Abstract
A recent study (Tannan et al., 2006) showed that pre-exposure of a skin region to a 5 sec 25 Hz flutter stimulus (“adaptation”) results in an approximately 2-fold improvement in the ability of neurologically healthy human adults to localize mechanical stimulation delivered to the same skin region that received the adapting stimulation. Tannan et al. (Tannan et al., 2006) proposed that tactile spatial discriminative performance is improved following adaptation because adaptation is accompanied by an increase in the spatial contrast in the response of contralateral primary somatosensory cortex (SI) to mechanical skin stimulation – an effect identified in previous imaging studies of SI cortex in anesthetized non-human primates (e.g., Simons et al., 2005; Tommerdahl et al., 2002; Whitsel et al., 1989).
In the experiments described in this report, a paradigm identical to that employed previously by Tannan et al. (2006) was used to study adults with autism. The results demonstrate that although cutaneous localization performance of adults with autism is significantly better than the performance of control subjects when the period of adapting stimulation is short (i.e., 0.5 sec), tactile spatial discriminative capacity remained unaltered in the same subjects when the duration of adapting stimulation was increased (to 5 sec). Both the failure of prior history of tactile stimulation to alter tactile spatial localization in adults with autism, and the better-than-normal tactile localization performance of adults with autism when the period of adaptation is short are concluded to be attributable to the deficient cerebral cortical GABAergic inhibitory neurotransmission characteristic of this disorder.
1. Introduction
In a recent paper in this Journal (Tannan et al., 2006), we reported that in healthy adult subjects spatial localization of a 25 Hz flutter stimulus on the skin of the hand improves substantially following adaptation. In that study we delivered an adapting stimulus (0.5 or 5 sec) to a randomly selected location on the skin, and in 2 subsequent temporal intervals either the standard or a test skin flutter stimulus (the 25 Hz standard and test stimuli were equal in amplitude) was delivered either at the locus of the adapting stimulus, or at another randomly located skin site. Subjects then were queried as to which interval contained the standard stimulus. The observations revealed that long-duration (5 sec) adaptation improved spatial acuity by nearly 2-fold relative to performance under the short-duration (0.5 sec) adaptation condition. This considerable improvement in performance was hypothesized to be due to adaptation-induced pericolumnar lateral inhibitory interactions which lead to a more focused (“spatially funneled”) primary somatosensory cortical response to mechanical skin stimulation (Simons et al., 2005; Tommerdahl et al., 2002; Whitsel et al., 1989; Juliano et al., 1989).
The above-described human psychophysical findings appear fully consistent with published theoretical accounts of the effects of repetitive skin stimulation on the SI cortical response to stimulation. More specifically, observations obtained in the pioneering neurophysiological recording studies led Mountcastle and colleagues to advance the proposal (Mountcastle and Darian-Smith, 1968; LaMotte and Mountcastle, 1975; LaMotte and Mountcastle, 1979) that a subject’s ability to localize a mechanical stimulus on the skin depends on stimulus-evoked dynamic (time-dependent) pericolumnar lateral inhibitory interactions which increase the spatial contrast between regions of SI cortex activated differentially by stimulus-evoked afferent drive. The idea that a subject’s ability to accurately perceive the spatial locus of a mechanical skin stimulus depends on the spatial contrast of the activity profile that a stimulus evokes in the responding region of contralateral SI cortex has received wide acceptance, but the continuing absence of direct experimental support for this concept motivated us to re-evaluate the phenomenon using optical intrinsic signal imaging methods (OIS imaging; Tommerdahl et al., 2002; Simons et al., 2005; Simons et al., 2006) to investigate the effect of different durations of repetitive mechanical stimulation of a skin site on the spatial profile of SI activation. Briefly summarized, we found that exposure of a skin site to a temporally extended (> 0.5 sec) 25 Hz vibrotactile stimulus is consistently accompanied by a dramatic but stereotypical sequence of changes in the global SI activity pattern – while activation in SI initially occurred in much of the extensive region that receives input from the stimulated skin site, with continuing stimulation not only did the activated region shrink in size, but the surviving region of activation came to be bounded by one or more regions where activity was reduced to significantly below-background values (Tommerdahl et al., 2002; Simons et al., 2005; Simons et al., 2006). In addition, evidence obtained in in vivo and in vitro imaging studies indicates unambiguously that GABA-mediated cortical inhibitory processes are responsible for the time-dependent spatial contraction of the SI activation pattern that accompanies the delivery of repetitive thalamocortical afferent drive (e.g., as can be achieved using temporally extended vibrotactile stimulation (Juliano et al., 1989), or repetitive electrical stimulation of thalamocortical afferents (Kohn et al., 2000)).
Experimental evidence provided by others led the authors to consider the possibility that individuals diagnosed with autism would not exhibit the adaptation-induced enhancement of tactile spatial localization characteristic of neurologically normal subjects. First, Casanova and colleagues have reported that neocortical minicolumnar size in autism is significantly reduced in ways that could reduce within-network communication (Casanova et al., 2006). Second, suppressed GABAergic inhibition has been suggested to be a common feature of the autistic brain (Hussman, 2001). For example, parietal cortex in subjects with autism exhibits a ~ 50% reduction in protein levels of the enzymes that synthesize GABA, glutamic acid decarboxylase (GAD) 65 and 67 (Fatemi et al., 2002). Both of these observations appear to be fully consistent with the frequently reported hypersensitivity (Cascio et al., 2007; Blakemore et al., 2006) and abnormally widespread (Belmonte et al., 2004) cerebral cortical response to skin stimulation in autism. If parietal cortical pericolumnar lateral inhibitory interactions are, in fact, deficient in subjects with autism, and if adaptation-induced spatial funneling of the primary somatosensory cortical response to repetitive skin stimulation is achieved via GABA-mediated postsynaptic inhibitory mechanisms intrinsic to primary somatosensory cortex, subjects with autism should not be expected to exhibit the adaptation-induced improvement of tactile spatial localization capacity that occurs reliably in neurologically normal subjects. The goal of the present study was to obtain evidence bearing on this possibility using the same human psychophysical procedures and instrumentation described by Tannan et al. (Tannan et al., 2006).
2. Results
A two-interval forced-choice (2IFC) tracking protocol (“2 up – 1 down”; 2 correct answers are followed by a decrease of the distance between the standard and test stimuli; the distance is increased following an incorrect answer) was used to determine spatial localization threshold under two different durations of adapting stimulation (0.5 sec vs. 5.0 sec) for subjects with autism. Five sessions (two runs in each session) were conducted for each of the four subjects with autism. Exemplary individual averaged plots for two control subjects (previously published in Tannan et al., 2006) are compared with data from the four individual subjects with autism in Figure 1. Note that in each of the data sets from the control subjects, there was a marked improvement in spatial localization tracking performance that occurred when the task was preceded by a 5 sec duration adapting stimulus. In contrast, the plots of the data obtained from the individuals with autism show that there is no discernable difference between the results generated by the two different adapting stimulus conditions.
Figure 1.
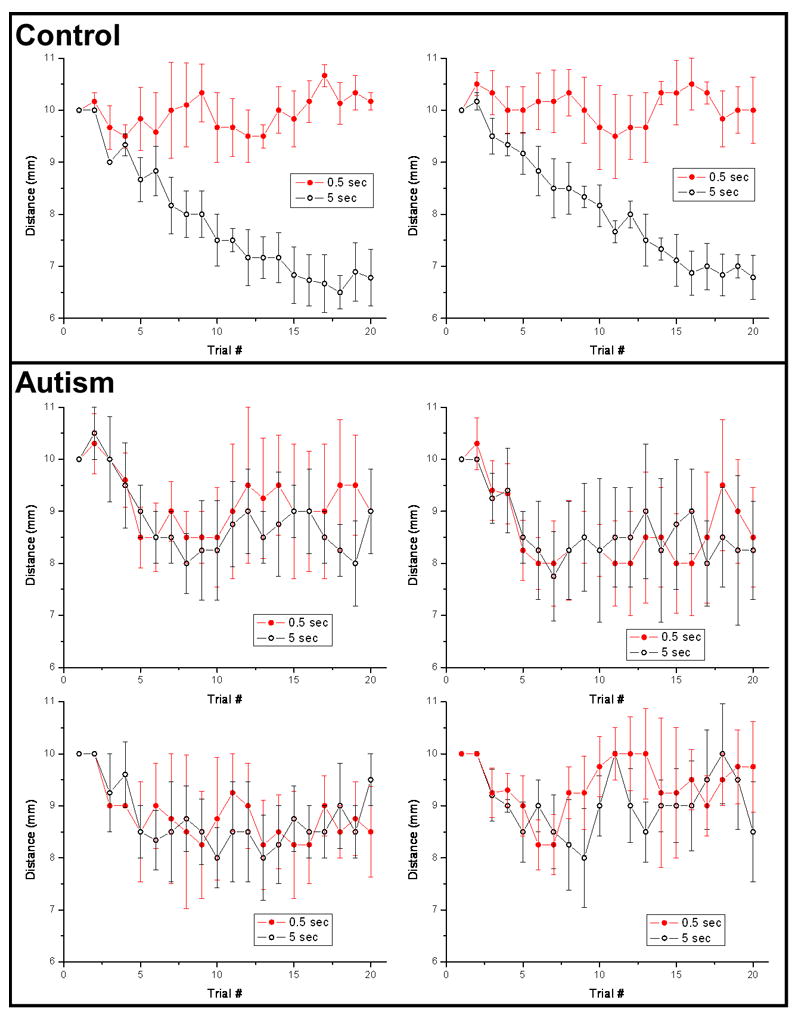
Spatial localization tracking plots, under 2 conditions of adapting stimulus duration, were averaged for individual subjects. Top panel: Tracking data for two subjects from the control group, obtained in a previously reported study (Tannan et al., 2006). Note that spatial localization performance is distinctly better in the 5.0 sec vs. 0.5 sec duration adapting stimulus condition. Bottom panel: Tracking plots for each of the 4 subjects with autism.
To determine across-subject consistency, the tracking data collected under each condition for the subjects with autism (20 sessions in total) were averaged. As seen in the middle panel of Figure 2, the localization distance of these subjects tracked to 8 – 9 mm for both the 0.5 sec and 5 sec durations of adapting stimulation. To summarize, in subjects with autism, the localization of tactile skin flutter stimulation did not change with a 5 sec adaptor compared to that with a 0.5 sec adaptor. In order to more directly compare the responses measured under each of the stimulus conditions, the tracking values obtained from the last five trials across all subjects with autism were averaged, shown in the right panel of Figure 2. These values were 8.983 ± 0.534 mm for the short-duration adaptor and 9.133 ± 0.511 mm for the long-duration adaptor (mean ± std err). Averaged spatial localization tracking performance obtained from control subjects in the previous study (Tannan et al., 2006) are shown in the left panel of Figure 2 for comparison, as are the localization distance values for the controls in the right panel of Figure 2: 10.283 ± 0.280 for the short-duration adaptor, and 6.983 ± 0.366 for the long-duration adaptor.
Figure 2.
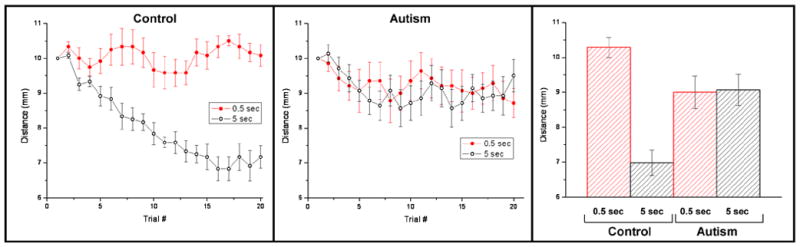
Spatial localization under 2 conditions of adapting stimulus duration for adults with and without autism. Data displayed from the control subjects (left panel; previously reported (Tannan et al., 2006)), contrasts markedly from the data displayed in the middle panel which was obtained from observations of subjects with autism. Note that subjects with autism, although they clearly outperformed the controls in the 0.5 sec adapting condition, did not improve with the 5.0 sec adapting condition. Panel at far right summarizes the findings with averages of the last 5 trials.
ANOVA testing was performed on the data averaged across all subjects with autism; the null hypothesis evaluated was that mean tactile localization performance is not significantly different when the duration of adapting stimulation is 0.5 or 5.0 sec. The results (F = 0.21416; p > 0.05) confirmed that tactile localization performance of subjects with autism does not alter significantly when the duration of adapting stimulation is increased from 0.5 to 5.0 sec. In addition, the analysis revealed that at either duration of adapting stimulation (for 5.0 sec adaptation F = 96.17924, p < 0.01; for 0.5 sec adaptation F = 15.23406, p < 0.01) mean tactile localization performance for control subjects is significantly different than that of the subjects with autism.
3. Discussion
The present study evaluated the effects of adaptation on spatial localization of a 25 Hz flutter stimulus on the dorsal surface of the attended hand in adults with autism. Tactile localization performance did not change (i.e., spatial acuity was the same) when the duration of adapting stimulation was changed from 5.0 to 0.5 sec. Thus, although in normal subjects longer duration (5.0 sec) adaptation results in a nearly 2-fold improvement in spatial acuity relative to that measured under the short-duration (0.5 sec) adaptation condition (Tannan et al., 2006), duration of exposure to adapting stimulation fails to modify spatial acuity in subjects with autism. Also notable was the finding that when the duration of adaptation was short (0.5 sec), subjects with autism actually outperformed subjects without autism. This superior performance not only demonstrates that the subjects with autism could clearly understand and perform the tracking task, but it points out a major difference between subjects with autism and the control population: i.e., the subjects with autism exhibit no improvement in tactile localization performance when the duration of adapting stimulation is increased.
The observed improvement in tactile spatial localization performance that in control subjects accompanies increasing stimulus duration is presumed to reflect the spatial separation of the responses of primary somatosensory cortex to dual-site skin stimulation. If this widely-held presumption is correct, the findings obtained in the present study raise the possibility that local corticocortical functional connectivity in subjects with autism may be substantially abnormal. While a number of findings have demonstrated that long-range functional connectivity in subjects with autism is very different from that present in the general population (for review, see Polleux and Lauder, 2004; Herbert, 2005), it is unlikely that the different observations obtained from subjects with autism and control subjects reported in this study are attributable to differences in long-range corticocortical connections. Instead, the improved tactile spatial localization that accompanies an increase in duration of adapting stimulation most likely would occur as a result of local dynamic corticocortical interactions that operate to improve the contrast of the primary somatosensory cortical response to a repetitive stimulus through lateral inhibition. Based on this perspective, the measures of tactile localization performance described in this paper appear to reflect the deficit in short-range parietal corticocortical connectivity identified in subjects with autism by Casanova and colleagues (Casanova et al., 2002, 2003; 2006). Such changes in connectivity could lead to the imbalance in excitation and inhibition that others have predicted underlies the neocortical hyperexcitability and unstable activity in cortical networks characteristic of autism (Polleux and Lauder, 2004; Rubenstein and Merzenich, 2003).
There is substantial evidence that in autism, cerebral cortical information processing not only is abnormal in the “high-order” neocortical areas that underlie language and social interaction skills, but also is deficient in the “lower-order” areas which receive and carry out the initial processing of the thalamocortical afferent activity evoked by sensory stimulation. Abnormalities of the stimulus-evoked response of primary sensory cortex in subjects with autism have been demonstrated using psychophysical testing procedures and neurophysiological recording methods. For example, some subjects with autism exhibit hyper-arousal to natural sensory input, and a decreased ability to select among competing sensory inputs (Belmonte and Yurgelun-Todd, 2003; Gomot et al., 2002; Kootz et al., 1981; Plaisted et al., 2003). Furthermore, regions of cortex devoted to stimulus-driven sensory processing display excessive responsivity in subjects with autism, and exhibit little-to-no specificity in response to either the location or modality of sensory stimulation (Belmonte et al., 2004; Baron-Cohen and Belmonte, 2005). Although many reports acknowledge a widespread neocortical dysfunction in autism, it is less widely recognized that the dysfunction is non-uniform – e.g., the excessive responsivity of primary sensory cortex to peripheral stimulation is accompanied (in the same subject) by abnormally low activation/responsivity in cortical regions devoted to higher-order information processing (Belmonte et al., 2004; Belmonte and Yurgelun-Todd, 2003).
Consideration of the above-described observations, together with the relatively recent demonstration that autism is associated with mutation in regions centered around the GABAA-β3 receptor subunit gene, led some researchers (Belmonte et al., 2004; Polleux and Lauder, 2004) to suggest that the neocortical dysfunction in this disorder may be attributable to a deficiency during early development in GABA-mediated synaptic neurotransmission. According to this view, the excessive excitatory neurotransmission within low-order neocortical processing areas that accompanies insufficient GABA-mediated neocortical inhibition leads to the experience-driven abnormalities in neocortical functional connectivity characteristic of autism. More specifically, in subjects that develop autism, abnormal neocortical information processing strategies attributable to excess excitation in the early-developing low-order processing areas are viewed to direct the formation (via activity-dependent neuroplasticity) of a long range inter-areal connectivity incapable of supporting the between-area communication and neuronal synchronization essential for normal social interaction and language processing skills (Belmonte et al., 2004; Just et al., 2004). Consistent with this perspective are: (i) the recommendation that drugs that promote GABAergic CNS synaptic neurotransmission be considered for early intervention and as potential therapeutic agents for autism (Belmonte et al., 2004; Bethea and Sikich, 2007); and (ii) the neuromechanistic proposal that an increased ratio of excitation to inhibition in CNS information processing systems accounts for the principal features of autism (Rubenstein and Merzenich, 2003).
The results described in this paper used an approach (Tannan et al., 2006) that enables convenient and rapid acquisition of quantitative measures of somatosensory tactile spatial localization capacity – a somatosensory perceptual capacity that animal functional neuroimaging evidence strongly suggests reflects the status of GABA-mediated inhibitory neurotransmission in primary somatosensory cortex (Simons et al., 2005; Tommerdahl et al., 2002; Whitsel et al., 1989; Juliano et al., 1989). Additionally, the findings reveal statistically highly significant differences between the tactile localization performance of normal subjects and those with autism. The deficient tactile spatial localization performance of subjects with autism demonstrated in the present study appears consistent with the proposal that somatosensory cortical GABAergic inhibitory neurotransmission is deficient in autism. In addition, the inability of adapting stimulation to improve tactile localization performance in subjects with autism (presumably because of the failure of such stimulation to dynamically recruit GABA-mediated local lateral inhibitory interactions in the responding region of primary somatosensory cortex) provides a potential explanation for the widely-known, but poorly understood tendency for subjects with autism to perform poorly (relative to control subjects) on more complex tasks requiring spatial and/or temporal integration, but better-than-normal when the task emphasizes appreciation of local detail (for review, see Mottron et al., 2006). These considerations lead the authors to regard it as feasible that the better-than-normal performance of subjects with autism on tasks emphasizing appreciation of local detail may be due to the complete absence in these individuals of adaptation in primary somatosensory cortex. Critical to this view is the fact that the local GABA-mediated lateral inhibitory interactions we presume to underlie tactile adaptation manifest a non-zero level of activity in a normal subject – and, accordingly, even in the absence of recent skin stimulation normal primary somatosensory cortex is assumed to exhibit partial/incomplete adaptation.
To our knowledge, this study has provided the first objective metric that clearly defines a difference between the centrally-mediated somatosensory discriminative capacities of the general population and a sample of individuals with autism. We consider it likely that further studies of this type will provide valuable insights not only about the nature of the neocortical information processing defects that underlie autism, but other neurological disorders as well.
4. Experimental Procedure
The subjects were four Caucasian males between the ages of 21 and 42 years, clinically diagnosed with autism (i.e., Autistic Disorder or Asperger Disorder; DSM-IV-TR; APA, 2000), and naïve both to the study design and issue under investigation. They were recruited from the University of North Carolina Neurodevelopmental Disorders Research Center Subject Registry. All four individuals had been previously tested with the Autism Diagnostic Interview – Revised (ADI-R; LeCouteur et al., 2003), the Autism Diagnostic Observation Schedule - Module 4 (ADOS; Lord et al., 1999), as well as the Wechsler Abbreviated Scale of Intelligence (WASI; Wechsler, 1999). All four subjects met diagnostic criteria for autism on the ADI-R, and had average to above average intelligence (WASI Full Scale IQs ranged from 87 to 129). Education levels were as follows: One subject completed the 8th grade, two subjects completed high school, and one subject received a bachelor’s degree. Participants were screened for comorbid psychiatric diagnoses, peripheral injury, and other conditions that would affect somatosensation. The subjects gave informed consent and were paid $20/hour for their time. All procedures were reviewed and approved in advance by an institutional review board.
Sinusoidal vertical skin displacement stimuli were delivered to the dorsum of the hand using a vertical displacement stimulator (Cantek Metatron Corp., Canonsburg, PA) fitted with a Two-Point Stimulator (TPS). The TPS and its use are described in detail in two separate reports (Tannan et al., 2005a; Tannan et al., 2005b). A single probe tip (2 mm diameter) is positioned along a linear axis 20 mm long at incremental steps of 1 mm (step error of approximately 1%). The stimulator apparatus was mounted on an adjustable mechanical arm with lockable joints that was attached to a free-standing, rigid platform (fabricated locally) which enables convenient adjustment and maintenance of stimulus position. Spatial discrimination tasks generate similar psychometric functions at the fingertip and the hand dorsum, differing essentially only by an order of magnitude (Schlereth et al., 2001; Mahns et al., 2006). A transversally oriented linear array (20mm in length) on the hand dorsum was selected to receive the stimulation because: 1) innervation density across this skin region remains relatively constant, 2) the surface is easily accessible and permits convenient stimulator placement, 3) the surface is relatively flat, reducing confounds of skin curvature present at other potential sites of stimulation, and 4) it permits positioning of the subject’s arm and hand in a comfortable and stable position for the full duration of an experimental session.
The subject was seated in a chair with the right arm placed resting on an X-ray bag filled with glass beads. The investigator molded the bag to fit the contours of the subject’s arm, and when the subject was comfortable and the arm positioned to allow unimpeded access of the stimulator to the center of the dorsal surface of the right hand, the bag was made rigid by evacuating it of air (achieved by connecting the bag to a vacuum line). The subject was unable to see either the experimenter or the stimulator and stimulus-control instrumentation. White noise presented via headphones eliminated potential auditory cues. A micrometer permitted the stimulator transducer and probe assembly to be lowered towards the predefined skin site. The micrometer position at which the digital display on the stimulator controllers registered a 0.1 – 0.2 g change in resistive force was interpreted as the point at which the stimulator probe made initial contact with the skin.
A two-interval forced-choice (2IFC) tracking protocol was used to evaluate spatial localization. The subject was instructed to attend to the percept evoked by 25 Hz (100 μm peak-to-peak amplitude) vibrotactile stimulus to the right hand. Each trial consisted of three stimuli: 1) an adapting stimulus (either 5 or 0.5 sec in duration), 2) a standard stimulus (0.5 sec) delivered at the same site as the adapting stimulus, and 3) a test stimulus (0.5 sec) delivered to a skin site different from the standard stimulus. Duration of the inter-stimulus (ISI) and inter-trial (ITI) intervals was held constant for all runs at 2 and 30 sec, respectively. All stimuli were superimposed on a pedestal of skin indentation (500 μm), and following each stimulus the probe was retracted to a position 500 μm above the skin surface. Timing and stimulus position diagrams of the experimental protocol, shown in Figure 3, are reported in a recent study (Tannan et al., 2006). The adapting stimulus always was presented first during the Adapting Interval (AI) and was positioned randomly along the axis. The adapting stimulus was, in turn, followed by either the standard stimulus or the test stimulus (order of presentation was randomly determined on a trial-by-trial basis) during Interval 1 (I1). The third stimulus delivered in a trial (standard or test, whichever had not been presented), was applied during Interval 2 (I2). Subject feedback was provided during the Response Interval (RI).
Figure 3.
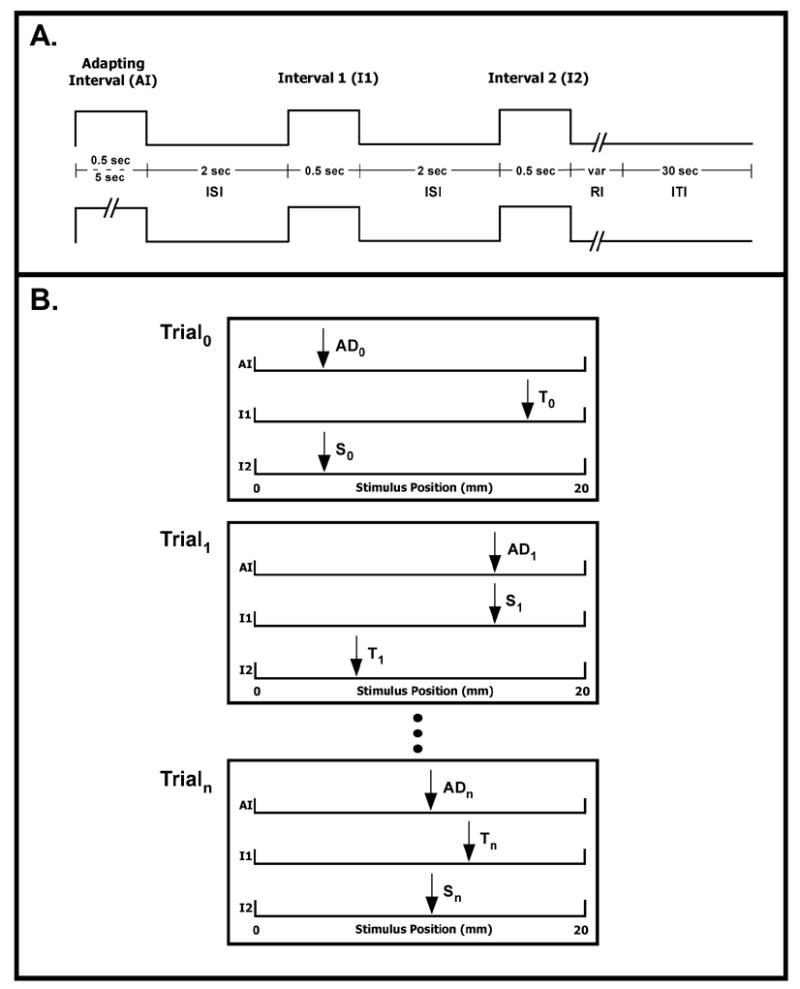
Stimulus position and timing diagram of experimental protocol. A. A 2IFC tracking protocol was used to determine a subject’s spatial localization threshold under two conditions of adapting stimulus duration (0.5 or 5 sec). B. In each trial, the subject was instructed to report which of the two post-adapting stimuli was in the same place as the adapting stimulus. A correct/incorrect response resulted in a decrease/increase in the distance by 1 mm. Each run consisted of 20 trials. (Tannan et al., 2006)
In each trial the adapting stimulus was delivered at a randomly selected locus within the 20 mm array. The distance between the standard and test stimuli (10 mm in the 1st trial at the start of each run) was determined on the basis of subject performance. The subject was instructed to report the interval during which the standard stimulus, delivered to the same skin site contacted by the adapting stimulus, was present. If the subject chose the correct interval, the distance between the skin sites contacted by the test and standard stimulus was reduced by 1 mm. If the incorrect interval was chosen, the distance was increased by 1 mm. This procedure was repeated for a minimum of 20 trials in an attempt to identify the minimally detectable separation (spatial localization threshold) between the test and standard stimuli under a given adaptation condition (5 sec vs. 0.5 sec). Order of the 2 adapting stimulus conditions (5 or 0.5 sec) within a session was randomized. Each subject completed 5 sessions (each session consisted of 2 runs).
Acknowledgments
This work was supported, in part by NIH R01 grant NS043375 (M. Tommerdahl, P.I.), and NIH R01 grant HD042168 (G. Baranek, P.I.). VT received salary support from NIH grant NS045685. CC was supported by NRSA T32 5T32HD040127-04. BLW was supported, in part, by NIH RO1 grant NS037501.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Baron-Cohen S, Belmonte MK. Autism: a window onto the development of the social and the analytic brain. Annu Rev Neurosci. 2005;28:109–26. doi: 10.1146/annurev.neuro.27.070203.144137. [DOI] [PubMed] [Google Scholar]
- 2.Bethea TC, Sikich L. Early pharmacological treatment of autism: a rationale for developmental treatment. Biol Psychiatry. 2007 Feb 15;61(4):521–37. doi: 10.1016/j.biopsych.2006.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belmonte MK, Allen G, Beckel-Mitchener A, Boulanger LM, Carper RA, Webb SJ. Autism and abnormal development of brain connectivity. J Neurosci. 2004 Oct 20;24(42):9228–31. doi: 10.1523/JNEUROSCI.3340-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belmonte MK, Yurgelun-Todd DA. Functional anatomy of impaired selective attention and compensatory processing in autism. Brain Res Cogn Brain Res. 2003 Oct;17(3):651–64. doi: 10.1016/s0926-6410(03)00189-7. [DOI] [PubMed] [Google Scholar]
- 5.Blakemore SJ, Tavassoli T, Calo S, Thomas RM, Catmur C, Frith U, Haggard P. Tactile sensitivity in Asperger syndrome. Brain Cogn. 2006 Jun;61(1):5–13. doi: 10.1016/j.bandc.2005.12.013. [DOI] [PubMed] [Google Scholar]
- 6.Casanova MF, Buxhoeveden D, Gomez J. Disruption in the inhibitory architecture of the cell minicolumn: implications for autism. Neuroscientist. 2004 Dec;9(6):496–507. doi: 10.1177/1073858403253552. [DOI] [PubMed] [Google Scholar]
- 7.Casanova MF, Buxhoeveden DP, Switala AE, Roy E. Minicolumnar pathology in autism. Neurology. 2002 Feb 12;58(3):428–32. doi: 10.1212/wnl.58.3.428. [DOI] [PubMed] [Google Scholar]
- 8.Casanova MF, van Kooten IA, Switala AE, van Engeland H, Heinsen H, Stenbusch HW, Hof PR, Trippe J, Stone J, Schmitz C. Minicolumnar abnormalities in autism. Acta Neuropathol (Berl) 2006 Sep;112(3):287–303. doi: 10.1007/s00401-006-0085-5. [DOI] [PubMed] [Google Scholar]
- 9.Cascio C, McGlone F, Folger S, Tannan V, Baranek G, Pelphrey K, Essick G. Tactile perception in adults with autism: a multidimensional psychophysical study. J Autism Dev Disord. 2007 doi: 10.1007/s10803-007-0370-8. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fatemi SH, Halt AR, Stary JM, Kanodia R, Schulz SC, Realmuto GR. Glutamic acid decarboxylase 65 and 67 kDa proteins are reduced in autistic parietal and cerebellar cortices. Biol Psychiatry. 2002 Oct 15;52(8):805–10. doi: 10.1016/s0006-3223(02)01430-0. [DOI] [PubMed] [Google Scholar]
- 11.Gomot M, Giard MH, Adrien JL, Barthelemy C, Bruneau N. Hypersensitivity to acoustic change in children with autism: electrophysiological evidence of left frontal cortex dysfunctioning. Psychophysiology. 2002 Sep;39(5):577–84. doi: 10.1017.S0048577202394058. [DOI] [PubMed] [Google Scholar]
- 12.Herbert MR. Large brains in autism: the challenge of pervasive abnormality. Neuroscientist. 2005 Oct;11(5):417–40. doi: 10.1177/0091270005278866. [DOI] [PubMed] [Google Scholar]
- 13.Hussman JP. Suppressed GABAergic inhibition as a common factor in suspected etiologies of autism. J Autism Dev Disord. 2001 Apr;31(2):247–8. doi: 10.1023/a:1010715619091. [DOI] [PubMed] [Google Scholar]
- 14.Juliano S, Whitsel BL, Tommerdahl M, Cheema SS. Determinants of patchy metabolic labeling in the somatosensory cortex of cats: a possible role for intrinsic inhibitory circuitry. J Neurosci. 1989 Jan;9(1):1–12. doi: 10.1523/JNEUROSCI.09-01-00001.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Just MA, Cherkassky VL, Keller TA, Minshew NJ. Cortical activation and synchronization during sentence comprehension in high-functioning autism: evidence of underconnectivity. Brain. 2004 Aug;127(8):1811–21. doi: 10.1093/brain/awh199. [DOI] [PubMed] [Google Scholar]
- 16.Kohn A, Metz CB, Quibrera PM, Tommerdahl M, Whitsel BL. Functional neocortical microcircuitry demonstrated with intrinsic signal optical imaging in vitro. Neuroscience. 2000;95(1):51–62. doi: 10.1016/s0306-4522(99)00385-1. [DOI] [PubMed] [Google Scholar]
- 17.Kootz JP, Marinelli B, Cohen DJ. Sensory receptor sensitivity in autistic children: response times to proximal and distal stimulation. Arch Gen Psychiatry. 1981 Mar;38(3):271–3. doi: 10.1001/archpsyc.1981.01780280039003. [DOI] [PubMed] [Google Scholar]
- 18.LaMotte RH, Mountcastle VB. Capacities of humans and monkeys to discriminate between vibratory stimuli of different frequency and amplitude: a correlation between neural events and psychophysical measurements. J Neurophysiol. 1975;38:539–59. doi: 10.1152/jn.1975.38.3.539. [DOI] [PubMed] [Google Scholar]
- 19.LaMotte RH, Mountcastle VB. Disorders in somesthesis following lesions of parietal lobe. J Neurophysiol. 1979;42:400–19. doi: 10.1152/jn.1979.42.2.400. [DOI] [PubMed] [Google Scholar]
- 20.LeCouteur A, Lord C, Rutter M. Autism Diagnostic Interview-Revised (ADI-R) Los Angeles: Western Psychological Corporation; 2003. [Google Scholar]
- 21.Lord C, Rutter M, Dilavore P, Risi S. The Autism Diagnostic Observation Schedule (ADOS) Los Angeles: Western Psychological Corporation; 1999. [Google Scholar]
- 22.Mahns DA, Perkins NM, Sahai V, Robinson L, Rowe MJ. Vibrotactile frequency discrimination in human hairy skin. J Neurophysiol. 2006;95:1442–50. doi: 10.1152/jn.00483.2005. [DOI] [PubMed] [Google Scholar]
- 23.Mottron L, Dawson M, Soulieres I, Hubert B, Burack J. Enhanced perceptual functioning in autism: an update, and eight principles of autistic perception. J Autism Dev Disord. 2006 Jan;36(1):27–43. doi: 10.1007/s10803-005-0040-7. [DOI] [PubMed] [Google Scholar]
- 24.Mountcastle VB, Darian-Smith I. Neural mechanisms in somesthesia. In: Mountcastle VB, editor. Medical Physiology. 2. Mosby; St. Louis: 1968. pp. 1372–423. [Google Scholar]
- 25.Plaisted K, Saksida L, Alcantara J, Weisblatt E. Towards an understanding of the mechanisms of weak central coherence effects: experiments in visual configural learning and auditory perception. Philos Trans R Soc Lond B Biol Sci. 2003 Feb 28;358(1430):375–86. doi: 10.1098/rstb.2002.1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Polleux F, Lauder JM. Toward a developmental neurobiology of autism. Ment Retard Dev Disabil Res Rev. 2004;10(4):303–17. doi: 10.1002/mrdd.20044. [DOI] [PubMed] [Google Scholar]
- 27.Rubenstein JL, Merzenich MM. Model of autism: increased ratio of excitation/inhibition in key neural systems. Genes Brain Behav. 2003 Oct;2(5):255–67. doi: 10.1034/j.1601-183x.2003.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlereth T, Magerl W, Treede R. Spatial discrimination thresholds for pain and touch in human hairy skin. Pain. 2001;92(1–2):187–94. doi: 10.1016/s0304-3959(00)00484-x. [DOI] [PubMed] [Google Scholar]
- 29.Simons SB, Chiu J, Favorov OV, Whitsel BL, Tommerdahl M. Duration-dependent response of SI cortex to flutter stimulation. J Neurophysiol. 2006 Oct 11; doi: 10.1152/jn.00513.2006. [DOI] [PubMed] [Google Scholar]
- 30.Simons SB, Tannan V, Chiu J, Favorov OV, Whitsel BL, Tommerdahl M. Amplitude-dependency of response of SI cortex to flutter stimulation. BMC Neurosci. 2005 Jun 21;6(1):43. doi: 10.1186/1471-2202-6-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tannan V, Dennis RG, Tommerdahl M. A novel device for delivering two-site vibrotactile stimuli to the skin. J Neurosci Methods. 2005a;147:75–81. doi: 10.1016/j.jneumeth.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 32.Tannan V, Dennis RG, Tommerdahl M. Stimulus-dependent effects on tactile spatial acuity. Behav Brain Funct. 2005b Oct 10;1:18. doi: 10.1186/1744-9081-1-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tannan V, Tommerdahl M, Whitsel BL. Vibrotactile adaptation enhances spatial localization. Brain Res. 2006 Aug 2;1102(1):109–16. doi: 10.1016/j.brainres.2006.05.037. [DOI] [PubMed] [Google Scholar]
- 34.Tommerdahl M, Favorov OV, Whitsel BL. Optical imaging of intrinsic signals in somatosensory cortex. Behav Brain Res. 2002;135:83–91. doi: 10.1016/s0166-4328(02)00159-6. [DOI] [PubMed] [Google Scholar]
- 35.Wechsler D. WASI Manual. San Antonio: Psychological Corporation; 1999. [Google Scholar]
- 36.Whitsel BL, Favorov OV, Tommerdahl M, Diamond M, Juliano S, Kelly D. Dynamic processes govern the somatosensory cortical response to natural stimulation. In: Lund JS, editor. Sensory Processing in the Mammalian Brain. Oxford Univ. Press; New York: 1989. pp. 79–107. [Google Scholar]


