Abstract
Targeted disruption of the single mutant K-ras allele in two human colorectal carcinoma cell lines (DLD-1 and HCT-116) leads to loss of tumorigenic competence in nude mice with retention of ability to grow indefinitely in monolayer culture. Because expression of the mutant K-ras oncogene in these cell lines is associated with marked up-regulation of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF), we sought to determine whether this potent angiogenesis inducer plays a role in K-ras-dependent tumorigenic competence. Transfection of a VEGF121 antisense expression vector into DLD-1 and HCT-116 cells resulted in suppression of VEGF/VPF production by a factor of 3- to 4-fold. The VEGF/VPF-deficient sublines, unlike the parental population or vector controls, were profoundly suppressed in their ability to form tumors in nude mice for as long as 6 months after cell injection. In contrast, in vitro growth of these sublines was unaffected, thus demonstrating the critical importance of VEGF/VPF as an angiogenic factor for HCT-116 and DLD-1 cells. Transfection of a full-length VEGF121 cDNA into two nontumorigenic mutant K-ras knockout sublines resulted in a weak but detectable restoration of tumorigenic ability in vivo in a subset of the transfectants, with no consistent change in growth properties in vitro. The findings indicate that mutant ras-oncogene-dependent VEGF/VPF expression is necessary, but not sufficient, for progressive tumor growth in vivo and highlight the relative contribution of oncogenes, such as mutant K-ras, to the process of tumor angiogenesis.
Expansion of solid tumors beyond microscopic sizes (e.g., 1–2 mm in diameter) requires the continuous recruitment of new blood vessels from preexisting vasculature, a process known as tumor angiogenesis (1). Two major types of event are thought to be necessary for small tumors being able to switch on angiogenesis (2, 3). The first involves a gain-of-function event in which certain angiogenic growth factor stimulators are induced or significantly up-regulated in tumor cells. These include vascular endothelial growth factor (VEGF), also known as vascular permeability factor (VPF), basic fibroblast growth factor (bFGF), and transforming growth factor β (TGF-β) or α (TGF-α), among a number of others. The second major event appears to be one of loss of function, namely, down-regulation of various endogenous angiogenesis inhibitors, such as thrombospondin or interferon α/β (2, 3). A combination of these two processes can result in a local excess in tumors of angiogenesis stimulators over inhibitors and, thus, “trip” the angiogenic switch at some point during tumor progression (2, 3).
The nature of the cellular and molecular changes that lead to these proangiogenic events in tumors remain poorly defined. However, it is interesting to note that the two major generic events at the genetic level associated with tumor development and progression—activation of dominantly acting oncogenes on the one hand and inactivation of tumor suppressor genes on the other (4)—are generally associated with gain- and loss-of-function events, respectively. In this regard, the hypothesis has been put forward that induction of tumor angiogenesis may be induced and regulated, at least in part, by precisely these same cancer-causing genetic changes (3, 5–7), possibly acting in conjunction with certain epigenetic/environmental influences such as hypoxia (8–10). Indeed, it is now known that endogenous inhibitors such as thrombospondin can be down-regulated by inactivating p53 mutations and deletions and that various proangiogenic growth factors such as TGF-β (3), TGF-α, or VEGF/VPF can be induced or strongly up-regulated by mutant ras oncogenes (3, 7). With respect to the latter findings, we have previously reported that VEGF/VPF- and mutant-ras-negative (immortalized but nontumorigenic) rat intestinal epithelial cells could be induced to express both high levels of VEGF/VPF and a tumorigenic phenotype by transfection of a mutant H-ras oncogene (11). Furthermore, the levels of VEGF/VPF mRNA and protein in two tumorigenic human colorectal carcinoma cell lines, DLD-1 and HCT-116, each known to carry a single mutant K-ras allele, were suppressed in vitro up to 4- to 5-fold when the mutant K-ras allele was disrupted by gene targeting methods (11). The mutant K-ras knockout sublines were also incapable of forming tumors in nude mice (11).
Given the importance of VEGF/VPF as an inducer of tumor angiogenesis (12, 13) and the necessity of angiogenesis for progressive growth of tumors in vivo, these results suggest that one of the growth promoting effects, or functions, of ras oncogene activation may be to facilitate tumor angiogenesis (7, 11). This may also be the case for other common oncogenic changes that are known to be important for tumor development, e.g., overexpression of the epidermal growth factor (EGF) receptor or the erbB2/HER-2/neu protooncogene (9). The possible impact of oncogenes as angiogenesis stimulators may also help explain why various cytostatic signal transduction inhibitory drugs, such as EGF-receptor-neutralizing antibodies or RAS farnesyltransferase inhibitors, sometimes appear to express potent antitumor cytotoxic-like effects in vivo (where angiogenesis is required) but not in cell culture (where it is not) (9).
Despite our results (11), and those of others (6, 8, 14–17), showing a significant inducing effect of mutant ras on VEGF/VPF expression, it has not yet been established whether such an association actually contributes to the tumorigenic phenotype through a proangiogenic effect. The purpose of this article is to present results that strongly implicate that it does. We first determined, by using an antisense approach, that VEGF/VPF production by the DLD-1 and HCT-116 human colorectal cell lines is indeed necessary for tumor angiogenesis and tumorigenic growth in nude mice. We then restored high levels of VEGF/VPF production in the nontumorigenic VEGF/VPF-deficient mutant K-ras knockout sublines of DLD-1 and HCT-116 and assessed their tumor forming ability in vivo. The results indicate that mutant ras-dependent induction of VEGF/VPF is necessary, but not sufficient, for tumorigenicity of these two human colorectal carcinoma cell lines.
MATERIALS AND METHODS
Cell Lines and Culture Conditions.
Human colon cancer cell lines DLD-1 and HCT-116 and their respective variant sublines (DKs-8 and HKh-2) in which the activated K-ras gene was disrupted have been characterized (18). All cell lines were maintained in DMEM supplemented with 10% fetal bovine serum (GIBCO/BRL).
Establishment of Constructs and Transfection.
The human VEGF121 cDNA was cloned in either an antisense or sense orientation into the simian virus 40 promoter-driven mammalian expression vector pZeoSV (Invitrogen). The full-length VEGF121 cDNA insert (441 bp), which was provided by R. Bicknell (19), was inserted directly between the HindIII and SpeI sites for sense orientation (pZeoSV-S-VEGF, Fig. 1A) or first cloned into the EcoRV–BamHI sites of pcDNA3.1 vector and then into HindIII–XhoI sites of the pZeoSV vector (pZeoSV-AS-VEGF, Fig. 1B), for antisense orientation.
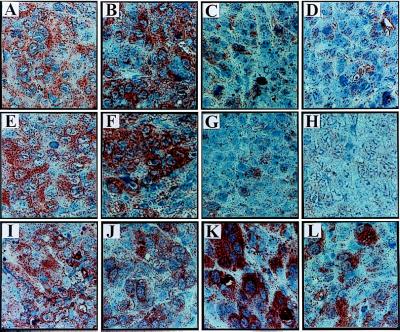
Figure 1.
In situ expression of VEGF protein in inoculates of DLD-1- and HCT-116-derived sublines 4 days after tumor cell injection. Clones were designated as AS for antisense VEGF transfectants, S for sense, and V for vector control transfectants respectively. (A) Parental DLD-1 cells. (B) DLD-V1. (C) DLD-AS2. (D) DLD-AS6. (E) Parental HCT-116 cells. (F) HCT-V1. (G) HCT-AS9. (H) HCT-AS18. (I) Mutant K-ras knockout-derived VEGF sense clone DKs-S1. (J) DKs-S6. (K) DKs-S10. (L) HKh-S3. Clones DLD-AS3 and HCT-AS22 ressembled their AS transfected sister cell lines (data not shown).
Transfection was performed by using Lipofectin essentially as described by the supplier (GIBCO/BRL). Zeocin-resistant colonies were selected with optimized concentrations of Zeocin (100 μg/ml for DLD-1, 300 μg/ml for HCT-116, and 400 μg/ml for DKs-8 and HKh-2 cells).
RNA Isolation and Northern Blot Analysis.
Total RNA was isolated from cultured cells by using the Trizol reagent (GIBCO/BRL/Life Science Technologies), essentially as described by the manufacturer. Polyadenylylated RNA was isolated as described (8). The blots were hybridized with the 0.4-kb VEGF probe or glyceraldehyde-3-phosphate dehydrogenase probe (1.25 kb).
Determination of in Vitro Tumor Cell Growth, Plating Efficiency, and Soft Agar Colony Formation.
For in vitro cell growth analysis, cells were seeded into 24-well plates (Nunc) at 2 × 104 cells per well. The medium was changed every other day. On the days indicated, cells were harvested and counted by using a Coulter Counter (Coulter).
To assess plating efficiency, 500 cells suspended in DMEM containing 10% fetal bovine serum were plated into 60-mm dishes in triplicate. The dishes were incubated for 14 days, and colonies were fixed in Carnoy’s fixative, stained in 0.1% crystal violet, and scored.
The soft agar (anchorage independent) growth assay was performed essentially as described (18). Briefly, 3 × 103 cells were suspended in 1 ml of DMEM containing 0.3% agar (GIBCO/BRL) and 10% fetal bovine serum and applied on top of a presolidified 0.6% agar (1 ml) in a six-well dish (Nunc). Triplicate plates were prepared for each clone. After 3 weeks of incubation, colonies larger than 0.1 mm in diameter were scored.
Tumorigenicity Assay.
Cells were injected subcutaneously into 8- to 12-week-old female nude athymic BALB/c mice. The resulting tumors were measured twice a week by using a Vernier’s calliper and tumor volume (mm3) was calculated by using the standard formula (11).
Measurement of Human VEGF, bFGF, and TGF-β1 Protein Levels in Conditioned Medium or Cytoplasmic Fraction by ELISA.
Commercially available ELISA kits (R & D Systems) for human VEGF, bFGF, and TGF-β were used to quantitate the level of each protein in conditioned medium according to the manufacturer’s instructions, as described (11). To maintain cells under hypoxic-like conditions, cobalt chloride (CoCl2) was added into the medium at 50 μM for DLD-1 cell line and at 100 μM for HCT-116 cell line (20). It should be pointed out that the effect of CoCl2 is not identical to true hypoxia and, therefore, we will refer to this treatment as “hypoxia-like” condition and to CoCl2 as “hypoxia mimetic” (21). Samples for TGF-β were acid-activated and neutralized to activate latent TGF-β and generate the immunoreactive form just before ELISA analysis. To prepare conditioned medium and cytoplasmic fraction for bFGF ELISA, cells were cultured to confluence in 100-mm dishes (Nunc) and medium was conditioned for another 48 h with addition of heparin (50 μg/ml, GIBCO/BRL) to liberate bFGF (22). After conditioned medium was collected, the cells were counted. Subsequently, the cells were frozen and thawed (on ice) three times, sonicated, and centrifuged at 25,000 × g for 20 min (22). Protein concentration of each sample was determined by using BIO-RAD protein assay reagent (BIO-RAD).
Immunohistochemistry.
At various times after tumor cell injection, solid tumors were removed, fixed in 10% formalin, and paraffin-embedded. The sections (4 μm) were boiled for 2 min in 10 mM citrate buffer at pH 6.0. The sections were then coated with 10% goat serum and incubated overnight at 4°C with either a rabbit anti-VEGF antibody, A20 (Santa Cruz Biotechnology) diluted 1:100, or a rabbit polyclonal anti-Ki67 antibody (Novocastra Laboratories, Newcastle-Upon-Tyne, UK) diluted 1:1,000 in the antibody diluting buffer (Dako). After washing in PBS, biotinylated goat anti-rabbit antibody was added for 30 min at room temperature, followed by the avidin-horseradish peroxidase complex. The immunoreactivity was visualized with 3-amino-9-ethylcarbazole (Zymed) for VEGF staining, and with diaminobenzidine tetrahydrochloride (Boehringer Mannheim) for Ki67 staining. Preincubation of the anti-VEGF antibodies with a specific blocking peptide (Santa Cruz Biotechnology) completely abrogated the staining, demonstrating the required specificity.
RESULTS
Inhibition of Endogenous VEGF Expression Suppresses Mutant K-ras-Dependent Tumorigenicity of Human Colorectal Cancer Cells.
In two colorectal cancer cell lines, DLD-1 and HCT-116, disruption of the single mutant K-ras oncogenic allele resulted in almost complete suppression of tumor-forming capacity in nude mice and in a concomitant several fold reduction in VEGF synthesis (11). To assess the potential impact of the latter event on mutant K-ras-dependent tumorigenic growth properties of these cancer cells, VEGF-deficient sublines of both DLD-1 and HCT-116 were generated by transfection of an antisense VEGF121 expression vector. From these two cell lines a total of 14 and 18 Zeocin-resistant clones, respectively, were initially isolated, all of which were then screened for expression of VEGF protein by ELISA and examined for expression of VEGF antisense mRNA (data not shown). To ensure that reduced VEGF protein expression observed in selected clones will not be overridden under hypoxic conditions commonly present within solid tumor masses, we scrutinized these clones further by examining their VEGF production profiles in the presence of cobalt chloride, which is known to biochemically mimic hypoxia-like conditions (8, 20, 23, 24). Subsequently, a number of clones that maintained low VEGF levels even under such hypoxic conditions were injected into nude mice, and after 4–6 days of prevascular growth, the inocula were excised and examined in situ for VEGF expression by specific immunohistochemical staining (Fig. 1). This procedure yielded four VEGF-deficient clones of the DLD-1 cell line (DLD-AS2, DLD-AS5, DLD-AS6, and DLD-AS9) and three such clones from the HCT-116 cell line (HCT-AS9, HCT-AS18, and HCT-AS22). All of the clones demonstrated significant reduction in VEGF expression in vitro and in vivo but exhibited no detectable changes in their in vitro growth properties (Tables 1 and 2). In addition to pooled parental populations and “empty” vector transfected clones (DLD/V1 and HCT/V1), which were used throughout the subsequent experiments as controls, eight Zeocin-resistant clones were randomly isolated from either DLD-1 or HCT-116 cell lines transfected with the control vector, and those were analyzed for VEGF production. In no case was VEGF found to be down-regulated (data not shown). Thus the reduced VEGF levels in the antisense clones was not due to random clonal selection and variation.
Table 1.
Production of VEGF, bFGF, and TGF-β1 by antisense or sense VEGF transfectants and their control colorectal cancer cell lines
| Cell line | bFGF
|
TGF-β1, pg/ml | |||
|---|---|---|---|---|---|
| VEGF, pg/ml
|
Cytosol, pg/mg* | CM, pg/ml | |||
| −CoCl2 | +CoCl2 | ||||
| DLD-1 | 4,376 ± 69 | 6,354 ± 482 | 4.9 | 2.3 | 1,051 ± 206 |
| DLD-V1 | 4,499 ± 30 | 6,618 ± 230 | 4.9 | 2.5 | 1,063 ± 118 |
| DLD-AS2 | 2,005 ± 806 | 2,267 ± 858 | 5.0 | 2.5 | 553 ± 31 |
| DLD-AS2tum+ | 2,951 ± 103 | ND | 6.1 | 3.6 | 1,083 ± 60 |
| DLD-AS5 | 2,203 ± 92 | 2,395 ± 90 | 4.7 | 0.9 | 813 ± 252 |
| DLD-AS6 | 1,895 ± 188 | 2,762 ± 891 | 3.2 | 0.8 | 1,049 ± 209 |
| DLD-AS9 | 1,853 ± 91 | 2,446 ± 141 | 2.9 | 1.1 | 1,056 ± 191 |
| DLD-AS9tum+ | 2,990 ± 281 | ND | 3.7 | 1.5 | 1,001 ± 95 |
| HCT-116 | 8,069 ± 45 | 8,681 ± 549 | 6.3 | 3.7 | 2,637 ± 38 |
| HCT-V1 | 8,096 ± 135 | 8,234 ± 172 | 5.7 | 4.0 | 2,661 ± 172 |
| HCT-AS9 | 1,940 ± 415 | 3,077 ± 180 | 5.4 | 8.5 | 2,044 ± 52 |
| HCT-AS18 | 3,016 ± 602 | 3,850 ± 62 | 5.3 | 4.5 | 2,664 ± 178 |
| HCT-AS18tum+ | 2,223 ± 401 | ND | 4.2 | 3.8 | 1,963 ± 27 |
| HCT-AS22 | 5,692 ± 286 | 6,993 ± 708 | 3.8 | 3.8 | 3,090 ± 144 |
| HCT-AS22tum+ | 8,609 ± 510 | ND | 4.9 | 6.2 | 2,845 ± 39 |
| DKs-8 | 1,359 ± 164 | 2,868 ± 239 | 3.0 | 1.1 | 769 ± 60 |
| DKs-V1 | 1,160 | ND | 3.3 | 0.8 | 712 ± 14 |
| DKs-S1 | 10,844 ± 2042 | ND | 6.5 | 2.3 | 995 ± 496 |
| DKs-S6 | 10,090 ± 2022 | ND | 5.4 | 1.7 | 800 ± 94 |
| DKs-S10 | 9,940 ± 1386 | ND | 3.8 | 0.6 | 889 ± 54 |
| DKs-S10tum+ | 10,125 ± 1074 | 10,900 ± 3875 | 4.6 | 1.2 | 1,556 ± 609 |
| HKh-2 | 1,747 ± 155 | 1,758 ± 108 | 7.6 | 3.7 | 1,353 ± 190 |
| HKh-V1 | 1,680 | ND | 6.3 | 4.0 | 1,281 ± 34 |
| HKh-S3 | 5,500 ± 537 | ND | 7.9 | 5.4 | 1,020 ± 41 |
| HKh-S8 | 7,224 ± 79 | ND | 2.8 | 3.4 | 1,275 ± 78 |
VEGF levels in supernatants were obtained from 3 to 4 × 105 tumor cells cultured in 24-well plates for 24 h with (+) or without (−) CoCl2 stimulation. Determinations from at least four experiments were similar; data are the mean ± SD. bFGF levels in supernatants or cytoplasmic fractions obtained from confluent cultures in 100-mm dishes for 48 h in the presence of heparin (50 μg/ml). Cytoplasmic fractions were obtained by freezing and thawing three times from the same cells used to obtain supernatant. TGF-β1 samples were acid-activated and neutralized just before ELISA analysis. CM, conditioned medium; ND, not determined.
Normalized to protein concentration.
Table 2.
Characteristics of antisense and sense VEGF transfected colorectal cancer cell lines
| Cell line |
In vitro
|
In vivo
|
||||
|---|---|---|---|---|---|---|
| Doubling time, h | Plating efficiency, % | Colony formation in soft agar, % | No. mice with tumor/no. mice injected | Tumor volume, mm3 | Days after injection | |
| DLD-1 | 33.6 | 40.2 | 15.6 | 6/6 | 1,074 ± 94 | 23 |
| DLD-V1 | 38.4 | 43.6 | 15.3 | 6/6 | 980 ± 41 | 23 |
| DLD-AS2 | 36.0 | 50.8 | 4.6 | 3/4 | 134 ± 132 | 31 |
| DLD-AS2tum+ | 28.8 | 54.2 | 3.5 | N/A | N/A | N/A |
| DLD-AS5 | 52.8 | 40.6 | 14.9 | 2/8 | 9 ± 13 | 45 |
| DLD-AS6 | 76.8 | 53.0 | 8.4 | 0/8 | 7 ± 6 | 45 |
| DLD-AS9 | 19.2 | 54.8 | 26.4 | 2/8 | 15 ± 27 | 45 |
| DLD-AS9tum+ | 16.8 | 74.6 | 25.2 | N/A | N/A | N/A |
| HCT-116 | 57.6 | 56.6 | 18.6 | 6/6 | 466 ± 229 | 31 |
| HCT-V1 | 62.4 | 52.8 | 18.8 | 6/6 | 456 ± 215 | 31 |
| HCT-AS9 | 57.6 | 51.2 | 17.3 | 0/8 | 0 | 45 |
| HCT-AS18 | 55.2 | 51.4 | 18.3 | 6/8 | 134 ± 208 | 45 |
| HCT-AS18tum+ | 48.0 | 40.8 | 20.7 | N/A | N/A | N/A |
| HCT-AS22 | 81.6 | 38.0 | 11.2 | 3/8 | 64 ± 76 | 45 |
| HCT-AS22tum+ | 38.4 | 56.6 | 13.3 | N/A | N/A | N/A |
| DKs-8 | 57.6 | 54.8 | 0 | 0/12 | 0 | 160 |
| DKs-V1 | 57.6 | 56.0 | 0 | 0/12 | 0 | 160 |
| DKs-S1 | 100.8 | 30.8 | 0 | 2/10 | 59 ± 154 | 35 |
| DKs-S6 | 40.8 | 14.2 | 0 | 2/16 | 43 ± 145 | 160 |
| DKs-S10 | 26.4 | 22.0 | 0 | 7/16 | 109 ± 132 | 69 |
| DKs-S10tum+ | 48.0 | 34.6 | 0 | N/A | N/A | N/A |
| HKh-2 | 48.0 | 34.6 | 0 | 0/10 | 0 | 160 |
| HKh-V1 | 50.4 | 40.0 | 0 | 0/8 | 0 | 160 |
| HKh-S3 | 64.8 | 21.8 | 0 | 1/18 | 17 ± 72 | 160 |
| HKh-S8 | 57.6 | 23.4 | 0 | 5/14 | 51 ± 4 | 160 |
To determine the ability of tumors to form in vivo, approximately 1 × 106 DLD-1, HCT-116, and their transfected cell lines or 1 × 107 DKs-8, HKh-2, and their transfected cell lines were injected subcutaneously into BALB/c nude mice and the number of mice forming tumors was recorded. Results are from two experiments with similar results. Data for tumor volume are the mean ± SD. N/A, not applicable.
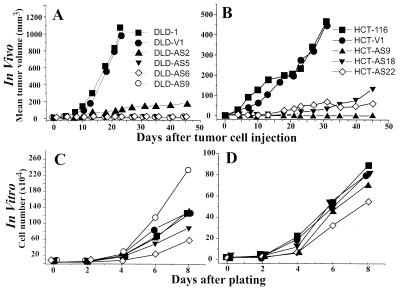
The tumor forming capacity of the VEGF-deficient sublines isolated from both DLD-1 and HCT-116 was found to be profoundly suppressed. Fig. 2 A and B shows that although the parental cell lines and their respective control vector transfectants readily formed progressively growing tumors upon subcutaneous injection, the antisense VEGF transfected clones either failed to form tumors altogether or formed small outgrowths that remained in a dormant state for more than 6 months (data not shown) or, in some cases, eventually regressed. In contrast, in vitro growth rates of both VEGF-deficient and proficient cell lines were relatively similar (Fig. 2 C and D). In this regard, some clonal variability was observed among DLD-1-derived sublines, but this bore no resemblance to their respective tumorigenic properties. In addition, such parameters as in vitro plating efficiency and soft agar colony formation were also evaluated in all the cell lines and found to be either essentially unchanged or exhibited only small and random variability. Production of other growth factors known to regulate mitogenesis and angiogenesis such as bFGF or TGF-β remained unchanged in the antisense VEGF transfectants, even though in the presence of mutant K-ras, production of the latter was mildly but consistently up-regulated (Table 1, compare HCT-116 and DLD-1 with their respective mutant K-ras knockouts, HKh-2 and DKs-8). These results indicate that the suppression of the tumorigenic phenotype in VEGF-deficient clones is not related to changes in their intrinsic growth properties or conceivable autocrine activity of growth factors including VEGF itself (25). This interpretation is consistent with the results of immunohistochemical staining revealing a significant Ki67-positive cell fraction in both control and the antisense VEGF-transfected cells within a 6-day-old inoculum (data not shown). In the latter case, however, there was a 2.4-fold increase in apoptotic fraction of cells, positive for terminal deoxynucleotidyltransferase-mediated UTP end labeling (data not shown), that may account, at least in part, for the lack of apparent tumor growth.
Figure 2.
In vivo and in vitro growth curves of antisense VEGF-expressing DLD-1 or HCT-116 cell lines. For in vivo experiments, 1 × 106 cells from antisense VEGF-expressing clones, a vector control transfected clone, or their parental cell lines were injected s.c. into nude mice (A and B). For in vitro experiments, 2 × 104 cells were seeded into 24-well plates. At the indicated day, cells were harvested and counted. Data points are the average of triplicate determinations (C and D). (A and C) ▪, DLD-1; •, DLD-V1; ▴, DLD-AS2; ▾, DLD-AS5; ⋄, DLD-AS6; ○, DLD-AS9. (B and D) ▪, HCT-116; •, HCT-V1; ▴, HCT-AS9; ▾, HCT-AS18; ⋄, HCT-AS22. In each case, the experiment was done two or three times with similar results and results of a representative experiment are shown.
Reversal of VEGF-Deficient Phenotype in VEGF Antisense Transfectants That Formed Tumors in Vivo.
In a small number of cases, injection of VEGF-deficient cell lines into mice led, after a long latency period, to formation of aggressively growing tumors. From four such tumors, which will be referred to as DLD-AS2tum+, DLD-AS9tum+, HCT-AS18tum+, and HCT-AS22tum+, cell lines were established in culture and their in vitro properties were analyzed (Tables 1 and 2). Three of four tumor-derived cell lines exhibited a significant increase in VEGF protein production, as shown by ELISA, which was attained without loss of their Zeocin resistance (data not shown) or major changes in their in vitro growth and colony-forming properties (Tables 1 and 2). In one case (HCT-AS18tum+), VEGF production remained low without a compensatory increase in production of bFGF or TGF-β1 (Table 1). The molecular basis for initiation of effective angiogenesis is, in this latter case, unknown. No change in VEGF production was observed in the DKs-S10tum+ cell line derived from a tumor that arose after injection of mutant-K-ras-negative DKs-8 cells engineered to overexpress VEGF121. In this context, it is interesting to note that reexpression of higher levels of VEGF cosegregated with reacquisition of tumor-forming ability, albeit not in every case.
Incomplete Restitution of the Mutant K-ras-Dependent Tumorigenic Phenotype by Overexpression of Exogenous VEGF.
To establish whether mutant K-ras-dependent up-regulation of VEGF is sufficient for expression of the mutant-K-ras-dependent tumorigenic phenotype, in the DLD-1 and HCT-116 cell lines, their respective nontumorigenic mutant K-ras knockout sublines (DKs-8 and HKh-2) were transfected with a VEGF121 expression vector. A total of nine and seven Zeocin-resistant clones were isolated, respectively, and screened for overexpression of the exogenous 1.9-kb VEGF transcript readily recognizable by total RNA Northern blot analysis (data not shown). Clones identified as highly positive (three and two of the DLD-1 and HCT-116 parental cell lines, respectively) were subjected to analysis for VEGF protein production by ELISA (Table 1) and by in vivo immunohistochemistry, as described (Fig. 1). In the latter assay, conspicuous cytoplasmic VEGF-specific staining was observed in all clones analyzed. The VEGF-overexpressing clones designated as DKs-S1, -S6, and -S10 or HKh-S3 and -S8 secreted at least 4.3–8.6 times greater amounts of VEGF protein into their conditioned medium than their respective parental counterparts or vector controls. Again, eight of such Zeocin-resistant randomly isolated control clones transfected with an empty vector were initially examined, and in no case was up-regulation of VEGF detected (data now shown).
None of the mice injected with DKs-8 or HKh-2 cell lines or their vector control clones developed progressively growing tumors within 160 days. Only very small palpable lesions developed initially but these regressed spontaneously (Table 2). On the other hand, the VEGF-overexpressing DKs-8 and HKh-2 cell lines demonstrated small but appreciable increase in tumor formation in vivo (Table 2). All such tumors, particularly DKs-S10 were slow growing, highly vascularized (data not shown), and stained strongly for VEGF (Fig. 1). No major changes in expression of selected growth factors other than VEGF were observed with the exception of a cell line established from the DKs-S10 tumor (DKs-S10tum+) that produced increased amounts of TGF-β1 compared with both its parental VEGF transfectant clone and the original DKs-8 cell line (Table 1).
In vitro growth rate varied among VEGF overexpressing and control clones derived from DKs-8 cell line but there was no correlation with their levels of VEGF production or in vivo tumorigenicity. No such variation was observed in the case of HKh-2 derivatives (Table 2). These observations suggest that although tumorigenicity of cell lines engineered to overexpress VEGF is noticeably increased, this alone cannot fully compensate for the absence of the mutant K-ras oncogene.
DISCUSSION
The results of our experiments represent another important step toward establishing a contributory role for oncogenes, such as mutant K-ras, in tumor angiogenesis. Previously, we had reported that one of the consequences of disruption of the mutant K-ras allele in two different colorectal carcinoma cell lines was a significant down-regulation of VEGF—a finding that could account, at least in part, for the loss of tumorigenicity in these knockout cell lines (6, 8, 11, 14–17). This hypothesis assumes that VEGF functions as a major proangiogenesis factor in the DLD-1 and HCT-116 cell lines. Indeed, on the basis of the results presented in this paper, this would appear to be the case because every one of the six VEGF121 antisense transfected clones obtained from DLD-1 or HCT-116 either failed to form tumors in nude mice or were profoundly suppressed in their ability to do so. Progressively expanding tumors failed to form even when the mice were monitored for as long as 6 months after tumor cell injection. All of these clones produced between 2 and 4 times less VEGF protein in cell culture, compared with their respective controls. In contrast to this severe in vivo growth defect, their growth in vitro was not in any way impaired. This strongly suggests that the in vivo growth defect was due to suppression of tumor angiogenesis and that VEGF is a critical mediator of this process for both DLD-1 and HCT-116 cells. Immunohistochemistry clearly showed the antisense transfectants were also strongly suppressed for VEGF expression in vivo.
The fact that only a 2- to 4-fold reduction in VEGF protein was sufficient to cause such a profound defect in tumorigenic competence is consistent with previous findings of Cheng et al. (26) who found that 3-fold reductions in VEGF levels, also induced by an antisense transfection strategy, caused a similar profound loss of tumorigenic competence for a human glioblastoma in nude mice. It is also noteworthy that disruption of only one VEGF allele causes such a significant suppression of vasculogenesis and angiogenesis in mouse embryos that they fail to survive full term in utero (27).
After establishing the importance of VEGF for the growth of DLD-1 and HCT-116 cells in vivo, we then determined the effects of restoring high levels of VEGF expression in two mutant K-ras knockout (and VEGF suppressed) sublines (18) obtained from DLD-1 and HCT-116. This was done by using a VEGF “sense” gene transfection strategy. This resulted in a weak but detectable degree of tumorigenicity in a subset of the VEGF121 transfectants, the extent of which may, if anything, be an underestimate. The reason for this is that down-regulation of VEGF with the VEGF121 antisense expression vector would result in suppression of all VEGF isoforms, whereas restoration of VEGF121 expression in the mutant K-ras knockout sublines with the VEGF121 sense expression construct would not affect levels of the VEGF165, VEGF189, or VEGF206 isoforms. If any of the latter isoforms possess qualitative effects in stimulating angiogenesis that are distinct from VEGF121, then even very high levels of VEGF121 may not stimulate angiogenesis to the degree observed in the mutant K-ras-positive parental cell lines.
Thus, taken together with the antisense transfection findings, the results show that K-ras-oncogene-dependent VEGF expression is necessary, but clearly not sufficient, for progressive in vivo growth of the HCT-116 and DLD-1 cell lines. This conclusion is consistent with the notion that several different (and direct) growth promoting functions of ras gene mutation/activation are possible—in addition to stimulating growth indirectly through angiogenesis. For example, besides stimulating tumor cell proliferation in a direct manner, e.g., by increasing the production of autocrine growth factors such as TGF-α (28) or cell cycle control elements such as cyclin D1 (29), mutant ras oncogenes can function to suppress high levels of apoptosis induced in epithelial cells forced to grow in an anchorage-independent (30) or multicellular (31) context. In this regard, it is known that the anchorage-independent growth ability of the DLD-1- and HCT-116-derived mutant K-ras knockout sublines is severely suppressed (18). It is conceivable that this may be due to elevated levels of apoptosis. As a result, disruption of mutant K-ras may simultaneously compromise cellular growth, survival, and angiogenesis-inductive properties. Restoration of VEGF production, however, would have no effect on reversing the intrinsic growth and survival defects in such cells.
Our results may have some bearing on the explanation of why therapeutic agents that putatively block Ras oncoprotein function appear to exert potent anti-tumor cytotoxic-like effects in vivo (32, 33) but not in monolayer cell culture (34, 35). Such drugs (e.g., Ras farnesyltransferase inhibitors) could function in vivo, in part, by suppressing angiogenesis as a consequence of down-regulation of factors such as VEGF (11). However, this would have no antitumor consequence in cell culture where angiogenesis is clearly neither required nor expressed. A cytotoxic effect in vivo could occur secondarily to an antiangiogenic effect of the drug treatment. For example, suppression of VEGF can lead to regression of established but newly formed (immature) blood vessels in the eye or in tumors (24, 36) and such an effect can lead to tumor regression after ischemic tumor cell death (36). Tumor cell death could also conceivably occur in a direct fashion as a result of compromising the survival function of the mutant ras oncogene in tumor cells growing in a three-dimensional multicellular context (30, 31). Indeed, evidence for a strong and direct tumor cell pro-apoptotic function of Ras inhibitors in vivo in mice bearing solid tumors was recently reported by Barrington et al. (37).
Although our experimental analysis has focused on mutant ras oncogenes, we have noted that other dominantly acting (proto)-oncogenic changes, including mutant erbB2/neu/HER-2, overexpressed normal erbB2/neu/HER-2, and overexpressed EGF receptors can induce, or up-regulate, VEGF in a manner similar to mutant ras (9). Treatment of appropriate human target tumor cells with neutralizing monoclonal antibodies to EGF receptor or erbB2/neu/HER-2 can result in down-regulation of VEGF, both in vitro and in vivo (9). Thus a generic function of oncogenes may be to help facilitate tumor angiogenesis. If so, some kind of tumor angiogenesis inhibitory effect may ensue as a consequence of therapy in vivo with agents that block aberrant signal transduction pathways mediated by mutant oncogenes or overexpressed protooncogenes.
Acknowledgments
The excellent secretarial assistance of Cassandra Cheng, Lynda Woodcock, and Frances Hogue is gratefully acknowledged. This work was supported by grants to R.S.K. from the Medical Research Council of Canada and the National Institutes of Health (CA-41223). R.S.K. is a Terry Fox Scientist of the National Cancer Institute of Canada. F.O. was supported in part by a Sunnybrook Trust fellowship. B.L. was supported by an award from the French National Institute of Health and Medical Research.
Footnotes
This paper was submitted directly (Track II) to the Proceedings Office.
Abbreviations: VEGF, vascular endothelial growth factor; VPF, vascular permeability factor; bFGF, basic fibroblast growth factor; TGF, transforming growth factor; EGF, epidermal growth factor.
References
- 1.Folkman J. N Engl J Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Folkman J. Cell. 1996;86:353–364. doi: 10.1016/s0092-8674(00)80108-7. [DOI] [PubMed] [Google Scholar]
- 3.Bouck N, Stellmach V, Hsu S C. Adv Cancer Res. 1996;69:135–174. doi: 10.1016/s0065-230x(08)60862-3. [DOI] [PubMed] [Google Scholar]
- 4.Fearon E R, Vogelstein B. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- 5.Bouck N. Cancer Cells. 1990;2:179–185. [PubMed] [Google Scholar]
- 6.Volpert O V, Dameron K M, Bouck N. Oncogene. 1997;14:1495–1502. doi: 10.1038/sj.onc.1200977. [DOI] [PubMed] [Google Scholar]
- 7.Rak J, Filmus J, Finkenzeller G, Grugel S, Marme D, Kerbel R S. Cancer Metastasis Rev. 1995;14:263–277. doi: 10.1007/BF00690598. [DOI] [PubMed] [Google Scholar]
- 8.Mazure N M, Chen E Y, Yeh P, Laderoute K R, Giaccia A J. Cancer Res. 1996;56:3436–3440. [PubMed] [Google Scholar]
- 9.Viloria-Petit A M, Rak J, Hung M-C, Rockwell P, Goldstein N, Kerbel R S. Am J Pathol. 1997;151:1523–1530. [PMC free article] [PubMed] [Google Scholar]
- 10.Mukhopadhyay D, Tsiokas L, Zhou X-M, Foster D, Brugge J S, Sukhatme V P. Nature (London) 1995;375:577–581. doi: 10.1038/375577a0. [DOI] [PubMed] [Google Scholar]
- 11.Rak J, Mitsuhashi Y, Bayko L, Filmus J, Sasazuki T, Kerbel R S. Cancer Res. 1995;55:4575–4580. [PubMed] [Google Scholar]
- 12.Risau W. Cancer Metastasis Rev. 1996;15:149–151. doi: 10.1007/BF00437466. [DOI] [PubMed] [Google Scholar]
- 13.Dvorak H F, Brown L F, Detmar M, Dvorak A M. Am J Pathol. 1995;146:1029–1039. [PMC free article] [PubMed] [Google Scholar]
- 14.Grugel S, Finkenzeller G, Weindel K, Barleon B, Marme D. J Biol Chem. 1995;270:25915–25919. doi: 10.1074/jbc.270.43.25915. [DOI] [PubMed] [Google Scholar]
- 15.Arbiser J L, Moses M A, Fernandez C A, Ghiso N, Cao Y, Klauber N, Frank D, Brownlee M, Flynn E, Parangi S, et al. Proc Natl Acad Sci USA. 1997;94:861–866. doi: 10.1073/pnas.94.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papkoff J. J Biol Chem. 1997;272:4536–4543. [PubMed] [Google Scholar]
- 17.Larcher F, Robles A I, Duran H, Murillas R, Quintanilla M, Cano A, Conti C J, Jorcano J L. Cancer Res. 1996;56:5391–5396. [PubMed] [Google Scholar]
- 18.Shirasawa S, Furuse M, Yokoyama N, Sasazuki T. Science. 1993;260:85–88. doi: 10.1126/science.8465203. [DOI] [PubMed] [Google Scholar]
- 19.Zhang H-T, Craft P, Scott P A E, Ziche M, Weich H A, Harris A L, Bicknell R. J Natl Cancer Inst. 1995;87:213–219. doi: 10.1093/jnci/87.3.213. [DOI] [PubMed] [Google Scholar]
- 20.Minchenko A, Salceda S, Bauer T, Caro J. Cell Mol Biol Res. 1994;40:35–39. [PubMed] [Google Scholar]
- 21.Shima D T, Deutsch U, D’Amore P A. FEBS Lett. 1995;370:203–208. doi: 10.1016/0014-5793(95)00831-s. [DOI] [PubMed] [Google Scholar]
- 22.Kandel J, Bossy-Wetzel E, Radvanyi F, Klagsbrun M, Folkman J, Hanahan D. Cell. 1991;66:1095–1104. doi: 10.1016/0092-8674(91)90033-u. [DOI] [PubMed] [Google Scholar]
- 23.Saleh M, Stacker S A, Wilks A F. Cancer Res. 1996;56:393–401. [PubMed] [Google Scholar]
- 24.Alon T, Hemo I, Itin A, Pe’er J, Stone J, Keshet E. Nat Med. 1995;1:1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- 25.Gitay-Goren H, Halaban R, Neufeld G. Biochem Biophys Res Commun. 1993;190:702–709. doi: 10.1006/bbrc.1993.1106. [DOI] [PubMed] [Google Scholar]
- 26.Cheng S Y, Huang H J, Nagane M, Ji X D, Wang D, Shih C C, Arap W, Huang C M, Cavenee W K. Proc Natl Acad Sci USA. 1996;93:8502–8507. doi: 10.1073/pnas.93.16.8502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrara N. Breast Cancer Res Treat. 1995;36:127–137. doi: 10.1007/BF00666035. [DOI] [PubMed] [Google Scholar]
- 28.Buick R N, Filmus J, Quaroni A. Exp Cell Res. 1987;170:300–309. doi: 10.1016/0014-4827(87)90308-9. [DOI] [PubMed] [Google Scholar]
- 29.Filmus J, Robles A I, Shi W, Wong M J, Colombo L L, Conti C J. Oncogene. 1994;9:3627–3633. [PubMed] [Google Scholar]
- 30.Lebowitz P, Sakamuro D, Prendergast G C. Cancer Res. 1997;57:708–713. [PubMed] [Google Scholar]
- 31.Rak J, Mitsuhashi Y, Erdos V, Huang S-N, Filmus J, Kerbel R S. J Cell Biol. 1995;131:1587–1598. doi: 10.1083/jcb.131.6.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kohl N E, Wilson F R, Mossier S D, Giuliani E, deSolms S J, Conner M W, Anthony N J, Holtz W J, Gomez R P, Lee T-J, et al. Proc Natl Acad Sci USA. 1994;91:9141–9145. doi: 10.1073/pnas.91.19.9141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kohl N E, Omer C A, Conner M W, Anthony N J, Davide J P, deSolms S J, Giuliani E A, Gomez R P, Graham S L, Hamilton K, et al. Nat Med. 1995;1:792–797. doi: 10.1038/nm0895-792. [DOI] [PubMed] [Google Scholar]
- 34.Kohl N E, Mosser S D, deSolms S J, Giuliani E A, Pompliano D L, Graham S L, Smith R L, Scolnick E M, Oliff A, Gibbs J B. Science. 1993;260:1934–1942. doi: 10.1126/science.8316833. [DOI] [PubMed] [Google Scholar]
- 35.James G L, Golstein J L, Brown M S, Rawson T E, Somers T C, McDowell R S, Crowley C W, Lucas B K, Levinson A D, Marsters J J C. Science. 1993;260:1937–1942. doi: 10.1126/science.8316834. [DOI] [PubMed] [Google Scholar]
- 36.Benjamin L E, Keshet E. Proc Natl Acad Sci USA. 1997;94:8761–8766. doi: 10.1073/pnas.94.16.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barrington R E, Subler M A, Rands E, Omer C A, Miller P J, Hundley J E, Koester S K, Troyer D A, Bearss D J, Conner M W. Mol Cell Biol. 1998;18:85–92. doi: 10.1128/mcb.18.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]