Abstract
Background
Glycogen synthase kinase 3 (GSK3) encodes a serine/threonine protein kinase, is known to play roles in many biological processes. Two closely related GSK3 isoforms encoded by distinct genes: GSK3α (51 kDa) and GSK3β (47 kDa). In previously studies, most GSK3 inhibitors are not only inhibiting GSK3, but are also affecting many other kinases. In addition, because of highly similarity in amino acid sequence between GSK3α and GSK3β, making it difficult to identify an inhibitor that can be selective against GSK3α or GSK3β. Thus, it is relatively difficult to address the functions of GSK3 isoforms during embryogenesis. At this study, we attempt to specifically inhibit either GSK3α or GSK3β and uncover the isoform-specific roles that GSK3 plays during cardiogenesis.
Results
We blocked gsk3α and gsk3β translations by injection of morpholino antisense oligonucleotides (MO). Both gsk3α- and gsk3β-MO-injected embryos displayed similar morphological defects, with a thin, string-like shaped heart and pericardial edema at 72 hours post-fertilization. However, when detailed analysis of the gsk3α- and gsk3β-MO-induced heart defects, we found that the reduced number of cardiomyocytes in gsk3α morphants during the heart-ring stage was due to apoptosis. On the contrary, gsk3β morphants did not exhibit significant apoptosis in the cardiomyocytes, and the heart developed normally during the heart-ring stage. Later, however, the heart positioning was severely disrupted in gsk3β morphants. bmp4 expression in gsk3β morphants was up-regulated and disrupted the asymmetry pattern in the heart. The cardiac valve defects in gsk3β morphants were similar to those observed in axin1 and apcmcr mutants, suggesting that GSK3β might play a role in cardiac valve development through the Wnt/β-catenin pathway. Finally, the phenotypes of gsk3α mutant embryos cannot be rescued by gsk3β mRNA, and vice versa, demonstrating that GSK3α and GSK3β are not functionally redundant.
Conclusion
We conclude that (1) GSK3α, but not GSK3β, is necessary in cardiomyocyte survival; (2) the GSK3β plays important roles in modulating the left-right asymmetry and affecting heart positioning; and (3) GSK3α and GSK3β play distinct roles during zebrafish cardiogenesis.
Background
Glycogen synthase kinase 3 (GSK3) encodes a multifunctional serine/threonine protein kinase, which is ubiquitously expressed in organisms ranging from yeasts to mammals [1-3]. GSK3 is, therefore, very important in the cellular signaling network. In addition to playing pivotal roles in the canonical Wnt and PI3K-PKB/AKT pathways, it has been shown to phosphorylate glycogen synthase, eLF2B, NFAT, c-jun, CyclinD1, NF-kB, as well as many others [4]. GSK3 is involved in many biological processes, including cell survival, tumorigenesis, and developmental patterning.
There are two closely related GSK3 isoforms encoded by distinct genes: GSK3α (51 kDa) and GSK3β (47 kDa) [5]. The difference in size is due to a glycine-rich extension at the N-terminus of GSK3α. GSK3α and GSK3β are highly homologous within their kinase domains [6]. Homologues of GSK3 isoforms from species as distant from each other as flies, zebrafishes and humans display over 90% sequence similarity within the kinase domain [7,8].
Despite that GSK3α and GSK3β share common substrates, their expression patterns, substrate preferences, regulation, and cellular functions are not identical [1,6,9,10]. In vitro study reveals that GSK3α and GSK3β are inactivated by phosphorylation of a specific N-terminal serine residue (Ser-21 in GSK3α; Ser-9 in GSK3β) catalyzed by either MAPKAP kinase-1/or p70S6K [11,12], whereas protein kinase C phosphorylates and partially inhibits GSK3β, but not GSK3α [13]. In humans, only GSK3α is deactivated by insulin during physiological conditions [14,15], whereas supraphysiological insulin injection in the rat leads to deactivation of both GSK3α and GSK3β [15,16]. Although differential regulations by the two isoforms of GSK3 were proposed, the exact roles of GSK3α and GSK3β and endogenous targets of such regulation remain to be investigated.
Several groups have identified small-molecule GSK3 inhibitors [17,18]. Most drugs bind to the ATP pocket of GSK3 and compete with ATP. However, these inhibitors are not only inhibiting GSK3, but are also affecting CDK kinase (2 and 5) and many other kinases. In addition, there appears to be only a single amino acid difference (Glu196 in GSK3α, Asp133 in GSK3β), making it difficult to identify an inhibitor that can be selective against GSK3α or GSK3β [19]. This finding is why it is difficult to analyze the exact roles of GSK3α and GSK3β in vitro and in vivo.
Recent years, numerous studies indicate that GSK3 negatively regulates cardiac hypertrophy [20-22]. Despite that GSK3β functions as a negative regulator of cardiac hypertrophy, GSK3 also plays an important role in regulating cardiac development. Transgenic mice over-expressing GSK3β in the heart have impairments of postnatal cardiomyocyte growth and abnormal cardiac contractile function [23]. In Xenopus, injection of gsk3β mRNA in embryos induces expression of Nkx2.5 and Tbx5 [24]. Oral treatment with lithium, a mood-stabilizing drug that is inhibitory for GSK3, in pregnant women showed a higher incidence of congenital heart defects in babies [25-27]. These findings prove that GSK3 might be involved in heart development. Unfortunately, disruption of the gsk3β gene in mice results in embryonic lethality caused by severe liver degeneration [9], and no report is available to demonstrate that cardiac defects are happened in GSK3β mutants. Thus, whether the roles of GSK3α and GSK3β in different species are conserved remain to be investigated. Moreover, the roles of GSK3 in cardiac development are still unclear. Also, whether GSK3α and GSK3β play similar roles in heart development is ambiguous.
We have previously identified two zebrafish homologues related to mammalian GSK3: zebrafish GSK3α and GSK3β [8]. In this report, we have taken advantage of the zebrafish system to address the distinct roles of GSK3α and GSK3β during heart development of zebrafish. Our findings suggest that, in zebrafish, GSK3α, but not GSK3β, is necessary in cardiomyocyte survival; whereas the GSK3β isoform plays important roles during zebrafish cardiogenesis, modulating the left-right asymmetry and affecting heart positioning.
Results
Knockdown of gsk3α and gsk3β display similar heart defects in the embryos
To address the role of GSK3 during zebrafish cardiogenesis, we designed gsk3α- and gsk3β-MO for specifically inhibiting the translation of gsk3α and gsk3β, respectively. When the protein lysate was extracted from gsk3α – and gsk3β-MO-injected embryos at 24 hours postfertilization (hpf), Western blot analysis was performed by using isoform-specific antibodies. Results showed that the protein levels of GSK3α and GSK3β were largely reduced in the protein extracts from gsk3α – and gsk3β -morphants, respectively (Fig. 1), suggesting that the MOs we designed in this study were isoform-specific.
Figure 1.

Injection of translation inhibitors gsk3α- and gsk3β -MO into embryos can specifically reduce the protein levels of GSK3α and GSK3β, respectively. The total protein lysate extracted from seven zebrafish embryos at 24 hpf was loaded on each lane and analyzed by western blot. The antibody used is indicated in the left of each blot. Anti-GSK3 antibody enables to recognize both GSK3α and GSK3β proteins; anti-α-tubulin antibody was used as a loading control. The protein levels of GSK3α and GSK3β were reduced greatly in the protein lysates extracted from the gsk3α – and gsk3β -MO-injected embryos, respectively.
Similar morphological defects of the heart were observed in gsk3α- and gsk3β-MO-injected zebrafish embryos at 72 hpf, such as a thin and string-like shape, pericardial edema, and blood pooling (Fig. 2F, G, I, J). These defects occurred initially in some 2 days postfertilization (dpf) morphants, and then were predominantly observed in most 3- and 4-dpf morphants. Although the heart defects were similar between gsk3α- and gsk3β-MO-injected zebrafish embryos, the defects of the gsk3α morphants were more severe than those of the gsk3β morphants (Fig. 2B, C, F vs. 2D, G). Around 40% of the gsk3α morphant defects were lethal due to an absent body axis during 24 hpf (Fig. 2B), and the remainder of the surviving gsk3α morphants suffered from an incomplete formation of axis (Fig. 2C), suggesting that gsk3α and gsk3β may function differently during cardiogenesis, although they cause similar heart defects. We also noticed that the percentage of heart abnormalities was dependent on the concentration of the injected gsk3α- and gsk3β-MO (Table 1). When 0.5 ng of gsk3α-MO was injected into 1-celled stage embryos, we found that 41.8% (n = 146) of embryos displayed a string-like-shape heart; whereas when 2 ng gsk3α-MO were injected, 88.2% (n = 212) of embryos appeared similar heart defect. Similarly, 2 ng gsk3β-MO caused 30.2% (n = 126) of embryos to suffer a string-like-shape heart at 72 hpf; whereas 6 ng gsk3β-MO caused 87.5% (n = 288) of embryos to have similar heart defect. These results indicated that the effect of gsk3-MO on embryogenesis was dosage-dependent and specific.
Figure 2.
The morphological defects in gsk3α and gsk3β morphants. Wild-type embryos (A, E, H), gsk3α (B, C, F, I), and gsk3β (D, G, J) morphants. The 24 hpf gsk3α morphants have mild (C) to severe (B) defects in axis formation. At 72 hpf, both gsk3α and gsk3β morphants displayed pericardial edema (F, G, I, J) and an unlooped, stretched heart (I, J).
Table 1.
Morphological phenotypes of zebrafish embryos derived from fertilized eggs injected with different materials
| Injected materials | Concentration | Number of embryos surviving among number of injected eggs | Wild-type phenotype | Abnormal Heart Development |
| gsk3α -MO | 0.5 ng | 146/155 (94.2%) | 85 (58.2%) | 61 (41.8%) |
| gsk3α -MO | 1 ng | 176/191 (92.1%) | 61 (34.6%) | 115 (65.4%) |
| gsk3α -MO | 2 ng | 212/273 (77.7%) | 25 (11.8%) | 187 (88.2%) |
| gsk3β -MO | 2 ng | 126/129 (97.7%) | 88 (69.8%) | 38 (30.2%) |
| gsk3β -MO | 4 ng | 137/144 (95.1%) | 69 (50.4%) | 68 (49.6%) |
| gsk3β -MO | 6 ng | 288/314 (91.7%) | 36 (12.5%) | 252 (87.5%) |
| axin1-MO | 6 ng | 151/164 (92.1%) | 27 (17.9%) | 124 (82.1%) |
| dsRed mRNA | 100 pg | 68/68 (98.8%) | 83 (100%) | 0 (0%) |
| Control MO | 2 ng | 105/108 (97.2%) | 101 (96.2%) | 4 (3.8%) |
| Control MO | 6 ng | 112/125 (89.6%) | 103 (93.6%) | 7 (6.4%) |
| gsk3α -mRNA_gsk3α -MO | 50 pg + 2 ng | 59/69 (85.5%) | 18 (30.5%) | 41 (69.5%) |
| gsk3α -mRNA_gsk3α -MO | 100 pg + 2 ng | 93/118 (78.8%) | 51 (54.8%) | 42 (45.2%) |
| gsk3β -mRNA_gsk3α -MO | 50 pg + 2 ng | 72/90 (80.0%) | 9 (12.5%) | 63 (87.5%) |
| gsk3β -mRNA_gsk3α -MO | 100 pg + 2 ng | 91/134 (67.2%) | 5 (5.5%) | 86 (94.5%) |
| gsk3β -mRNA_gsk3β -MO | 50 pg + 6 ng | 73/77 (94.8%) | 45 (61.6%) | 28 (38.4%) |
| gsk3β -mRNA_gsk3β -MO | 100 pg + 6 ng | 128/144 (89.0%) | 96 (75.0%) | 32 (25%) |
| gsk3α -mRNA_gsk3β -MO | 50 pg + 6 ng | 83/109 (76.1%) | 15 (18.1%) | 68 (81.9%) |
| gsk3α -mRNA_gsk3β -MO | 100 pg + 6 ng | 92/127 (72.4%) | 8 (8.7%) | 84 (91.3%) |
| dsRed-mRNA_gsk3α -MO | 100 pg + 2 ng | 83/96 (86.5%) | 7 (8.4%) | 76 (91.6%) |
| dsRed-mRNA_gsk3β -MO | 100 pg + 6 ng | 75/82 (91.4%) | 13 (17.3%) | 62 (82.7%) |
Fertilized eggs were injected at the 1-cell stage, and then gsk3α morphants were observed at 36 to 48 hpf; gsk3β morphants were observed the heart positioning at 24 to 36 hpf. Results are from three independent experiments. dsRed mRNA: served as a negative control.
Heart defects caused by gsk3α- and gsk3β-MO are induced differently
We investigated whether the MO-induced defects could be rescued by co-injecting synthetic gsk3α or gsk3β-mRNA with its corresponding MOs, and vice versa. Results showed that co-injection of gsk3α-MO with synthetic gsk3α-mRNA could effectively rescue the defects caused by the injection of gsk3α-MO alone (Table 1). Similarly, the gsk3β-MO-induced defects were rescued by injection of gsk3β-mRNA. However, the synthetic gsk3α-mRNA did not rescue the gsk3β-MO-induced phenotype, and vice versa (Table 1). This evidence clearly demonstrates that two isoforms of GSK3 are necessary for heart development, but the function of GSK3α and GSK3β is not redundant, suggesting that GSK3α and GSK3β play specific roles in cardiogenesis during zebrafish development.
We injected either gsk3α- or gsk3β-MO into embryos derived from the transgenic line Tg(cmlc2: gfp), in which the GFP is expressed specifically in heart, resulting in a good material to monitor cardiac development of zebrafish [29]. In the wild-type embryos, the heart precursor cells completed their in situ formation, elongated, and jogged to the left at 24 hpf; started looping at 30 hpf; and completed looping at 48 hpf [34]. However, we observed that heart development was retarded, failing to elongate at 24 hpf (Fig. 3B) and even ceasing at heart-cone stage without further morphogenesis to a heart tube at [30-36] hpf (Figs. 3E, H) in the gsk3α-MO-injected embryos. We observed defective hearts as stretched to a thin and string-like shape at 72 hpf (Fig. 3L). Nevertheless, unlike in gsk3α morphants, elongation of the heart tube in gsk3β morphants at 24 hpf was as normal as in wild-type zebrafish (Fig. 3C), but heart looping was incomplete from 30 to 36 hpf (Figs. 3F, I), resulting in a stretched heart at 72 hpf (Fig. 3M).
Figure 3.
The cardiac defects induced by the knockdown of zebrafish GSK3α and GSK3β. Anti-sense morpholino oligonucleotide (MO), which was designed to specifically inhibit the translation of either gsk3α-(gsk3α-MO) or gsk3β-mRNA (gsk3β-MO), was injected into one-celled stage embryos and the heart morphology was observed at the stage as indicated. The elongation of heart tube was normally developed at 24 hpf in the wild-type (A) and in the gsk3β morphants (C); whereas the heart of gsk3α morphant did not elongate to from a heart-tube (B). The wild-type (D) and gsk3β morphant's heart (F) developed normally at 30 hpf, but the heart of gsk3α morphant was still retardant development at 30 hpf (E), and even ceased at heart-cone stage at 36 hpf (F). Compared to the wild-type (G), however, the heart positioning was abnormally in the gsk3β morphant at 36 hpf (I, J). Eventually, both gsk3α and gsk3β morphants displayed an unlooped and stretched heart (L, M). The heart morphology of embryos injected with the control MO was also observed at 72 hpf (N). a: atrium; v: ventricle.
In addition, we have designed to an experiment for using a standard negative control morpolino (MO) injection: 5'-CCTCTTACCTCAGTTACAATTTATA-3' (Gene Tools, USA). This oligo has no target, no significant biological activity. After 2 and 6 ng of this control MO were injected, no any defects were observed at 24 hpf. The morphology and development of heart appeared normally (see Additional file 1 and Figure 3N). These results reveal that the defects induced by the gsk3α- and gsk3β-MO are specific in this study.
Effect of GSK3 on the number of cardiomyocytes is isoform-specific
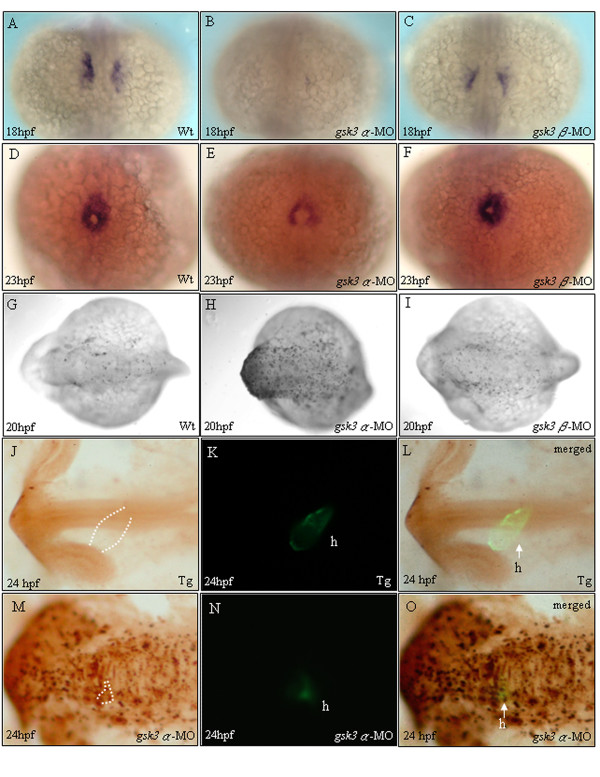
Compared to that of wild-type and gsk3β morphants, the GFP signals in cardiomyocytes of gsk3α morphants were greatly reduced (Fig. 3B). To investigate whether the reduced GSK3α level affects the cardiomyocyte number, we used a cardiomyocyte marker, cardiac myosin light chain 2 (cmlc2), to detect cells at heart-field and heart-cone stages. We found that the number of cmlc2-positive cells was greatly reduced in gsk3α morphants at both heart-field and heart-cone stages (Fig. 4B, E), indicating that the cardiomyocyte number was greatly reduced in the gsk3α morphants. These results suggest that the retarded heart development in gsk3α morphants is due to the decreased number of cardiomyocytes during early cardiogenesis. In contrast, gsk3β morphants displayed normal cmlc2 staining (Fig. 4C, F), indicating that cardiomyocyte number remains unchanged in gsk3β morphants. These results also clearly demonstrate that GSK3α and GSK3β play distinct roles during cardiogenesis.
Figure 4.
The heart defects in gsk3α morphants weredue to a reduced cardiomyocyte population size. Dorsal (A-I) and lateral (J-O) views of embryos stained by in situ hybridization (A-F) and TUNEL assay (G-L). Whole mount in situ hybridization staining with cmlc2 at 18 and 23 hpf received that gsk3α-MO causes a repressive influence on cardiomyocyte formation (B, E). The heart defect in gsk3α morphants was due to the reduction of cardiomyocyte population size. However, gsk3β morphants display normal cardiomyocyte formation (C, F) compared to wild-type embryos (A, D). TUNEL labeling was evident throughout the head of gsk3a-MO-injected embryos (H), especially in the head, but was limited in the head of controls (G) and gsk3β morphants (I). Compared to embryos derived from the transgenic line, Tg(cmlc2:EGFP), which has heart-specific GFP (K), we observed that the heart of gsk3α morphant did not elongate to form a heart-tube and the GFP signal was very faint at 24 hpf (N). Panels L and O are the merged images from J and K, and M and N, respectively. The apoptotic signals were co-localized with the heart-specific GFP signal, indicating that the reduced cardiomyocyte numbers was due to apoptosis in heart (O). h: heart.
Apoptosis occurs in the head of gsk3α knockdown embryos
The pronounced degeneration in the head of gsk3α morphants at [18-30] hpf were also observed (Fig. 2C). To confirm whether the reduced cardiomyocyte number in gsk3α morphants was due to apoptosis, the embryos were analyzed by Terminal deoxynucleotidyl Transferase Biotin-dUTP Nick End Labeling (TUNEL) assay after MO injection. In wild-type embryos at 20 hpf, apoptosis was low (Fig. 4G). However, in gsk3α morphants at the same stage, apoptosis was pronounced throughout the axis, especially in the head (Fig. 4H) but was limited in the head of controls (G) and gsk3β morphants (I). Moreover, In gsk3a-morphants, the GFP signal was very faint at 24 hpf (Fig. 4N). The apoptotic signals were co-localized with the heart-specific GFP signal, indicating that the reduced cardiomyocyte numbers was due to apoptosis in heart (Fig. 4O). Taken together, the heart defects in gsk3α morphants was due to the reduced number of cardiomyocytes, which results from apoptosis in the head.
GSK3β, but not GSK3α, is involved in the cardiac positioning
Although the heart of gsk3β-MO-injected embryos eventually becomes a string-like shape, we found that the cardiomyocyte development was not affected in the gsk3β morphants during early cardiac development, suggesting that GSK3β may play a unique role in cardiac morphogenesis. Whole-mount in situ hybridization of the cmlc2 probe at 36 hpf, outlining cardiac looping, was marked by a rightward bending in the ventricle in wild-type embryos (Fig. 5A). However, no looping was observed in gsk3β morphants (Fig. 5B–D). Upon detailed analysis of the early (jogging) and late (looping) stages of cardiac positioning in the gsk3β morphant heart (Table 2), we found that heart positioning was severely disrupted in gsk3β morphants and that the extent of the defect was proportional to the amount of gsk3β-MO we injected. The majority of gsk3β morphant hearts failed to jog (69.9%; 65/93). Moreover, this defect was frequently accompanied by no looping (45.3%) or L-looping (14.4%) of the heart tube, compared to wild-type, which has correct left-jogging (93.1%; 67/72) and D-looping (92.4%; 122/132). These results indicate that knockdown of GSK3β resulted in a severe disruption of jogging and looping of cardiac positioning. However, we found that the ventricle-specific marker vmhc and the atrium-specific marker amhc were normally transcribed in gsk3β morphants (Fig. 5E, G vs. 5F, H), suggesting that GSK3β might not affect the chamber-specific pattern of gene expression, although normal heart looping was not completed. We also noted that the heart positioning in gsk3α morphants was delayed but that correct jogging (left-jog) and looping (D-loop) were observed at [36-48] hpf, indicating that GSK3α was not involved in heart positioning.
Figure 5.
Cardiac positioning is gsk3β-dependent, but the chamber-specific patterning is not. A-C, E-H) ventral view, (D) dorsal view of wild-type (A, E, G), and gskβ morphants (B-D, F, H) at 36 hpf. in situ hybridization with cmlc2 staining revealed that randomized looping was observed in gsk3β morphants (B-D). The expression of vmhc (F) and amhc (H) appeared normal in gsk3β morphants.
Table 2.
Knockdown GSK3β levels that disrupt normal cardiac jogging and looping
| No. of embryos | Left-jog | No-jog | Right-jog | No. of embryos | D-loop | No-loop | L-loop | |
| Uninjected | 72 | 93% | 2.8% | 4.2% | 132 | 92.5% | 3% | 4.5% |
| 2 ng gsk3α -MO | 63 | 87.3% | 7.9% | 4.8% | 74 | 93.2% | 4.1% | 2.7% |
| 1 ng gsk3α -MO | 58 | 93.1% | 5.2% | 1.7% | 90 | 94.5% | 2.2% | 3.3% |
| 6 ng gsk3β -MO | 93 | 17.2% | 69.9% | 12.95 | 159 | 40.3% | 45.3% | 14.4% |
| 4 ng gsk3β -MO | 86 | 22.1% | 67.4% | 10.5% | 101 | 40.6% | 44.6% | 14.8% |
| 2 ng gsk3β -MO | 62 | 45% | 50% | 5% | 107 | 70.1% | 22.4% | 7.5% |
gsk3α morphants were analyzed for heart positioning at 36 to 48 hpf; gsk3β morphants were analyzed for heart positioning at 24 to 36 hpf.
GSK3β mediates bmp4 and lefty-1 in cardiac positioning and is required for left-right patterning
Cardiac bmp4 is an integral component involved in the asymmetric signaling pathway and interprets left-right information for the zebrafish embryo heart [35]. The bmp4 transcripts became markedly asymmetric, with far more on the left side than on the right side of the heart ring at 20 hpf (Fig. 6A, B), just before jogging. This left-predominant asymmetry persists through the stages of jogging (25 hpf, Fig. 6G). However, the pattern of bmp4 expression in gsk3β morphants was symmetrical before jogging (Fig. 6D, E) and ectopic around the heart-tube stage at 25 hpf, thereby disrupting the pattern of left-predominant asymmetry (Fig. 6H, I). Moreover, another asymmetric marker, lefty-1 [36], lost its expression domain in the left side of the midline in gsk3β morphants (Fig. 6C, F). We propose that GSK3β mediates bmp4 and lefty-1 in cardiac positioning.
Figure 6.
Heart asymmetry was affected in gsk3β morphants. Normally, bmp4 transcripts accumulate predominantly on the left side of the heart tube at 20 hpf (A, B), and the left-predominant bmp4 asymmetry persists through the stages of jogging (G). However, in gsk3β morphants, the expression of bmp4 becomes symmetrical at 20 hpf (B, D). In gsk3β morphants, in which the heart fails to jog, bmp4 is more evenly distributed in the heart region (H, I). The left-sided lefty-1 domain was greatly reduced in gsk3β morphant hearts at 16 hpf (F). All are dorsal views. B, E are higher magnifications of A, D, respectively. Lines mark the midline. L, embryo left.
GSK3β modulates valve formation and heart position through Wnt/β-catenin signaling
Many morphological defects of heart were found in the gsk3β-MO-injected zebrafish embryos. Moreover, when we used the valve markers bmp4 and versican to detect the gsk3β-MO-injected embryos at 60–72 hpf, we found that these valve markers were markedly up-regulated in the heart (Fig. 7A–D), suggesting that GSK3β might also be involved in the formation of cardiac valves. Thus, we used a two-photon fluorescence image to directly observe the valve formation of embryos derived from the transgenic zebrafish line Tg(cmlc2:Hc-RFP; 28). The yellow color shown in our nonlinear microscopy image (valves and red blood cells) is corresponding to the image modality taken by the Third-Harmonic-Generation Microscopy. Valves were normally formed in the wild-type embryos (Fig. 7E), but valves of embryos injected with gsk3β-MO were totally absent (Fig. 7F).
Figure 7.
GSK3β modulates zebrafish cardiac valve formation. Whole-mount in situ hybridization with bmp4 and versican staining reveals that cardiac valve development was affected in gsk3β morphants. At 60–72 hpf,versican (A, B) and bmp4 (C, D) expression was greatly up-regulated in gsk3β morphants. Tg(cmlc2: Hc-RFP) embryos were injected with gsk3β-MO and observed by in vivo two-photon fluorescence imaging of a live transgenic zebrafish heart at 100 hpf. The endocardial cells and blood are labeled yellow; the Hc-GFP-positive myocardial cells are labeled red. Valves are clearly observed in wild-type embryos (E; white arrows), but not in gsk3β morphants (F). b, blood cells; V, ventricle; A, atrium.
Hurlstone et al [37] reported that cardiac valve formation is severely affected in zebrafish APC mutants (apcmcr). Furthermore, when axin1, another key component in the Wnt pathway, is knocked down, either a reduction or absence of heart positioning of the heart tube was frequently observed (see Additional file 2A–D). GSK3 is known to be important in the canonical Wnt pathway, and the defective valves and hearts in gsk3β-MO-injected embryos were identical to those observed in the apcmcr mutants and axin1 morphants, suggesting that GSK3β modulates cardiac development through Wnt/β-catenin signaling.
Discussion
GSK3β knockout mice display an embryonic lethal phenotype, indicating that GSK3α does not compensate for the loss of GSK3β [9]. Furthermore, the activity of GSK3α, but not GSK3β, is required for the production of amyloid-β in the brain of patients with Alzheimer's disease [38]. All these findings indicate that there may be isoform-specific functions of GSK3, although they exhibit similar biochemical and substrate properties in vitro [7]. However, to clearly delineate the biological functions of these two GSK3 isoforms in vivo is difficult and little reported. In this report, we study the roles of GSK3α and GSK3β in the cardiogenesis of zebrafish using a loss-of-function approach. The loss of either GSK3α or GSK3β results in abnormal development of heart. Yet, the role that GSK3 plays in cardiogenesis is in an isoform-specific manner. GSK3α plays a role in the survival of cardiomyocytes, whereas GSK3β plays a role in heart left-right biased positioning, modulated through the Wnt/β-catenin signaling pathway.
GSK3α and GSK3β play different roles during zebrafish embryogenesis
Although, knockdown of gsk3α and gsk3β causes similar defective phenotypes, such as an unlooped and stretched heart, pericardial edema, blood pooling. We used gsk3α-MO and gsk3β-MO in the transgenic zebrafish line Tg(cmlc2:GFP), in which GFP is expressed in the myocardium specifically, to modulate and observe, in real-time, the different defective phenotypes. The hearts of gsk3α morphants failed to elongate at 24 hpf. We prove that the heart defects induced by the gsk3α-MO are due to a decreased number of cardiomyocytes. On the other hand, the gsk3β-MO-injected embryos develop normally before the onset of cardiac jogging. Defective heart positioning is observed after 26 hpf. Rescue experiments revealed that GSK 3α and GSK3β do not function redundantly. Taken together, we conclude that each isoform of GSK3 plays its own distinct role during cardiogenesis of zebrafish.
GSK3α, but not GSK3β, is involved in apoptosis during early embryogenesis
GSK3 plays an important role in the regulation of apoptosis/cell survival through the activation of caspase3 [39,41,42]. These findings support a role of GSK3β in controlling apoptosis. Many studies reporting the affect of GSK3β on apoptosis have been confirmed by using GSK3 inhibitors, including lithium, the first known inhibitor, and many synthetic ones [43-45]. However, these inhibitors have many effects on cells and are not isoform-specific. Thus, whether GSK3α and GSK3β function redundantly or distinctly on cell survival is still ambiguous. In our study, extensive apoptosis is observed throughout the head region in the gsk3α morphants. On the other hand, only slight apoptosis is noticed in the gsk3β morphants, suggesting that GSK3α, but not GSK3β, is greatly involved in apoptosis during early embryogenesis. Moreover, embryos that are co-injected with gsk3α-MO and gsk3β mRNA do not show reduced apoptosis, suggesting that GSK3α and GSK3β do not function redundantly in cell survival.
GSK3α-mediated apoptosis may not be implicated in Wnt signaling
The Wnt signaling are involved in cell proliferation and in apoptosis [46-49]. On the other hand, PKB/Akt, a major regulator of GSK3, also triggers a network that regulates cell cycle progression through inactivation of GSK3β [50]. It has been shown that PKB/Akt promotes cell survival in cardiac myocytes [51,52]. In zebrafish, apcmcr mutant's hearts are morphologically normal during early cardiogenesis, but they fail to undergo looping morphogenesis [37]. Both apcmcr and axin1 mutants (mbl) display cardiac defects that are similar to those of gsk3β morphants. However, no information is provided about apoptosis in apcmcr and mbl mutants. In this report, we find that apoptosis occurs in the head of gsk3α morphants. In addition, the axin1-MO-injected embryos and the mbl mutant of zebrafish have defects of looping morphogenesis in the heart, which are similar to defects occurring in the gsk3β morphants but are unlike defects occurring in the gsk3α morphants (see Additional file 2). Therefore, we know that GSK3α may not mediate apoptosis implicated in Wnt signaling because apoptotic signals do not increase in axin1 morphants (data not shown). It is worth studying which pathway is implicated in GSK3α-mediated apoptosis.
gsk3β regulates bmp4 during cardiac development through Wnt/β-catenin signaling
The phenotypes of apcmcr and mbl mutants are similar to our results in that inhibition of GSK3β also causes unlooping heart tube, pericardial edema, and blood pooling [37]. In addition, valve development is totally lost in gsk3β morphants (Fig. 7), which is similar to that of apcmcr mutants. Ectopic expression of bmp4 in the heart at 24–72 hpf and ectopic expression of versican in the valve at 60–72 hpf are also observed in the apcmcr mutant and in the gsk3β morphant (Figs. 7A–D). Moreover, the retention of bmp4 symmetry is associated with disordered jogging, and we observe that bmp4 retention occurred in the gsk3β morphant. In addition, bmp4 is downstream of Wnt/β-catenin signaling in several systems [53,54]. Therefore, it is reasonable to conclude that GSK3β might regulate zebrafish cardiac development by means of the canonical Wnt/β-catenin signaling pathway.
GSK3β may be involved in the regulation of T-box genes during cardiogenesis
Our study reveals that knockdown of gsk3β causes a string-like heart. This phenotype is similar to the heartstrings mutant, caused by mutation of the tbx5 [55]. Patients with Holt-Oram syndrome, one of the autosomal dominant human "heart-hand" disorders, are caused by mutations of tbx5 [56]. Both loss and gain of tbx5 functions result in an absence of heart looping and an alteration in cardiac-specific genes [57,58]. In our study, we demonstrate that gsk3β morphants appear to have multiple heart defects, such as a non-looping or reversed looping heart, slower heart rate, and no blood circulation (Figs. 3, 4). In addition, after we probe with fin markers, we prove that the pectoral fin of the GSK3β morphant fails to differentiate (see Additional file 3). In chick, Tbx5 and Tbx4 trigger limb initiation through activation of the Wnt/Fgf signaling cascade [59]. Therefore, we propose that GSK3β and Tbx5 might be involved in the same regulatory mechanism during cardiogenesis.
Zebrafish is an alternative, promising model animal to study GSK3-specific inhibitors in vivo
GSK3 is a target of prominent drugs for treating many diseases, including Alzheimer's disease and diabetes mellitus. Substrate-competitive inhibitors, which compete for the substrate binding site of the kinase, are more likely to be highly specific inhibitors. Several ATP-competitive inhibitors of GSK3 have also been defined [17,18]. However, the development of new drug not only requires the identification of the target, but also requires validation in an in vivo system. Recently, Atilla-Gokcumen et al., [60] performed phenotypic experiments in zebrafish embryo which is served as an in vivo experiment to analyse the functions of novel GSK3 inhibitor, organometallic reagent (R)-7. In this study, we clearly distinguish the morphological defects in zebrafish GSK3α- and GSK3β-knockdown embryos. Therefore, these findings will surely provide new criteria for the in vivo validation of potential isoform-specific inhibitors of GSK3.
Different biological function of GSK3 isoform in species
In this report, we have defined that GSK3α and GSK3β play distinct roles during zebrafish cardiogenesis. Moreover, the defective valves and hearts in gsk3β-MO-injected embryos were identical to those observed in the apcmcr mutants and axin1 morphants, suggesting that GSK3β modulates cardiac development through Wnt/β-catenin signaling. In addition, GSK3 is a critical regulator of Wnt signaling mechanism, several recent studies have shown that the components of the Wnt signaling play an important role in heart development [3]. However, heart defects are not reported in the GSK3β-knockout mice. One of reasons is that mice GSK3α might function redundantly to GSK3β during the heart development of mice. We also notice that the expression profiles of GSK3β in zebrafish and in Xenopus are different: zebrafish gsk3β is weakly detected until 50–60% epiboly, but Xenopus gsk3β is expressed strongly and constantly throughout embryogenesis [61,62]. Taken together, although GSK3 isoforms share highly conserved in their functional domain, the biological functions of GSK3 isoforms in different species are not identical.
Conclusion
Our data indicate that GSK3α and GSK3β play distinct roles during zebrafish embryogenesis. GSK3α, but not GSK3β, is necessary in cardiomyocyte survival; GSK3β plays an important role in regulating left-right biased heart positioning during the cardiogenesis in zebrafish. We also demonstrate that the cardiac valve defects observed in gsk3β morphants were similar to those observed in axin1 and apcmcrmutants, suggesting that GSK3β modulates valve formation and heart position through Wnt/β-catenin signaling. Finally, the defects of gsk3α morphant embryos cannot be rescued by gsk3β mRNA, and vice versa, demonstrating that GSK3α and GSK3β are not functionally equivalent. Thus, we conclude that GSK3α and GSK3β play distinct roles during zebrafish cardiogenesis.
Methods
Zebrafish maintenance and observation
The zebrafish AB strain, transgenic lines Tg(cmlc2:Hc-RFP) and Tg(cmlc2:GFP) were raised and staged as previously described (28–30). The heart formation were observed under a fluorescent stereomicroscope MZ FLIII (Leica) and two-photon fluorescence microscope and Third-Harmonic-Generation Microscopy [28].
Knockdown microinjection of zebrafish embryos
The following morpholino antisense oligonucleotides (MOs) were obtained from Gene Tools: gsk3α-MO, CCGCTGCCGCTCATTTCGGGTTGCA; gsk3β-MO, GTTCTGGGCCGACCGGACATTTTTC; axin1-MO, GCTAATGCGGTCATATCTCCTCTGC; standard negative control-MO, CCTCTTACCTCAGTTACAATTTATA. All MOs were prepared at a stock concentration of 1 mM and diluted to the desired concentration for microinjection into each embryo.
Western blot
The embryos were dechorionated and deyolked with two extra washing steps as described in Link et al. [31]. Deyolked samples were dissolved in 2 μl of 2 × sodium dodecyl sulfate (SDS) sample buffer per embryo and incubated for 5 min at 95°C. After full-speed centrifugation for 1 min in a microcentrifuge to remove insoluble particles, samples were loaded on a 12% SDS gel (seven embryos per lane). Antibodies used were anti-GSK3 (Santa Cruz, SC-7291, 1:750) and anti-α-tubulin (Sigma-Aldrich, T9026, 1:750).
Whole-mount in situ hybridization
Whole-mount in situ hybridization techniques have been described previously [32]. The probes were digoxigenin-labeled, after which we cloned their partial DNA fragments.
mRNA preparation for the rescue experiment
Capped mRNAs of gsk3α, gsk3β, and RFP were synthesized according to the protocol of the manufacturer (Epicentre). The resultant mRNAs were diluted to 44 ng/μl with distilled water. Approximately 2.3 nl was injected into one-cell stage embryos.
Detection of apoptotic cell death
The apoptosis assay was performed using The DeadEnd™ Colorimetric TUNEL System (Promega) and has been described previously [33].
Authors' contributions
HCL designed and performed all the experiments, analysis the data, and prepared the manuscript. JNT and WCC carried out the Western blot analysis; KYL, PYL and WYT took care of fish handling including morpholino injections. CCC and CKS performed the two-photon fluorescence microscope imaging; HJT was a P.I. of this project. All authors read and approved the final manuscript.
Supplementary Material
Morphological phenotypes of zebrafish embryos derived from fertilized eggs injected with standard control morpholino. The standard negative control-MO has no target, no significant biological activity, and are commonly used in many studies (Hultman et al., 2007; Besser et al., 2007; Nixon et al., 2005). After 2 and 6 ng of this control MO were injected, no any defects were observed at 24 hpf. The morphology and development of heart appeared normally.
Similar cardiac defects in the axin1 and the gsk3β morphants. Axin1-MO or gsk3β-MO were microinjcetd to And observed under dissecting microscope by bright filed (A, C) or fluorescence (B, D). Incomplete looping of the heart tube was also observed in axin1 mutant heart.
Arrested pectoral fin bud induction in gsk3β morphants. At 72 hpf, wild-type pectoral fins elongate (A), but gsk3β morphants have still not developed fin buds (arrows; D). Whole mount in situ hybridization with shh and dlx2 staining reveal that the developed of fin bud were affected in gsk3β morphants. At 36 hpf, wild-type embryos continue shh (B) and dlx2 (C) expression in the developing bud mesenchyme, but in gsk3β morphants, the shh and dlx2 expression is greatly decreased.
Acknowledgments
Acknowledgements
We are grateful to Dr. Anna-Pavlina Haramis for providing the bmp4 and versican probes, to Drs. Christine and Bernard Thisse for providing the lefty-1 probe. This work was partially supported by the National Science Council, Republic of China, under grand no. NCS-95-2313-B-002-067-My2 (HJT), and partially supported by National Health Research Institute, grand no NHRI-EX96-9201EI (CKS).
Contributor Information
Huang-Chieh Lee, Email: allen628@ms35.hinet.net.
Jen-Ning Tsai, Email: zgsk3@yahoo.com.tw.
Pei-Yin Liao, Email: pei-in@yahoo.com.tw.
Wei-Yuan Tsai, Email: r90243005@ms90.ntu.edu.tw.
Kai-Yen Lin, Email: r95b43014@ntu.edu.tw.
Chung-Cheng Chuang, Email: ching101@ms9.hinet.net.
Chi-Kuang Sun, Email: sun@cc.ee.ntu.edu.tw.
Wen-Chang Chang, Email: bcwchang@ccvax.sinica.edu.tw.
Huai-Jen Tsai, Email: hjtsai@ntu.edu.tw.
References
- Woodgett JR. Molecular cloning and expression of glycogen synthase kinase-3/factor A. EMBO J. 1990;9:243–248. doi: 10.1002/j.1460-2075.1990.tb07419.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plyte SE, Hughes K, Nikolakaki E, Pulverer BJ, Woodgett JR. Glycogen synthase kinase-3: functions in oncogenesis and development. Biochim Biophys Acta. 1992;1114:147–162. doi: 10.1016/0304-419x(92)90012-n. [DOI] [PubMed] [Google Scholar]
- Hardt SE, Sadoshima J. Glycogen synthase kinase-3beta: a novel regulator of cardiac hypertrophy and development. Circ Res. 2002;90:1055–1063. doi: 10.1161/01.RES.0000018952.70505.F1. [DOI] [PubMed] [Google Scholar]
- van Amerongen R, Berns A. Re-evaluating the role of Frat in Wnt-signal transduction. Cell Cycle. 2005;4:1065–1072. [PubMed] [Google Scholar]
- Woodgett JR. cDNA cloning and properties of glycogen synthase kinase-3. Methods Enzymol. 1991;200:564–577. doi: 10.1016/0076-6879(91)00172-s. [DOI] [PubMed] [Google Scholar]
- Woodgett JR. Judging a protein by more than its name: GSK-3. Sci STKE. 2001 doi: 10.1126/stke.2001.100.re12. 2001 RE12. [DOI] [PubMed] [Google Scholar]
- Ali A, Hoeflich KP, Woodgett JR. Glycogen synthase kinase-3: properties, functions, and regulation. Chem Rev. 2001;101:2527–2540. doi: 10.1021/cr000110o. [DOI] [PubMed] [Google Scholar]
- Tsai JN, Lee CH, Jeng H, Chi WK, Chang WC. Differential expression of glycogen synthase kinase 3 genes during zebrafish embryogenesis. Mech Dev. 2000;91:387–391. doi: 10.1016/S0925-4773(99)00300-7. [DOI] [PubMed] [Google Scholar]
- Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR. Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature. 2000;406:86–90. doi: 10.1038/35017574. [DOI] [PubMed] [Google Scholar]
- Liang MH, Chuang DM. Regulation and function of glycogen synthase kinase-3 isoforms in neuronal survival. J Biol Chem. 2007;282:3904–3917. doi: 10.1074/jbc.M605178200. [DOI] [PubMed] [Google Scholar]
- Sutherland C, Cohen P. The alpha-isoform of glycogen synthase kinase-3 from rabbit skeletal muscle is inactivated by p70 S6 kinase or MAP kinase-activated protein kinase-1 in vitro. FEBS Lett. 1994;338:37–42. doi: 10.1016/0014-5793(94)80112-6. [DOI] [PubMed] [Google Scholar]
- Sutherland C, Leighton IA, Cohen P. Inactivation of glycogen synthase kinase-3 beta by phosphorylation: new kinase connections in insulin and growth-factor signalling. Biochem J. 1993;296:15–19. doi: 10.1042/bj2960015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goode N, Hughes K, Woodgett JR, Parker PJ. Differential regulation of glycogen synthase kinase-3 beta by protein kinase C isotypes. J Biol Chem. 1992;267:16878–16882. [PubMed] [Google Scholar]
- Nikoulina SE, Ciaraldi TP, Mudaliar S, Mohideen P, Carter L, Henry RR. Potential role of glycogen synthase kinase-3 in skeletal muscle insulin resistance of type 2 diabetes. Diabetes. 2000;49:263–271. doi: 10.2337/diabetes.49.2.263. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, Nielsen P, Kiens B, Richter EA. Regulation of glycogen synthase kinase-3 in human skeletal muscle: effects of food intake and bicycle exercise. Diabetes. 2001;50:265–269. doi: 10.2337/diabetes.50.2.265. [DOI] [PubMed] [Google Scholar]
- Markuns JF, Wojtaszewski JF, Goodyear LJ. Insulin and exercise decrease glycogen synthase kinase-3 activity by different mechanisms in rat skeletal muscle. J Biol Chem. 1999;274:24896–24900. doi: 10.1074/jbc.274.35.24896. [DOI] [PubMed] [Google Scholar]
- Cohen P, Goedert M. GSK3 inhibitors: development and therapeutic potential. Nat Rev Drug Discov. 2004;3:479–487. doi: 10.1038/nrd1415. [DOI] [PubMed] [Google Scholar]
- Meijer L, Flajolet M, Greengard P. Pharmacological inhibitors of glycogen synthase kinase 3. Trends Pharmacol Sci. 2004;25:471–480. doi: 10.1016/j.tips.2004.07.006. [DOI] [PubMed] [Google Scholar]
- Bhat RV, Budd Haeberlein SL, Avila J. Glycogen synthase kinase 3: a drug target for CNS therapies. J Neurochem. 2004;89:1313–1317. doi: 10.1111/j.1471-4159.2004.02422.x. [DOI] [PubMed] [Google Scholar]
- Antos CL, McKinsey TA, Frey N, Kutschke W, McAnally J, Shelton JM, Richardson JA, Hill JA, Olson EN. Activated glycogen synthase-3beta suppresses cardiac hypertrophy in vivo. Proc Natl Acad Sci U S A. 2002;99:907–912. doi: 10.1073/pnas.231619298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badorff C, Ruetten H, Mueller S, Stahmer M, Gehring D, Jung F, Ihling C, Zeiher AM, Dimmeler S. Fas receptor signaling inhibits glycogen synthase kinase 3s and induces cardiac hypertrophy following pressure overload. J Clin Invest. 2003;109:373–381. doi: 10.1172/JCI200213779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haq S, Choukroun G, Kang ZB, Ranu H, Matsui T, Rosenzweig A, Molkentin JD, Alessandrini A, Woodgett J, Hajjar R, Michael A, Force T. Glycogen Synthase Kinase-3s Is a Negative Regulator of Cardiomyocyte Hypertrophy. J Cell Biol. 2000;151:117–129. doi: 10.1083/jcb.151.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael A, Haq S, Chen X, Hsich E, Cui L, Walters B, Shao Z, Bhattacharya K, Kilter H, Huggins G, Andreucci M, Periasamy M, Solomon RN, Liao R, Patten R, Molkentin JD, Force T. Glycogen synthase kinase-3beta regulates growth, calcium homeostasis, and diastolic function in the heart. J Biol Chem. 2004;279:21383–21393. doi: 10.1074/jbc.M401413200. [DOI] [PubMed] [Google Scholar]
- Foley AC, Mercola M. Heart induction by Wnt antagonists depends on the homeodomain transcription factor Hex. Genes Dev. 2005;19:387–396. doi: 10.1101/gad.1279405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Francannet C, Pradat P, Robert E. The epidemiology of cardiovascular defects, part 2: a study based on data from three large registries of congenital malformations. Pediatr Cardiol. 2003;24:222–235. doi: 10.1007/s00246-002-9402-5. [DOI] [PubMed] [Google Scholar]
- Shader RI, Greenblatt DJ. Lithium and the newborn heart. J Clin Psychopharmacol. 1990;10:311. doi: 10.1097/00004714-199010000-00001. [DOI] [PubMed] [Google Scholar]
- Zierler S. Related Maternal drugs and congenital heart disease. Obstet Gynecol. 1985;65:155–165. [PubMed] [Google Scholar]
- Tsai TH, Lin CY, Tsai HJ, Chen SY, Tai SP, Lin KH, Sun CK. Biomolecular imaging based on far-red fluorescent protein with a high two-photon excitation action cross section. Opt Lett. 2006;31:930–932. doi: 10.1364/OL.31.000930. [DOI] [PubMed] [Google Scholar]
- Huang CJ, Tu CT, Hsiao CD, Hsieh FJ, Tsai HJ. Germ-line transmission of a myocardium-specific GFP transgene reveals critical regulatory elements in the cardiac myosin light chain 2 promoter of zebrafish. Dev Dyn. 2003;228:30–40. doi: 10.1002/dvdy.10356. [DOI] [PubMed] [Google Scholar]
- Kimmel CB, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. Stages of embryonic development of the zebrafish. Dev Dyn. 1995;203:253–310. doi: 10.1002/aja.1002030302. [DOI] [PubMed] [Google Scholar]
- Link V, Shevchenko A, Heisenberg CP. Proteomics of early zebrafish embryos. BMC Dev Biol. 2006;6:1. doi: 10.1186/1471-213X-6-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HC, Huang HY, Lin CY, Chen YH, Tsai HJ. Foxd3 mediates zebrafish myf5 expression during early somitogenesis. Dev Biol. 2006;290:359–372. doi: 10.1016/j.ydbio.2005.11.032. [DOI] [PubMed] [Google Scholar]
- Lin CY, Yung RF, Lee HC, Chen WT, Chen YH, Tsai HJ. Myogenic regulatory factors Myf5 and Myod function distinctly during craniofacial myogenesis of zebrafish. Dev Biol. 2006;299:594–608. doi: 10.1016/j.ydbio.2006.08.042. [DOI] [PubMed] [Google Scholar]
- Stainier DY. Zebrafish genetics and vertebrate heart formation. Nat Rev Genet. 2001;2:39–48. doi: 10.1038/35047564. [DOI] [PubMed] [Google Scholar]
- Chen JN, van Eeden FJ, Warren KS, Chin A, Nusslein-Volhard C, Haffter P, Fishman MC. Left-right pattern of cardiac BMP4 may drive asymmetry of the heart in zebrafish. Development. 1997;124:4373–4382. doi: 10.1242/dev.124.21.4373. [DOI] [PubMed] [Google Scholar]
- Ramsdell AF. Left-right asymmetry and congenital cardiac defects: getting to the heart of the matter in vertebrate left-right axis determination. Dev Biol. 2005;288:1–20. doi: 10.1016/j.ydbio.2005.07.038. [DOI] [PubMed] [Google Scholar]
- Hurlstone AF, Haramis AP, Wienholds E, Begthel H, Korving J, Van Eeden F, Cuppen E, Zivkovic D, Plasterk RH, Clevers H. The Wnt/beta-catenin pathway regulates cardiac valve formation. Nature. 2003;425:633–637. doi: 10.1038/nature02028. [DOI] [PubMed] [Google Scholar]
- Phiel CJ, Wilson CA, Lee VM, Klein PS. GSK-3alpha regulates production of Alzheimer's disease amyloid-beta peptides. Nature. 2003;423:435–439. doi: 10.1038/nature01640. [DOI] [PubMed] [Google Scholar]
- Viatour P, Dejardin E, Warnier M, Lair F, Claudio E, Bureau F, Marine JC, Merville MP, Maurer U, Green D, Piette J, Siebenlist U, Bours V, Chariot A. GSK3-mediated BCL-3 phosphorylation modulates its degradation and its oncogenicity. Mol Cell. 2004;16:35–45. doi: 10.1016/j.molcel.2004.09.004. [DOI] [PubMed] [Google Scholar]
- King TD, Bijur GN, Jope RS. Caspase-3 activation induced by inhibition of mitochondrial complex I is facilitated by glycogen synthase kinase-3beta and attenuated by lithium. Brain Res. 2001;919:106–114. doi: 10.1016/S0006-8993(01)03005-0. [DOI] [PubMed] [Google Scholar]
- Song L, De Sarno P, Jope RS. Central role of glycogen synthase kinase-3beta in endoplasmic reticulum stress-induced caspase-3 activation. J Biol Chem. 2002;277:44701–44708. doi: 10.1074/jbc.M206047200. [DOI] [PubMed] [Google Scholar]
- Stoica BA, Movsesyan VA, Lea PM, 4th, Faden AI. Ceramide-induced neuronal apoptosis is associated with dephosphorylation of Akt, BAD, FKHR, GSK-3beta, and induction of the mitochondrial-dependent intrinsic caspase pathway. Mol Cell Neurosci. 2003;22:365–382. doi: 10.1016/S1044-7431(02)00028-3. [DOI] [PubMed] [Google Scholar]
- Klein PS, Melton DA. A molecular mechanism for the effect of lithium on development. Proc Natl Acad Sci U S A. 1996;93:8455–8459. doi: 10.1073/pnas.93.16.8455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eldar-Finkelman H. Glycogen synthase kinase 3: an emerging therapeutic target. Trends Mol Med. 2002;8:126–132. doi: 10.1016/S1471-4914(01)02266-3. [DOI] [PubMed] [Google Scholar]
- Martinez A, Castro A, Dorronsoro I, Alonso M. Glycogen synthase kinase 3 (GSK-3) inhibitors as new promising drugs for diabetes, neurodegeneration, cancer, and inflammation. Med Res Rev. 2002;22:373–384. doi: 10.1002/med.10011. [DOI] [PubMed] [Google Scholar]
- Sommer L. Checkpoints of melanocyte stem cell development. Sci STKE. 2005 doi: 10.1126/stke.2982005pe42. 205 pe42. [DOI] [PubMed] [Google Scholar]
- Willert K, Brown JD, Danenbergn E, Duncan AW, Weissman IL, Reya T, Yates JR, 3rd, Nusse R. Wnt proteins are lipid-modified and can act as stem cell growth factors. Nature. 2003;423:448–452. doi: 10.1038/nature01611. [DOI] [PubMed] [Google Scholar]
- Chen S, Guttridge DC, You Z, Zhang Z, Fribley A, Mayo MW, Kitajewski J, Wang CY. Wnt-1 signaling inhibits apoptosis by activating beta-catenin/T cell factor-mediated transcription. J Cell Biol. 2001;152:87–96. doi: 10.1083/jcb.152.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You L, He B, Uematsu K, Xu Z, Mazieres J, Lee A, McCormick F, Jablons DM. Inhibition of Wnt-1 signaling induces apoptosis in beta-catenin-deficient mesothelioma cells. Cancer Res. 2004;64:3474–3478. doi: 10.1158/0008-5472.CAN-04-0115. [DOI] [PubMed] [Google Scholar]
- Liang J, Slingerland JM. Multiple roles of the PI3K/PKB (Akt) pathway in cell cycle progression. Cell Cycle. 2003;2:339–345. [PubMed] [Google Scholar]
- Brar BK, Stephanou A, Knight R, Latchman DS. Activation of protein kinase B/Akt by urocortin is essential for its ability to protect cardiac cells against hypoxia/reoxygenation-induced cell death. J Mol Cell Cardiol. 2002;34:483–492. doi: 10.1006/jmcc.2002.1529. [DOI] [PubMed] [Google Scholar]
- Germack R, Griffin M, Dickenson JM. Activation of protein kinase B by adenosine A1 and A3 receptors in newborn rat cardiomyocytes. J Mol Cell Cardiol. 2004;37:989–999. doi: 10.1016/j.yjmcc.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Barrow JR, Thomas KR, Boussadia-Zahui O, Moore R, Kemler R, Capecchi MR, McMahon AP. Ectodermal Wnt3/beta-catenin signaling is required for the establishment and maintenance of the apical ectodermal ridge. Genes Dev. 2003;17:394–409. doi: 10.1101/gad.1044903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill TP, Taketo MM, Birchmeier W, Hartmann C. Multiple roles of mesenchymal beta-catenin during murine limb patterning. Development. 2006;133:1219–1229. doi: 10.1242/dev.02298. [DOI] [PubMed] [Google Scholar]
- Garrity DM, Childs S, Fishman MC. The heartstrings mutation in zebrafish causes heart/fin Tbx5 deficiency syndrome. Development. 2001;129:4635–4645. doi: 10.1242/dev.129.19.4635. [DOI] [PubMed] [Google Scholar]
- Li QY, Newbury-Ecob RA, Terrett JA, Wilson DI, Curtis AR, Yi CH, Gebuhr T, Bullen PJ, Robson SC, Strachan T, Bonnet D, Lyonnet S, Young ID, Raeburn JA, Buckler AJ, Law DJ, Brook JD. Holt-Oram syndrome is caused by mutations in TBX5, a member of the Brachyury (T) gene family. Nat Genet. 1997;15:21–29. doi: 10.1038/ng0197-21. [DOI] [PubMed] [Google Scholar]
- Liberatore CM, Searcy-Schrick RD, Yutzey KE. Ventricular expression of tbx5 inhibits normal heart chamber development. Dev Biol. 2000;223:169–180. doi: 10.1006/dbio.2000.9748. [DOI] [PubMed] [Google Scholar]
- Bruneau BG, Nemer G, Schmitt JP, Charron F, Robitaille L, Caron S, Conner DA, Gessler M, Nemer M, Seidman CE, Seidman JG. A murine model of Holt-Oram syndrome defines roles of the T-box transcription factor Tbx5 in cardiogenesis and disease. Cell. 2001;106:709–721. doi: 10.1016/S0092-8674(01)00493-7. [DOI] [PubMed] [Google Scholar]
- Takeuchi JK, Koshiba-Takeuchi K, Suzuki T, Kamimura M, Ogura K, Ogura T. Tbx5 and Tbx4 trigger limb initiation through activation of the Wnt/Fgf signaling cascade. Development. 2003;130:2720–2739. doi: 10.1242/dev.00474. [DOI] [PubMed] [Google Scholar]
- Atilla-Gokcumen GE, Williams DS, Bregman H, Pagano N, Meggers E. Organometallic compounds with biological activity: a very selective and highly potent cellular inhibitor for glycogen synthase kinase 3. Chembiochem. 2006;7:1443–1450. doi: 10.1002/cbic.200600117. [DOI] [PubMed] [Google Scholar]
- Dominguez I, Itoh K, Sokol SY. Role of glycogen synthase kinase 3b as a negative regulator of dorsoventral axis formation in Xenopus embryos. Proc Natl Acad Sci USA. 1995;92:8498–8502. doi: 10.1073/pnas.92.18.8498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce SB, Kimelman D. Regulation of Spemann organizer formation by the intracellular kinase Xgsk-3. Development. 1995;121:755–765. doi: 10.1242/dev.121.3.755. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Morphological phenotypes of zebrafish embryos derived from fertilized eggs injected with standard control morpholino. The standard negative control-MO has no target, no significant biological activity, and are commonly used in many studies (Hultman et al., 2007; Besser et al., 2007; Nixon et al., 2005). After 2 and 6 ng of this control MO were injected, no any defects were observed at 24 hpf. The morphology and development of heart appeared normally.
Similar cardiac defects in the axin1 and the gsk3β morphants. Axin1-MO or gsk3β-MO were microinjcetd to And observed under dissecting microscope by bright filed (A, C) or fluorescence (B, D). Incomplete looping of the heart tube was also observed in axin1 mutant heart.
Arrested pectoral fin bud induction in gsk3β morphants. At 72 hpf, wild-type pectoral fins elongate (A), but gsk3β morphants have still not developed fin buds (arrows; D). Whole mount in situ hybridization with shh and dlx2 staining reveal that the developed of fin bud were affected in gsk3β morphants. At 36 hpf, wild-type embryos continue shh (B) and dlx2 (C) expression in the developing bud mesenchyme, but in gsk3β morphants, the shh and dlx2 expression is greatly decreased.