Summary
In this short communication, we introduce a-cyclodextrin as a new probe to study mechanisms of adhesive interactions. We show that this cyclic polysaccharide, that consists of 6 glucosyl residues linked by alpha-1, 4 bonds, was the only sugar of 22 tested that dramatically blocked a specific cellular interaction in the sea urchin embryo (p < 0.001 compared with non-sugar controls). 150–400 embryos were sampled for each concentration of each sugar tested. Mechanisms of cellular interactions have been studied for almost a century and they still remain poorly understood. Cyclodextrin is an exciting new tool that can be utilized for investigating these mechanisms.
Keywords: Alpha cyclodextrin, Sea urchin adhesive interactions
Introduction
Cell surface carbohydrates and complementary carbohydrate binding proteins may be involved in sea urchin gastrulation (Blackburn and Schnaar, 1983). Tunicamycin is an antibiotic that inhibits the first step in the synthesis of the lipid-linked oligosaccharides that function as intermediates in biosynthesis of N-linked glycoproteins (Heifetz and Lennarz, 1979). Tunicamycin-induced effects on intact cells and organisms have been attributed to changes in protein glycosylation (Guarnaccia et al., 1983) and tunicamycin has been reported to cause marked changes in morphology of cells cultured in vitro, alterations in cell recognition and adhesion, inhibition of cell differentiation, and decreases in cell-surface receptor binding (Guarnaccia et al., 1983). Tunicamycin blocked sea urchin embryogenesis at gastrulation as well as several later stages of development (Heifetz and Lennarz, 1979). Specific lectins have been shown to enter the blastocoel of the sea urchin embryo and bind to specific cell types (Latham, et al., 1998). Furthermore Lens culinaris (a glucose/mannose binding lectin) agglutinin has been found to bind to secondary mesenchyme cells on the archenteron tip and blastocoel roof components, possibly blocking the attachment of filopodial extensions from the secondary mesenchyme cells to the blastocoel roof, resulting in exogastrulation (Latham, et al., 1998; Latham, et al., 1999). These results suggest that glucose/mannose containing ligands and receptors for these ligands may mediate the attachment of secondary mesenchyme cells at the tip of the archenteron to the blastocoel roof.
We have previously found that inhibitors of glycoprotein/proteoglycan synthesis, tunicamycin and sodium selenate and specific glycosidases, inhibited archenteron organization, elongation, and attachment to the blastocoel roof in viable swimming sea urchin embryos (Khurrum, et al., 2004). Of the four types of biological molecules (proteins, lipids, carbohydrates and nucleic acids), carbohydrates are the least studied even though they are the most prominent cell surface exposed structures (Hardin, 1994). Here we continue our study on the possible role of sugars and sugar receptors in mediating the adhesion between the tip of the archenteron and blastocoel roof, a model cellular interaction in a NIH designated model organism that may offer insight into mechanism of adhesive control in higher organisms (Sea Urchin Genome Sequence Consortium, 2006; Davidson and Cameron, 2002; Davidson, 2006; Whittaker et al., 2006). If sugar binding receptors are involved in archenteron/ blastocoel roof attachment then free sugars might block binding of the archenteron to the blastocoel roof. We know that such molecules can enter intact, living embryos (Latham, et al., 1998). In this study 22 different free sugars were tested for their possible effects on gastrulation of sea urchin embryos. The sugars ranged from monosaccharides to trisaccharides and cyclic polysaccharide.
We show that only one sugar, the cyclic polysaccharide a-cyclodextrin that consists of 6 glucosyl residues linked by alpha-1, 4 bonds blocked the cellular interaction under study. This is a short communication that introduces a-cyclodextrin as a new probe for studying mechanisms of adhesive interactions. Its use could help identify the active sites that control adhesive interactions in a variety of systems.
Material and Methods
Artificial sea water (ASW)
ASW was prepared using the Bidwell and Spotte (1985) formula; pH was adjusted to 8.0.
Sugars/ Sigma catalog numbers
The following sugars were purchased from Sigma (St. Louis, MO): D (+) Xylose (X-3877), L(−) Xylose (X-1625), D (−)Arabinose (A-6085), L (+) Arabinose (A-3256), Rhamnose (R-3875), D-Ribose (R-7500), Galactose (G-0750), D-Fructose (F-0127), Glucose (G-1894), Maltose (M-5885), D(+) Trehalose (T-5251), Melibiose (M-5500), β-lactose (L- 3750), Cellobiose (C-7252), Melezitose (M-5375), Maltotriose (M-8378), D(+)Raffinose (R-0514), a-Cylcodextrin (C-4642), Mannopheptose (M-6909), Glucosamine (G- 4875), Galactosamine (G-0500), Sucrose (S-7903). Many of these sugars are not normally occurring in animals but their structures could tell us a great deal about adhesive interactions if used in a system such as this one, just as such sugars have been used in many studies examining lectin binding affinities.
Photographs
A Carl Zeiss MC80 DX photomicroscope (Oberkochen, Germany) was used to take photographs of the embryos using black and white Kodak T-Max 400, 24 exposure or 36 exposure films. Film was developed and the negatives were placed on CDs at the Darkroom photo lab, Northridge, CA.
Organisms/ sea urchins
Sea urchins used in these experiments were Strongylocentrotus purpuratus and Lytechinus pictus, purchased from Marinus Scientific, Garden Grove, CA. The sea urchins were maintained in refrigerated aquaria.
Gametes and fertilization
Gametes of Strongylocentrus purpuratus or Lytechinus pictus were obtained by intracoelomic injection of 3 ml of 0.55M KCl around the mouth area. Semen was collected dry directly from the surface of the male sea urchin by using a 200 μl pipette, placed into separate 1.5ml microcentrifuge tubes and stored on ice. Only viable, moving sperm were used in the experiments. The female sea urchins were placed on 50 ml beakers, filled with ASW and allowed to release their eggs. A small sample of eggs from each female was placed on a slide and observed with a Zeiss Axiolab microscope. Eggs were considered normal if they were round and revealed a pronucleus. Eggs that were oval or lysed were not used. The ASW in each beaker was aspirated and fresh ASW was added for a total of three times. At the last wash all the eggs were transferred to a 500 ml beaker and kept on ice. 3 ml of freshly diluted sperm (1.5ml concentrated sperm/5ml ASW) were added to 5ml of eggs suspended in 250ml of ASW in a 500 ml beaker. Once all the eggs were fertilized, ASW was aspirated off; the embryos were washed with fresh ASW twice. Embryos were transferred to a Pyrex tray containing 1 liter of ASW and incubated at 15°C for 24 hours.
Sugar solutions
Sugar solutions were prepared at 0.1 M, 0.2 M, 0.5 M, concentrations. As many sugars used at 0.5 M concentrations allowed development of normal swimming embryos, it is unlikely that sugar osmolarity played an important role in this study. The sugars were kept at 4°C and used within a week often on the same day. There was no difference in the effects of sugars used on the same day as they were prepared or used within a week of their preparation. Some sugars at the above concentrations were toxic and killed the embryos. These sugars were tested at 0.01 M, 0.02 M and 0.05M concentrations.
Embryo microassy
The blastula stage embryos (24 hours old) in the Pyrex trays were collected and transferred to five Petri dishes. About 15ml of embryos were transferred to each Petri dish using a wide-mouthed pipette. The Petri dishes were observed under a microscope. If dead embryos or unfertilized eggs were observed, those samples were not used in experiments. Only hatched viable swimming embryos were used. For each experiment, one 96 well microplate was prepared for each sugar tested and controls without added sugars. Each experiment was repeated a minimum of three times for each species of sea urchin. Using a wide mouth pipette, the 24 hour hatched blastula embryos were transferred to the microplates along with the sugar solutions (in ASW) or ASW without sugars to a total volume of 100 μl per well. 10 replicate wells for each sugar were tested in each microplate. The microplates were incubated for 24 hours in a 15 °C incubator. On the average there were approximately 8–12 embryos in each well. The microplate with the 48 hour gastrulated embryos were first observed under a microscope to see if embryos were viable and to spot any morphological deformities caused by sugars tested. Then the 48 hour embryos in the microplates were fixed with 6 μl of 10% formaldehyde in each well. The fixed embryos were counted, morphologies were recorded and the embryos were photographed. The morphological characteristics examined and recorded for the 48 hour embryos were: complete archenteron, unattached archenteron, exogastrulation and dead embryos. Although standard histochemical stains are not used, this paper focuses on cellular imaging and structural biochemistry.
Statistical analysis
All embryos from at least the three trials were added together and referred to as total sample size. 150–400 embryos were sampled for each concentration of each sugar tested. The percentages of each morphology were calculated. Standard deviation (SD) and standard error of the mean (SEM) for each concentration of each sugar were calculated. Graphpad software (San Diego, CA) and unpaired t-test were used in the analysis of significance. In all cases, where differences were observed, the differences between control and sugar samples were statistically significant (p < 0.001).
Results
With about 150–400 embryos sampled for each concentration of each sugar tested (listed in Material and Methods) all of the monosaccharides, disaccharides and trisaccharides had little or no effect on archenteron morphology or attachment. In all cases, at all sugar concentrations tested that did not kill the embryos, listed in the Material and Methods, the embryos were similar to control embryos without sugar exhibiting complete, attached archenterons (Fig. 1, 2). The only sugar that induced a dramatically different effect was a-cyclodextrin (0.01 M), a polymer consisting of 6 glucose residues linked by alpha 1, 4 bonds. The effects for a-cyclodextrin were nearly identical in both species with about 70% of the embryos displaying unattached archenterons (Fig. 1, 2). These embryos were viable and swimming.
Figure 1.
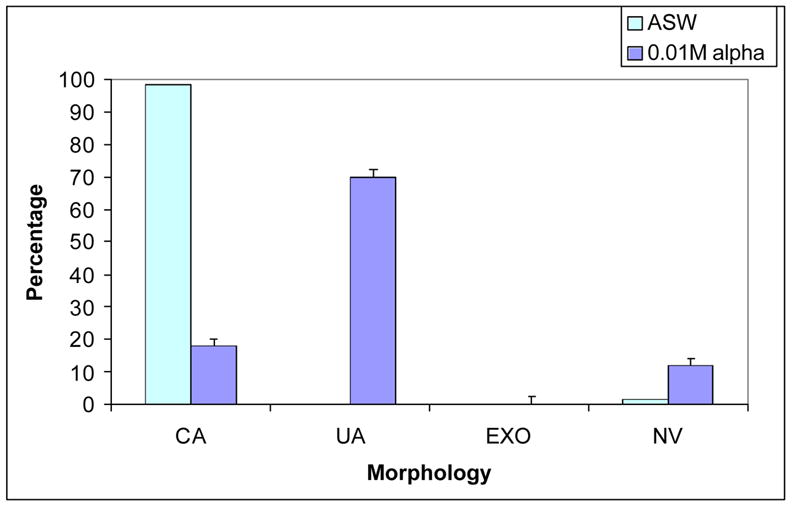
Effects of a-cyclodextrin (0.01 M) compared with ASW on 48 hr S.purpuratus embryos (top) and L.pictus embryos (bottom). Data are plotted as mean percentages of embryos exhibiting complete archenteron (CA), unattached archenteron (UA), exogastrulation (EXO) or not viable (NV) from 3 experiments in which over 200 embryos were assessed for each species. Unpaired t-test was used to compare mean percentages of a-cyclodextrin and ASW, P < 0.001. Error bars are Standard Error of the Mean.
Figure 2.
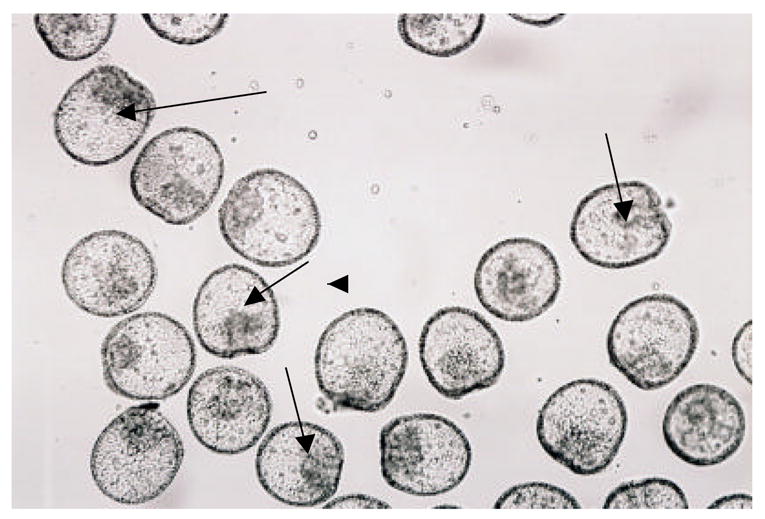
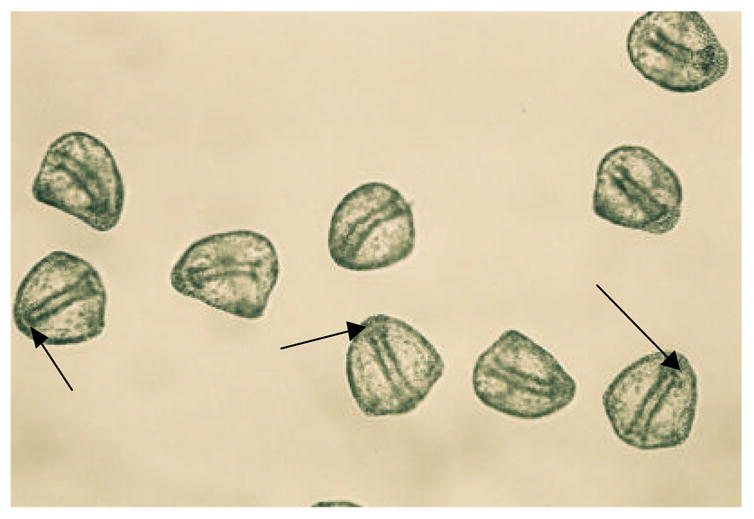
Photos of a-cyclodextrin (0.01 M) treated 48 hr L.pictus embryos (top) and 0.05M glucosamine treated 48 hr L.pictus embryos (bottom). The bottom photo is an example of all other sugar samples at all concentrations tested, and control ASW experiments where embryos were viable that generally appeared identical to the embryos in the bottom photo. Arrows in the top photo show unattached archenterons and in the bottom photo, attached archenterons. Magnification 100X.
Figure 1 provides a graphic representation of the results for a-cyclodextrin (0.01 M) compared with controls for both species of sea urchin. Both species displayed, nearly identical morphologies in the presence of a-cyclodextrin showing mostly unattached archenterons, a comparably small percentage of complete, attached archenterons and no exogastrulated archenterons. The results compared with controls were highly significant (p< 0.001).
Figure 2 provides photographs of a representative sample exhibiting complete, attached archenterons (similar for ASW and all sugars tested except for a-cyclodextrin) and a sample in a-cyclodextrin (0.01 M) exhibiting shortened or disorganized unattached archenterons. As can be seen in figure 2, embryos in a-cyclodextrin were very consistent in their appearance, showing almost identical morphologies to those found in embryos treated with glycosylation inhibitors or glucose polysaccharide degrading enzymes (Khurrum, et al., 2004).
Discussion
Past studies using the sea urchin model (Sea Urchin Genome Sequence Consortium, 2006; Davidson and Cameron, 2002; Davidson, 2006; Whittaker, et al., 2006) indicated that inhibitors of the synthesis of N-glycosidically linked glycoproteins or polysaccharide chain elongation (tunicamycin and sodium selenate respectively) and specific glycosidases arrest sea urchin embryos in the gastrula stage and inhibit archenteron organization, elongation and attachment to the blastocoel roof (Schneider et al., 1978; Kinoshita and Saiga, 1979; Heifetz and Lennarz, 1979; Akasaka et al., 1980; Khurrum et al., 2004). Single sea urchin gastrula cells bind to glucose/mannose-binding Lens culinaris and concanavalin A derivatized beads (Khurrum et al., 2004) and Lens culinaris binds to the surface of the archenteron and blastocoel roof causing exogastrulation, where the archenteron fails to attach to the blastocoel roof and everts out of the embryo proper ( Latham et al., 1998; Latham et al., 1999).
Here we show that of the 22 sugars tested only the 6-glucose polymer a-cyclodextrin (Garcia-Lopez, et al., 1999; Szejtli, et al., 1986) blocked archenteron elongation/ attachment to the blastocoel roof. If glucose like residues are involved in this cellular interaction, then why didn’t the other glucose containing saccharides tested here also block this interaction? It is well known that longer chain saccharides are often required to block interactions involving sugars and sugar binding receptors (Cambry et al., 2006) because the in vivo binding reaction often involves complex saccharides that bind strongly to receptors. Simple monosaccharides, disaccharides or even trisaccharides may not effectively compete with the in vivo cellular interaction.
This short communication introduces alpha cyclodextrin as a new probe for studying adhesive mechanisms. It is not intended to definitively identify how this molecule is acting in this system. We feel, however, that by introducing this probe here, many laboratories could use it in studies that might identify binding partners that will help elucidate mechanisms of adhesive interactions that have been notoriously refractory to study. Based on the literature, there are two possible natural binding partners for cyclodextrin: lectin-like glucose binding receptors (Garcia-Lopez, etal.1999) or phospholipids (Szejtli, et al., 1986). When the binding partners are identified, a major advance might be made in improved understanding of the molecular basis of cellular adhesion.
Acknowledgments
This work was supported by NIH NIGMS SCORE (S0648680), NIH MBRS RISE, NIH MARC and the Joseph Drown Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akasaka K, Amemiya S, Terayama H. Scanning electron microscopical study of the inside of sea urchin (Pseudocentrotus depressus): Effects of aryl beta-xyloside, tunicamycin, and deprivation of sulfate ions. Exp Cell Res. 1980;129:1–13. doi: 10.1016/0014-4827(80)90325-0. [DOI] [PubMed] [Google Scholar]
- Bidwell JP, Spotte S. Artificial Seawaters, Formulas and Methods. Jones and Bartlett Publishers, Inc.; Boston: 1985. p. 256. [Google Scholar]
- Blackburn C, Schnaar R. Carbohydrate-specific cell adhesion is mediated by immobilized glycolipids. The Journal of Biological Chemistry. 1983;258:1180–1188. [PubMed] [Google Scholar]
- Cambry I, Le Mercier M, Lefranc F, Kiss R. Galactin-1: a small protein with major functions. Glycobiology. 2006;16:137R–157R. doi: 10.1093/glycob/cwl025. [DOI] [PubMed] [Google Scholar]
- Davidson EH. The sea urchin genome: where will it lead us? Science. 2006;314:939–940. doi: 10.1126/science.1136252. [DOI] [PubMed] [Google Scholar]
- Davidson EH, Cameron RA. Arguments for sequencing the genome of the sea urchin Stronglycentrous purpuratus. www.genome.gov/pages/research/sequencing/SegProposals/SeaUrchin_Genome.prob.2002.
- Garcia-Lopez JJ, Hernandez-Mates F, Isar-Garcia J, Kim JM, Roy R, Santogo-Gonzales F, Vargas-Bernguel A. Synthesis of per-glycosylated β cyclodextrins having enhanced lectin binding affinity. J Org Chem. 1999;64:522–531. [Google Scholar]
- Guarnaccia S, Shaper J, Schnaar R. Tunicamycin inhibits ganglioside biosynthesis in neuronal cells. Proc Natl Acd Sci. 1983;80:1551–1555. doi: 10.1073/pnas.80.6.1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardin J. Local cell interactions and the control of gastrulation in the sea urchin embryo. Seminars in Developmental Biology. 1994;5:77–84. [Google Scholar]
- Heifetz A, Lennarz WJ. Biosynthesis of N-Glycosidically linked glycoproteins during gastrulation of sea urchin embryos. The Journal of Biological Chemistry. 1979;254:6119–27. [PubMed] [Google Scholar]
- Khurrum M, Hernandez A, Eskalaei M, Badali O, Coyle-Thompson C, Oppenheimer SB. Carbohydrate involvement in cellular interactions in sea urchin gastrulation. Acta Histochem. 2004;106:97–106. doi: 10.1016/j.acthis.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Kinoshita S, Saiga H. The role of proteoglycans in the development of sea urchins. I: abnormal development of sea urchins caused by the disturbance of proteoglycan synthesis. Exp Cell Res. 1979;123:229–36. doi: 10.1016/0014-4827(79)90463-4. [DOI] [PubMed] [Google Scholar]
- Latham V, Martinez A, Cazares L, Hamburger H, Tully MJ, Oppenheimer SB. Accessing the embryo interior without microinjection. Acta Histochem. 1998;100:193–200. doi: 10.1016/S0065-1281(98)80027-5. [DOI] [PubMed] [Google Scholar]
- Latham V, Tully M, Oppenheimer SB. A putative role for carbohydrates in sea urchin gastrulation. Acta Histochem. 1999;101:293–303. doi: 10.1016/S0065-1281(99)80030-0. [DOI] [PubMed] [Google Scholar]
- Schneider E, Nguyen N, Lennarz W. The effects of tunicamycin, an inhibitor of protein glycosylation, on embryonic development in the sea urchin embryo. J Biol Chem. 1978;253:2348–2355. [PubMed] [Google Scholar]
- Sea Urchin Genome Sequencing Consortium. The genome of the sea urchin Strongylocentrotus purpuratus. Science. 2006;314:941–952. doi: 10.1126/science.1133609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szejtli J, Csherhati T, Szogyi M. Interactions between cyclodextrins and cell membrane phospholipids. Carbohydr polymers. 1986;6:35–49. [Google Scholar]
- Whittaker CA, Bergeron CF, Whittle J, Brandhorst BP, Burke RD, Hynes RO. The echinoderm adhesome. Dev Biol. 2006 doi: 10.1016/j.ydbio.2006.07.044. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]


