav Neurosci. 2000;114(5):895–906. doi: 10.1037//0735-7044.114.5.895. [DOI] [PubMed] [Google Scholar]
- 18.Gallistel CR. The organization of learning. MIT Press; Cambridge, MA: 1990. [Google Scholar]
- 19.Hagan JJ, Salamone JD, Simpson J, Iversen SD, Morris RGM. Place navigation in rats is impaired by lesions of medial septum and diagonal band but not nucleus basalis magnocellularis. Behav Brain Res. 1988;27:9–20. doi: 10.1016/0166-4328(88)90105-2. [DOI] [PubMed] [Google Scholar]
- 20.Hepler DJ, Olton DS, Wenk GL, Coyle JT. Lesions in nucleus basalis magnocellularis and medial septal area of rats produce qualitatively similar memory impairments. J Neurosci. 1985;5(4):866–73. doi: 10.1523/JNEUROSCI.05-04-00866.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito R, Robbins TW, McNaughton BL, Everitt BJ. Selective excitotoxic lesions of the hippocampus and basolateral amygdala have dissociable effects on appetitive cue and place conditioning based on path integration in a novel Y-maze procedure. Eur J Neurosci. 2006;23(11):3071–80. doi: 10.1111/j.1460-9568.2006.04883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Janis LS, Glasier MM, Fulop Z, Stein DG. Intraseptal injections of 192 IgG saporin produce deficits for strategy selection in spatial-memory tasks. Behav Brain Res. 1998;90(1):23–34. doi: 10.1016/s0166-4328(97)00078-8. [DOI] [PubMed] [Google Scholar]
- 23.Jonasson Z, Cahill JF, Tobey RE, Baxter MG. Sexually dimorphic effects of hippocampal cholinergic deafferentation in rats. Eur J Neurosci. 2004;20(11):3041–53. doi: 10.1111/j.1460-9568.2004.03739.x. [DOI] [PubMed] [Google Scholar]
- 24.Karnovsky MJ, Roots A. A “direct-coloring” thiocholine method for cholinesterases. J Histochem Cytochem. 1964;12:219–21. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- 25.Kelsey JE, Landry BA. Medial septal lesions disrupt spatial mapping ability in rats. Behav Neurosci. 1988;102(2):289–93. doi: 10.1037//0735-7044.102.2.289. [DOI] [PubMed] [Google Scholar]
- 26.Kirby BP, Rawlins JN. The role of the septo-hippocampal cholinergic projection in T-maze rewarded alternation. Behav Brain Res. 2003;143(1):41–8. doi: 10.1016/s0166-4328(03)00005-6. [DOI] [PubMed] [Google Scholar]
- 27.Leanza G, Nilsson OG, Wiley RG, Bjorklund A. Selective lesioning of the basal forebrain cholinergic system by intraventricular 192 IgG-saporin: behavioural, biochemical and stereological studies in the rat. Eur J Neurosci. 1995;7(2):329–43. doi: 10.1111/j.1460-9568.1995.tb01068.x. [DOI] [PubMed] [Google Scholar]
- 28.Lehmann O, Bertrand F, Jeltsch H, Morer M, Lazarus C, Will B, et al. 5,7-DHT-induced hippocampal 5-HT depletion attenuates behavioural deficits produced by 192 IgG-saporin lesions of septal cholinergic neurons in the rat. Eur J Neurosci. 2002;15:1191–2006. doi: 10.1046/j.1460-9568.2002.02037.x. [DOI] [PubMed] [Google Scholar]
- 29.Lehmann O, Grottick AJ, Cassel JC, Higgins GA. A double dissociation between serial reaction time and radial maze performance in rats subjected to 192 IgG-saporin lesions of the nucleus basalis and/or the septal region. Eur J Neurosci. 2003;18(3):651–66. doi: 10.1046/j.1460-9568.2003.02745.x. [DOI] [PubMed] [Google Scholar]
- 30.Logsdon RG, Teri L, McCurry SM, Gibbons LE, Kukull WA, Larson EB. Wandering: a significant problem among community-residing individuals with Alzheimer' disease. J Gerontol B Psychol Sci Soc Sci. 1998;53(5):P294–9. doi: 10.1093/geronb/53b.5.p294. [DOI] [PubMed] [Google Scholar]
- 31.Maaswinkel H, Jarrard LE, Whishaw IQ. Hippocampectomized rats are impaired in homing by path integration. Hippocampus. 1999;9(5):553–61. doi: 10.1002/(SICI)1098-1063(1999)9:5<553::AID-HIPO9>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 32.Maaswinkel H, Whishaw IQ. Homing with locale, taxon, and dead reckoning strategies by foraging rats: sensory hierarchy in spatial navigation. Behav Brain Res. 1999;99(2):143–52. doi: 10.1016/s0166-4328(98)00100-4. [DOI] [PubMed] [Google Scholar]
- 33.Martin MM, Horn KL, Kusman KJ, Wallace DG. Medial septum lesions disrupt exploratory trip organization: evidence for septohippocampal involvement in dead reckoning. Physiol Behav. 2007;90(2–3):412–24. doi: 10.1016/j.physbeh.2006.10.007. [DOI] [PubMed] [Google Scholar]
- 34.McMahan RW, Sobel TJ, Baxter MG. Selective immunolesions of hippocampal cholinergic input fail to impair spatial working memory. Hippocampus. 1997;7:130–6. doi: 10.1002/(SICI)1098-1063(1997)7:2<130::AID-HIPO2>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- 35.M'Harzi M, Jarrard LE. Effects of medial and lateral septal lesions on acquisition of a place and cue radial maze task. Behav Brain Res. 1992;49(2):159–65. doi: 10.1016/s0166-4328(05)80160-3. [DOI] [PubMed] [Google Scholar]
- 36.Mittelstaedt ML, Mittelstaedt H. Homing by path integration in a mammal. Naturwissenschaften. 1980;67:566–7. [Google Scholar]
- 37.Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982 Jun 24;297(5868):681–3. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 38.Morris RG, Schenk F, Tweedie F, Jarrard LE. Ibotenate lesions of hippocampus and/or subiculum: dissociating components of allocentric spatial learning. Eur J Neurosci. 1990;2(12):1016–28. doi: 10.1111/j.1460-9568.1990.tb00014.x. [DOI] [PubMed] [Google Scholar]
- 39.Muller RU, Kubie JL. The effects of changes in the environment on the spatial firing of hippocampal complex-spike cells. J Neurosci. 1987;7(7):1951–68. doi: 10.1523/JNEUROSCI.07-07-01951.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muller RU, Kubie JL, Ranck JB., Jr Spatial firing patterns of hippocampal complex-spike cells in a fixed environment. J Neurosci. 1987;7(7):1935–50. doi: 10.1523/JNEUROSCI.07-07-01935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Murphy JJ. Instinct: a mechanical analogy. Nature. 1873;7:483. [Google Scholar]
- 42.Neitz J, Jacobs GH. Reexamination of spectral mechanisms in the rat (Rattus norvegicus) J Comp Psych. 1986;100(1):21–9. [PubMed] [Google Scholar]
- 43.O'Keefe J, Dostrovsky J. The hippocampus as a spatial map. Preliminary evidence from unit activity in the freely-moving rat. Brain Res. 1971;34(1):171–5. doi: 10.1016/0006-8993(71)90358-1. [DOI] [PubMed] [Google Scholar]
- 44.O'Keefe J, Nadel L. The hippocampus as a cognitive map. Oxford; Clarendon: 1978. [Google Scholar]
- 45.Parent MB, Baxter MG. Septohippocampal acetylcholine: involved in but not necessary for learning and memory? Learn Mem. 2004;11:9–20. doi: 10.1101/lm.69104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Perry EK, Perry RH, Blessed G, Tomlinson BE. Changes in brain cholinesterases in senile dementia of Alzheimer type. Neuropathol Appl Neurobiol. 1977;4(4):273–7. doi: 10.1111/j.1365-2990.1978.tb00545.x. 1978 Jul-Aug. [DOI] [PubMed] [Google Scholar]
- 47.Pizzo DP, Thal LJ, Winkler J. Mnemonic deficits in animals depend upon the degree of cholinergic deficit and task complexity. Exp Neurol. 2002;177:292–305. doi: 10.1006/exnr.2002.7993. [DOI] [PubMed] [Google Scholar]
- 48.Potegal M. Vestibular and neostriatal contributions to spatial orientation. In: Potegal M, editor. Spatial abilities: developmental and physiological foundations. Academic Press; New York: 1982. pp. 361–87. [Google Scholar]
- 49.Rawlins JN, Olton DS. The septo-hippocampal system and cognitive mapping. Behav Brain Res. 1982;5(4):331–58. doi: 10.1016/0166-4328(82)90039-0. [DOI] [PubMed] [Google Scholar]
- 50.Seguinot V, Maurer R, Etienne AS. Dead reckoning in a small mammal: the evaluation of distance. J Comp Physiol [A] 1993;173:103–13. doi: 10.1007/BF00209622. [DOI] [PubMed] [Google Scholar]
- 51.Shettleworth SJ. Cognition, evolution and behavior. Oxford University Press; New York, NY: 1998. [Google Scholar]
- 52.Sutherland RJ, Rodriguez AJ. The role of the fornix/fimbria and some related subcortical structures in place learning and memory. Behav Brain Res. 1989;32(3):265–77. doi: 10.1016/s0166-4328(89)80059-2. [DOI] [PubMed] [Google Scholar]
- 53.Suzuki S, Augerinos G, Black AH. Stimulus control of spatial behavior on the eight-arm maze in rats. Learn Mot. 1980;11:1–18. [Google Scholar]
- 54.Tetewsky SJ, Duffy CJ. Visual loss and getting lost in Alzheimer's disease. Neurology. 1999;52(5):958–65. doi: 10.1212/wnl.52.5.958. [DOI] [PubMed] [Google Scholar]
- 55.Vuckovich JA, Semel ME, Baxter MG. Extensive lesions of cholinergic basal forebrain neurons do not impair spatial working memory. Learn Mem. 2004;11(1):87–94. doi: 10.1101/lm.63504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Waite JJ, Chen AD, Wardlow ML, Wiley RG, Lappi DA, Thal LJ. 192 immunoglobulin G-saporin produces graded behavioral and biochemical changes accompanying the loss of cholinergic neurons of the basal forebrain and cerebellar Purkinje cells. Neuroscience. 1995;65(2):463–76. doi: 10.1016/0306-4522(94)00479-o. [DOI] [PubMed] [Google Scholar]
- 57.Wallace DG, Gorny B, Whishaw IQ. Rats can track odors, other rats, and themselves: implications for the study of spatial behavior. Behav Brain Res. 2002;131(1–2):185–92. doi: 10.1016/s0166-4328(01)00384-9. [DOI] [PubMed] [Google Scholar]
- 58.Wallace DG, Hines DJ, Pellis SM, Whishaw IQ. Vestibular information is required for dead reckoning in the rat. J Neurosci. 2002;22(22):10009–17. doi: 10.1523/JNEUROSCI.22-22-10009.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wallace DG, Hamilton DA, Whishaw IQ. Movement characteristics support a role for dead reckoning in organizing exploratory behavior. Anim Cogn. 2006;9(3):219–28. doi: 10.1007/s10071-006-0023-x. [DOI] [PubMed] [Google Scholar]
- 60.Wallace DG, Hines DJ, Whishaw IQ. Quantification of a single exploratory trip reveals hippocampal formation mediated dead reckoning. J Neurosci Meth. 2002;113(2):131–45. doi: 10.1016/s0165-0270(01)00489-7. [DOI] [PubMed] [Google Scholar]
- 61.Wallace DG, Whishaw IQ. NMDA lesions of Ammon's horn and the dentate gyrus disrupt the direct and temporally paced homing displayed by rats exploring a novel environment: evidence for a role of the hippocampus in dead reckoning. Eur J Neurosci. 2003;18(3):513–23. doi: 10.1046/j.1460-9568.2003.02772.x. [DOI] [PubMed] [Google Scholar]
- 62.Whishaw IQ. Time estimates contribute to food handling decisions by rats: implications for neural control of hoarding. Psychobiology. 1990;18(4):460–6. [Google Scholar]
- 63.Whishaw IQ. Place learning in hippocampal rats and the path integration hypothesis. Neurosci Biobehav Rev. 1998;22(2):209–20. doi: 10.1016/s0149-7634(97)00002-x. [DOI] [PubMed] [Google Scholar]
- 64.Whishaw IQ, Hines DJ, Wallace DG. Dead reckoning (path integration) requires the hippocampal formation: evidence from spontaneous exploration and spatial learning tasks in light (allothetic) and dark (idiothetic) tests. Behav Brain Res. 2001;127(1–2):49–69. doi: 10.1016/s0166-4328(01)00359-x. [DOI] [PubMed] [Google Scholar]
- 65.Whishaw IQ, Maaswinkel H. Rats with fimbria-fornix lesions are impaired in path integration: a role for the hippocampus in “sense of direction”. J Neurosci. 1998;18(8):3050–8. doi: 10.1523/JNEUROSCI.18-08-03050.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Whishaw IQ, Maaswinkel H, Gonzalez CL, Kolb B. Deficits in allothetic and idiothetic spatial behavior in rats with posterior cingulate cortex lesions. Behav Brain Res. 2001;118(1):67–76. doi: 10.1016/s0166-4328(00)00312-0. [DOI] [PubMed] [Google Scholar]
- 67.Whishaw IQ, Tomie JA. Perseveration on place reversals in spatial swimming pool tasks: further evidence for place learning in hippocampal rats. Hippocampus. 1997;7(4):361–70. doi: 10.1002/(SICI)1098-1063(1997)7:4<361::AID-HIPO2>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 68.Whishaw IQ, Tomie JA. Piloting and dead reckoning dissociated by fimbria-fornix lesions in a rat food carrying task. Behav Brain Res. 1997;89(1–2):87–97. doi: 10.1016/s0166-4328(97)00068-5. [DOI] [PubMed] [Google Scholar]
- 69.Wiley RG, Oeltmann TN, Lappi DA. Immunolesioning: selective destruction of neurons using immunotoxin to rat NGF receptor. Brain Res. 1991;562(1):149–53. doi: 10.1016/0006-8993(91)91199-b. [DOI] [PubMed] [Google Scholar]
- 70.Wrenn CC, Lappi DA, Wiley RG. Threshold relationship between lesion extent of the cholinergic basal forebrain in the rat and working memory impairment in the radial maze. Brain Res. 1999;847:284–98. doi: 10.1016/s0006-8993(99)02099-5. [DOI] [PubMed] [Google Scholar]


