Abstract
The matricellular protein secreted protein acidic and rich in cysteine (SPARC) modulates cell adhesion, proliferation, matrix deposition, and tissue remodeling. SPARC has been shown to regulate the expression of collagen type I and transforming growth factor-β1 in mesangial cells and to be highly expressed during tubulointerstitial fibrosis in rat angiotensin (ANG) II infusion models. We hypothesized that SPARC is a downstream effector of ANG II and that loss of host SPARC function provides a protective effect on renal damage and fibrosis associated with ANG II hypertension. Our results revealed that cultured primary mesangial cells displayed a concentration-dependent increase in SPARC expression in response to ANG II. After a 14-day chronic infusion of ANG II, hypertensive SPARC-null mice exhibited significantly attenuated levels of urinary and renal indicators of oxidative stress and inflammation and decreased renal perivascular and tubulointerstitial fibrosis relative to wild-type hypertensive controls. Moreover, the observed renal protective changes in SPARC-null mice were found to be independent of blood pressure. These results identify SPARC as an effector of ANG II signaling and suggest an important role for SPARC in mediating ANG II-induced oxidative stress, inflammation, and fibrosis.
Secreted protein acidic and rich in cysteine (SPARC), also known as BM-40 and osteonectin, is a 32-kd matricellular glycoprotein that modulates the interaction of cells with the extracellular matrix primarily through regulation of cell adhesion, proliferation, and matrix deposition.1 SPARC is highly expressed during morphogenesis and in tissues undergoing remodeling and repair.2 Activities of several growth factors with essential roles in kidney physiology and pathology are regulated by SPARC. Whereas the activities of platelet-derived growth factor, vascular endothelial growth factor, and insulin-like growth factor are negatively regulated by SPARC, a reciprocal positive autocrine feedback loop has been reported between SPARC and transforming growth factor (TGF)-β1 in glomerular mesangial cells.3 SPARC also regulates the expression of several secreted extracellular matrix proteins and matrix metalloproteinases (MMPs) in certain cell types.1,4 Hence, SPARC seems to possess the necessary biological functions required to regulate the balance between matrix deposition and degradation in the kidney.
The role of SPARC in kidney pathologies has not been studied extensively. In studies done in rats, SPARC was shown to be increased in response to ANG II-dependent hypertension, and the increased expression was in both the glomeruli and the tubulointerstitial cells.5,6 It has also been reported that patients with fibrotic renal injury show increased serum concentrations of SPARC.7 Moreover, SPARC-null mice have been reported to exhibit an amelioration of type 1 diabetes-induced nephropathy characterized by decreases in matrix deposition and TGF-β1 expression.8 In this study, we sought to characterize the role of SPARC in the kidney during ANG II-induced hypertension. To this end, we examined the results of chronic infusion of ANG II on kidney function and morphology in wild-type (SP+/+) and SPARC-null (SP−/−) mice. We found that SP−/− hypertensive mice had significantly decreased levels of urinary TGF-β1, diminished tubulointerstitial and perivascular collagen deposition, and significant attenuations in the levels of reactive oxygen species and inflammatory markers in their kidneys, relative to hypertensive SP+/+ mice. Moreover, the observed amelioration of renal damage in ANG II hypertensive SP−/− mice was shown to be independent of systemic blood pressure changes.
Materials and Methods
Animals
C57BL/6 × 129SvJ SP+/+ and SP−/− male mice (2 to 3 months old) were used in these studies and were a kind gift of Dr. E.H. Sage (Hope Heart Program, Benaroya Research Institute at Virginia Mason, Seattle, WA). Mice were backcrossed against wild-type C57BL/6 mice (The Jackson Laboratory, Bar Harbor, ME) for at least six generations before use in these studies. All experimental procedures were approved by the Laboratory Animal Services of the Medical College of Georgia.
Cell Culture
Primary human and mouse glomerular mesangial cells were purchased from Cambrex (Baltimore, MD) and Dominion Pharmakine (Bilbao, Spain), respectively. Cells were used from passages 3 to 8 and were maintained in specialty medium according to the manufacturer’s recommendations.
Reagents and Antibodies
Anti-human and anti-mouse SPARC antibodies were purchased from Hematological Technologies, Inc. (Essex, VT) and R&D Systems (Minneapolis, MN), respectively. For immunohistochemistry staining, the anti-mouse Mac3 antibodies were purchased from BD Biosciences (San Jose, CA) and Cedarlane Laboratories (Burlington, ON, Canada), and the anti-mouse F4/80 antibody was from AbD Serotec (Raleigh, NC). ANG II for in vitro and in vivo studies was obtained from Sigma (St. Louis, MO) and Phoenix Pharmaceuticals (Belmont, CA), respectively. Dihydroethidium (DHE), a dye used to assess the levels of superoxide radicals, and dichlorofluorescein (DCF), a hydrogen peroxide-specific dye, were obtained from Invitrogen (Carlsbad, CA).
Immunoblotting
Human and mouse mesangial cells (106) were serum-starved in Dulbecco’s modified Eagle’s medium/0.5% fetal bovine serum overnight (15 to 18 hours) before stimulation with ANG II (0 to 100 nmol/L) for 24 hours. Fifty micrograms of cell extracts were subjected to immunoblotting using primary antibodies against human and mouse SPARC as described previously.3
Angiotensin II Infusion and Measurement of Blood Pressure
All animal studies were performed in accordance with the Medical College of Georgia Animal Care and Use Committee. SP+/+ and SP−/− mice were randomly assigned to one of four treatment groups: SP+/+ normotensive (NT) (n = 4), SP+/+ hypertensive (HT) (n = 8), SP−/− normotensive (n = 4), and SP−/− hypertensive (n = 8). The mice in the hypertensive groups were anesthetized with 2% isoflurane, and sterile osmotic pumps (0.25 μl/hour, 14 days; Duret Corporation, Cupertino, CA) were implanted subcutaneously on day 1 of the treatment regimen. The osmotic pumps infused ANG II at a rate of 90 ng/day for a period of 2 weeks. Baseline systolic blood pressure was measured via tail cuff before placement of the pumps and on days 3, 7, 10, and 14 of treatment. Mice were placed in metabolic cages before the end of the treatment, and a 24-hour urine sample was collected. On day 14, animals were again anesthetized and euthanized for sample collection. Tissue collections included plasma, kidney, liver, and aorta. All samples were immediately frozen in liquid nitrogen and then stored at −80°C.
Biochemical Assays
To assess renal damage, the inflammatory and fibrotic responses were assessed in urine samples by enzyme-linked immunosorbent assay (ELISA) for microalbumin (Exocell, Inc., Philadelphia, PA), monocyte chemotactic protein 1 (MCP-1) (BD Biosciences), interleukin (IL)-1β, and active TGF-β1 (R&D Systems). Urinary protein excretion was determined with a standard Bradford assay as previously described.9 Urine samples were also analyzed for creatinine content using the QuantiChrom Creatinine Assay kit (BioAssay Systems, Hayward, CA) for normalization. Serum concentrations of ANG II were determined using an ANG II EIA kit (Cayman Chemical, Ann Arbor, MI).
Histology and Immunohistochemistry
Harvested kidneys were processed by snap-freezing in liquid nitrogen or by fixation either in methyl Carnoy’s fixative or in 10% neutral-buffered formalin (Fisher Scientific, Fairlawn, NJ). The kidneys fixed with methyl Carnoy’s and formalin were embedded in paraffin and cut into 5-μm sections. Frozen sections were placed in Optimal Cutting Temperature embedding medium (Tissue-Tek, Hatfield, PA) before sectioning. Masson’s Trichrome (Sigma) and Picrosirius red (Sigma) histological stains were used to stain for collagens I and III according to the manufacturer’s recommended protocols. To quantify the Masson’s Trichrome staining, 20 random images each from the HT groups (n = 5/group) were assigned random numbers and scored by blinded observers on a scale of 0 to 5 for collagen staining. For immunohistochemistry staining, the antibodies Mac3 and F4/80 were used to assess renal inflammation. Kidney sections embedded in paraffin were de-paraffinized with xylene and rehydrated in graded ethanol before being incubated in 0.3% H2O2 in methanol for 40 minutes to block endogenous peroxidases. Sections were then treated with AutoZyme (BioMedia Corp., Foster City, CA) for 20 minutes at 37°C before incubation with primary antibodies overnight. After washing with phosphate-buffered saline/0.5%Tween 20, the sections were incubated with a horseradish peroxidase-conjugated secondary antibody (Jackson ImmunoResearch Labs, Inc., West Grove, PA) for 1 hour before being developed with the Vectastain ABC Elite kit (Vector Laboratories, Burlingame, CA) and stable 3,3′-diaminobenzidine (ResGen, Huntsville, AL). Slides were then counterstained with hematoxylin and mounted. Images were acquired with a Leica microscope (DM5000) equipped with a Q-Imaging digital camera (Leica Microsystems, Wetzlar, Germany). Quantification of the Mac3 and F4/80 staining was accomplished by analyzing 20 random images from each of the HT groups (n = 5/group), and the number of positive staining cells per field were counted.
Gelatin Zymography
Urine samples were normalized to creatinine concentration, denatured in the absence of a reducing agent, and electrophoresed in 8% sodium dodecyl sulfate-polyacrylamide gel electrophoresis containing 0.1% gelatin (Sigma). Gels were then washed twice for 20 minutes in 2.5% Triton X-100 (Sigma), followed by incubation at 37°C overnight in a buffer consisting of 10 mmol/L Tris-HCl, pH 7.5, 1.25% Triton X-100, 10 mmol/L CaCl2, and 1 μmol/L ZnCl2. Gels were stained with 2.5% Coomassie blue (Fisher Scientific). Proteolysis was detected as a white zone in a blue background and photographed using a Kodak Gel Logic 100 imaging system (Eastman Kodak, Rochester, NY) and Kodak 1-D 3.6 software.
DHE and DCF Staining
Frozen kidney sections embedded in Optimal Cutting Temperature embedding medium were cut into 5-μm sections and mounted on glass slides. Before staining, the frozen slides were allowed to equilibrate to room temperature for 30 minutes and washed in Dulbecco’s phosphate buffer (phosphate-buffered saline; Sigma) for 5 minutes. The sections were then covered with either 10 μmol/L DHE or DCF, placed into a humidified chamber, and incubated in the dark at 37°C for 30 minutes. After the incubation was complete, the slides were washed in phosphate-buffered saline for 5 minutes and mounted with VectaShield Mounting Media for Fluorescence (Vector Laboratories). Images were acquired with a Leica microscope (DM5000) equipped with a Q-Imaging digital camera (Leica Microsystems) under fluorescent light. The staining was quantified by measuring pixel density of 40 random images from each of the HT groups (n = 5/group) using MetaMorph software (Molecular Devices Corp., Sunnyvale, CA).
Reverse Transcription-Polymerase Chain Reaction
Reverse transcription-polymerase chain reaction (RT-PCR) was used to assess the levels of mRNA expression in harvested kidneys. Total RNA was extracted using TRIzol Reagent (Invitrogen) according to manufacturer’s protocol and further purified with the RNeasy isolation kit (Qiagen, Valencia, CA). Two micrograms of RNA was reverse-transcribed using oligo-(dT) primer and Improm-II Reverse Transcriptase (Promega, Madison, WI) into cDNA for subsequent PCR analysis. PCR amplification was done using JumpStart TAQ (Sigma). PCR primer sequences along with the specific cycle number and annealing temperatures used are listed in Table 1. The general reaction conditions were an initial denaturation at 95°C for 4 minutes, followed by a variable number of cycles of 95°C for 30 seconds, the specific annealing temperature for 1 minute, 72°C for 90 seconds, and a final elongation at 72°C for 8 minutes. PCR products were visualized on 2% agarose gels containing ethidium bromide. Relative gene expression levels were quantified using Kodak 1D 3.6 software (Eastman Kodak) to measure band intensity. The intensity of the PCR product bands was normalized to ribosomal protein (rp)S6 expression.
Table 1.
PCR Primer Sequences, Annealing Temperatures, and Cycle Numbers Used in RT-PCR Experiments
| Gene | Forward primer | Reverse primer | Annealing temperature (°C) | Cycle number | Band size |
|---|---|---|---|---|---|
| p22phox | 5′-TTTCACACAGTGGTATTTCG-3′ | 5′-CGTAGTAATTCCTGGTGAGG-3′ | 55 | 35 | 170 |
| gp91phox | 5′-TCACATCCTCTACCAAAACC-3′ | 5′-CCTTTATTTTTCCCCATTCT-3′ | 55 | 35 | 198 |
| Nox-4 | 5′-GTTTTGGCAAGAAAACAGAC-3′ | 5′-GAAATAGAACTGGGTCCACA-3′ | 55 | 25 | 213 |
| p67phox | 5′-GGCCAAGTGAAAAACTACTG-3′ | 5′-GCCTCATAACTGAAGATTGC-3′ | 55 | 30 | 247 |
| p40phox | 5′-TGGAGATGTGATCTTCCTTC-3′ | 5′-CTAGCAGGTCTTTGAACAGG-3′ | 61 | 35 | 236 |
| p47phox | 5′-AGAACAGAGTCATCCCACAC-3′ | 5′-GCTACGTTATTCTTGCCATC-3′ | 55 | 30 | 167 |
| SOD-1 | 5′-TGCAGGACCTCATTTTAATC-3′ | 5′-TGCTCTCCTGAGAGTGAGAT-3′ | 55 | 25 | 153 |
| SOD-2 | 5′-TTACAACTCAGGTCGCTCTT-3′ | 5′-GCTGTCAGCTTCTCCTTAAA-3′ | 55 | 35 | 178 |
| SOD-3 | 5′-ATGTTGGCCTTCTTGTTCTA-3′ | 5′-GTGTCGCCTATCTTCTCAAC-3′ | 55 | 35 | 152 |
| MMP-2 | 5′-TTGGATATTTGCAATGCAGCC-3′ | 5′-AAGGTTGAAGGAAACGAGCGA-3′ | 50 | 35 | 300 |
| MMP-9 | 5′-CCATTTCGACGACGACGAGT-3′ | 5′-CCAAATTGCCGTCCTTATCGTA-3′ | 58 | 35 | 830 |
| MMP-14 | 5′-ACCAGGTACACTTGGTACATATAGGGC-3′ | 5′-GGGAATCTCACAGCTCGGTG-3′ | 56 | 35 | 212 |
| MMP-15 | 5′-GAACCTCTCCTCCCACGACAA-3′ | 5′-GAATCCACCACTTGGAAAGCG-3′ | 56 | 35 | 795 |
| TGF-β1 | 5′-ACCATCCATGACATGAACCG-3′ | 5′-GGTTGCGACCCACGTA-3′ | 56 | 30 | 390 |
| rpS6 | 5′-AAGCTCCGCACCTTCTATGAGA-3′ | 5′-TGACTGGACTCAGACTTAGAAGTAGA-3′ | 56 | 24 | 730 |
Statistical Analysis
ELISA data are presented as mean ± SEM. To determine statistical differences between groups with respect to the ELISA assays, a one-way analysis of variance was performed followed by Kruskal-Wallis post hoc test. For the systolic blood pressure experiment, statistical significance was determined using a two-way analysis of variance followed by a Kruskal-Wallis post hoc test to identify individual differences between specific groups and treatment times. Quantification of the staining and RT-PCR experiments are presented as mean ± SEM, and statistical differences were determined by a two-tailed t-test. A P value of less than 0.05 was considered as statistically significant for all data.
Results
Angiotensin II Regulates SPARC mRNA and Protein Expression in Primary Mesangial Cells
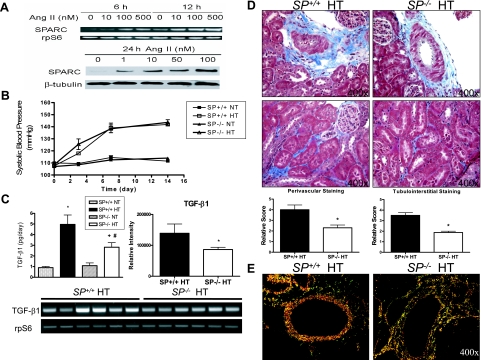
The reported transient increase in the expression of SPARC at sites of glomerular and tubulointerstitial injury and fibrosis, as well as by smooth muscle cells and cells in the adventitia of renal arteries in ANG II-infused rats with moderate hypertension,5 prompted us to test whether a direct effect of ANG II on SPARC expression can be documented in renal cells in culture. ANG II treatment of human (data not shown) and mouse primary mesangial cells (Figure 1A) resulted in a dose-dependent increase in SPARC mRNA expression as early as 6 hours after treatment and lasted up to 12 hours. SPARC protein levels also increased in a dose-dependent manner 24 hours after ANG II treatment of primary mouse mesangial cells (Figure 1A). These results indicate that SPARC expression can be regulated by ANG II. It is interesting that the regulation of expression of another matricellular protein, thrombospondin-1, by ANG II in human mesangial cells has been reported to be responsible for activation of latent TGF-β1, a known mediator of fibrosis and progressive renal disease.10
Figure 1.
SP−/− mice show attenuated urinary TGF-β1 levels, which correlated with decreases in renal collagen deposition in response to ANG II infusion. A: ANG II treatment results in a concentration-dependent increase in SPARC mRNA expression and protein levels in cultured mouse mesangial cells. mRNA levels were measured by RT-PCR after 6- and 12-hour ANG II treatment at varying concentrations. Protein levels were measured by Western blotting 24 hours after ANG II treatment at varying concentrations. Figures are representative of three independent experiments. B: Tail-cuff measurements of the blood pressures of normotensive (NT) (n = 4) and ANG II-hypertensive (HT) SP+/+ and SP−/− mice (n = 8) in a 14-day period. C: Left, ELISA assay was used to measure urinary levels of active TGF-β1 in the four experimental groups (*P < 0.05 versus SP+/+ NT, #P < 0.05 versus SP−/− NT, and +P < 0.05 versus SP+/+ HT). mRNA expression levels of renal TGF-β1 in hypertensive SP+/+ and SP−/− mice (n = 6 each) as determined by RT-PCR; results shown are representative of three independent experiments (right, quantification of the relative band intensities of TGF-β1 following normalization to corresponding rpS6 internal controls. *P < 0.05 versus SP+/+ HT. Bottom, representative agarose gel of PCR products). D: Determination of collagen deposition in kidneys from SP+/+ and SP−/− HT animals using Masson’s Trichrome staining. Top, perivascular staining of collagen (magnification, ×400). Bottom, tubulointerstitial staining of collagen (magnification, ×400). Graphs indicate the quantification of the Masson’s Trichrome staining by blinded scoring. *P < 0.05 versus SP+/+ HT. E: Picrosirius red staining of renal vessels under polarized light to measure perivascular collagen deposition in SP+/+ and SP−/− HT animals (magnification, ×400). Red color depicts mature collagen fibril staining (SP+/+ HT), and orange-green color indicates the presence of immature fibrils (SP−/− HT).
Loss of SPARC Expression Has No Effect on Blood Pressure in Angiotensin II Hypertension
A 14-day ANG II infusion resulted in a significant increase in the systolic blood pressure of both SP+/+ and SP−/− mice that reached a plateau at approximately 140 mmHg (Figure 1B). There was no significant difference in the blood pressure of SP+/+ and SP−/− mice in either normotensive or hypertensive groups. Therefore, the presence or absence of host SPARC does not affect basal or ANG II-induced increases in blood pressure. To confirm that the hypertensive groups had equal levels of circulating ANG II, we measured the serum concentration of ANG II (Supplemental Figure 1 at http://ajp.amjpathol.org). There were no significant differences in serum ANG II concentrations between the SP+/+ and SP−/− hypertensive groups. However, we cannot yet exclude the possibility that differences in renal levels of ANG II can account for the attenuations in renal damage observed in SP−/− hypertensive mice, relative to SP+/+ controls.
SP−/− Mice Show Diminished Levels of TGF-β1 and Collagen Deposition in Response to Angiotensin-Dependent Hypertension
SPARC is known to be involved in a positive autocrine feedback loop with the growth factor TGF-β1.3 Hence, we investigated the differences in urinary TGF-β1 levels in the four experimental groups (Figure 1C). No significant differences were seen in the urinary TGF-β1 levels of the SP+/+ and SP−/− normotensive groups (0.90 ± 0.11 and 1.1 ± 0.25 pg/day, respectively). However, there was a significant decrease (>57%) in the urinary TGF-β1 excretion levels of the SP−/− hypertensive group compared with their SP+/+ counterparts. In agreement with these findings, a significant decrease in the mRNA expression of TGF-β1 was observed in kidneys of hypertensive SP−/− (n = 6) relative to SP+/+ (n = 6) controls (Figure 1C). Because TGF-β1 is known to play a crucial role in extracellular matrix deposition and because SPARC is also known to have a stimulatory effect on collagen deposition, we used histological stains to assess the levels of collagen deposition in the hypertensive groups. As measured by Masson’s Trichrome and Picrosirius red staining (Figure 1, D and E, respectively), the SP−/− hypertensive kidneys showed decreased levels of perivascular and tubulointerstitial matrix, specifically that of collagen I and collagen III. These results strongly support the hypothesis that the loss of SPARC partially ameliorates the increase in TGF-β1 expression and deposition of extracellular matrix in the kidney in response to ANG II-induced hypertension.
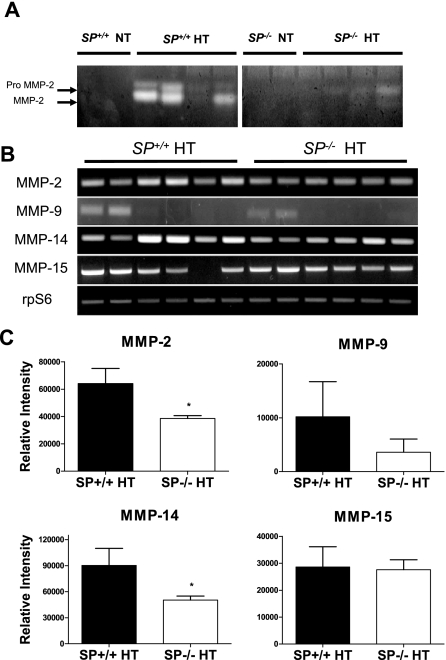
Up-Regulation of Urinary MMP-2 Activity in Response to Angiotensin II Hypertension Is Ameliorated in SP−/− Mice
ANG II has been shown to increase the expression of MMP-2 in rat glomeruli11 and in cultured human endothelial cells.12 We therefore examined the activity levels of MMP-2 in the urine of SP+/+ and SP−/− hypertensive groups. Gelatin zymography on the urine samples indicated a greater increase in both pro-MMP-2 and MMP-2 activity in the SP+/+ hypertensive animals compared with the SP−/− hypertensive counterparts (Figure 2A). In agreement with these findings, a significant decrease in the mRNA expression of MMP-2 and MMP-14 (MT1-MMP), the membrane-associated activator of MMP-2, was observed in kidneys of hypertensive SP−/−, relative to SP+/+ counterparts (n = 6 each) (Figure 2, B and C). These differences in MMP-2 activity levels are likely mediated by ANG II because blood pressure was not different between these groups, and no detectable activity was observed in normotensive groups (Figure 2A). Thus, our data indicate that there is an attenuation of ANG II-induced increase in renal mRNA expression and urinary MMP-2 activity in the SP−/− mice.
Figure 2.
SP−/− mice show an amelioration of urinary MMP-2 activity after ANG II infusion. A: Gelatin zymography revealed that SP+/+ mice exhibited a greater increase in urinary MMP-2 activity after ANG II infusion, compared with SP−/− mice. Figure is representative of three independent experiments. B: mRNA expression levels of renal MMPs in hypertensive SP+/+ and SP−/− mice (n = 6 each) as determined by RT-PCR; results shown are representative of three independent experiments. C: Quantification of the relative band intensities of MMPs after normalization to corresponding rpS6 internal controls. *P < 0.05 versus SP+/+ HT.
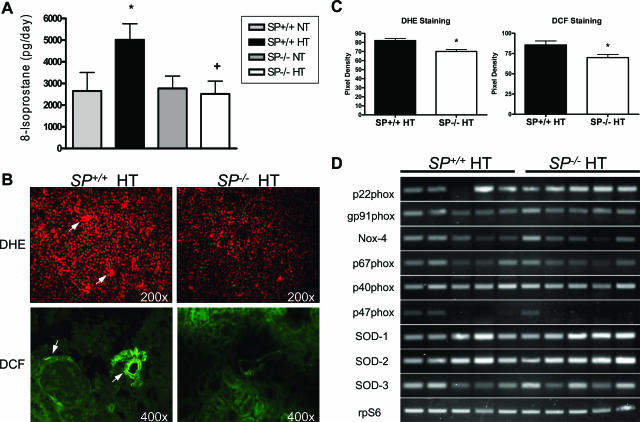
Angiotensin II Hypertension-Induced Increase in Reactive Oxygen Species Formation Is Attenuated in SP−/− Mice
Chronic hypertension caused by ANG II infusion has been shown to be associated with increased production of reactive oxygen species (ROS) in the peripheral vasculature and the kidney.13 Therefore, we investigated whether the loss of SPARC affected ROS production in response to ANG II hypertension. Urinary 8-isoprostane levels, an indicator of reactive oxygen species production, were measured for the four experimental groups (Figure 3A). Whereas 8-isoprostane levels were not significantly different in the normotensive groups, the SP−/− hypertensive group showed a significant (∼twofold) amelioration compared with their SP+/+ hypertensive counterparts. To confirm that the observed differences in reactive oxygen species levels were of renal origin, we assessed ROS production in the kidneys through DHE and DCF staining and by quantification of nicotinamide adenine dinucleotide phosphate [NAD(P)H] oxidase subunit expression levels by RT-PCR. DHE staining in kidney sections from the hypertensive groups showed increased superoxide staining in both cortex and medulla compared with the normotensive groups. Attenuation of this staining in SP−/− hypertensive group was shown to be statistically significant, relative to the SP+/+ hypertensive group (Figure 3, B and C). DCF staining indicated a statistically significant reduction in renal perivascular and peri-glomerular (podocyte-like) production of H2O2 in the SP−/− hypertensive group compared with the SP+/+ hypertensive group (Figure 3, B and C). Semiquantitative RT-PCR for the NAD(P)H oxidase subunits and the three isoforms of superoxide dismutase (SOD) in SP+/+ and SP−/− hypertensive kidneys (n = 5) showed no significant changes in expression of the NAD(P)H oxidase subunits in SP−/− mice (Figure 3D; Supplemental Figure 2 at http://ajp.amjpathol.org). Taken together, our data demonstrate that SP−/− mice display a decreased production of reactive oxygen species in response to ANG II-induced hypertension that is not the result of changes in the expression levels of renal NAD(P)H oxidase subunits or SODs.
Figure 3.
SP−/−mice have an attenuated increase in ROS production from ANG II infusion. A: Urinary levels of 8-isoprostane showed a reduction in oxidative stress in the SP−/− HT group (*P < 0.05 versus SP+/+ NT, and +P < 0.05 versus SP+/+ HT). B: Top, representative DHE staining of superoxide radicals in kidneys from SP+/+ and SP−/− HT animals. SP−/− HT mouse kidneys showed decreased levels of superoxide (magnification, ×200). Bottom, representative DCF staining of hydrogen peroxide in kidneys from SP+/+ and SP−/− HT animals. Staining indicates that SP−/− HT kidneys have reduced levels of hydrogen peroxide (magnification, ×400). Arrows indicate areas of increased ROS production. C: Quantification of the pixel density of DHE and DCF stainings. *P < 0.05 versus SP+/+ HT. D: Representative RT-PCR results examining the expression levels of NAD(P)H oxidase subunits as well as SOD isoforms in hypertensive SP+/+ and SP−/− mice (n = 5 each). Data shown are representative of three independent experiments.
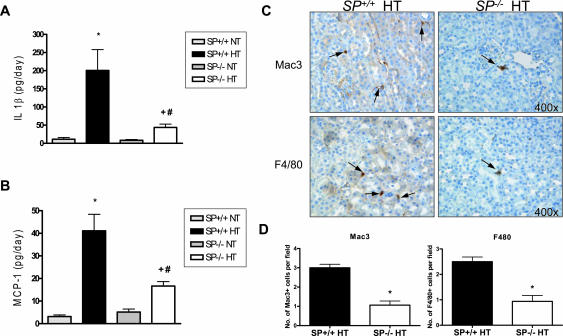
SP−/− Mice Have a Diminished Inflammatory Response to Angiotensin II Hypertension
It has been reported that hypertension results in an inflammatory response that exacerbates the disease state and associated end organ damage.14,15 Therefore, we measured the inflammatory response of the four experimental groups in this study. Measurements of urinary levels of IL-1β and MCP-1, known markers of inflammation, did not show a significant difference between SP+/+ and SP−/− NT groups (Figure 4, A and B). However, the SP+/+ ANG II HT group showed a significant (∼threefold) increase in the levels of urinary IL-1β compared with the SP−/− HT group (Figure 4A). A similar, albeit less pronounced, increase (∼1.8-fold) was seen in the SP+/+ HT urinary levels of MCP-1 (Figure 4B). To verify that the observed ameliorations in the urinary levels of inflammatory markers in the SP−/− hypertensive group were of renal origin, we assessed the presence of infiltrating macrophages in the kidney. Analysis of two markers of mature macrophages (Mac 3 and F4/80) showed significantly decreased staining in the SP−/− HT kidneys, relative to SP+/+ HT counterparts (Figure 4, C and D). Quantification of the Mac3 and F4/80 staining revealed a significant diminution (∼3- and ∼2.5-fold, respectively) in the number of macrophages infiltrating the tubulointerstitial space. These results, combined with the urinary levels of IL-1β and MCP-1, indicate that the loss of host SPARC decreases the renal inflammatory response in ANG II-dependent hypertension.
Figure 4.
SP−/− mice exhibit a decreased renal inflammatory response after ANG II infusion. A: ELISA assay of urinary IL-1β levels indicated that SP−/− HT animals have a diminished inflammatory response to ANG II compared with SP+/+ HT animals (*P < 0.05 versus SP+/+ NT, #P < 0.05 versus SP−/− NT, and +P < 0.05 versus SP+/+ HT). B: ELISA assay of urinary MCP-1 levels indicates that SP−/− HT animals have a diminished response to ANG II infusion (*P < 0.05 versus SP+/+ NT, #P < 0.05 versus SP−/− NT, and +P < 0.05 versus SP+/+ HT). C: Immunohistochemistry staining of Mac3 (top) and F4/80 (bottom) indicated that there is a reduced inflammatory response in the kidneys of SP−/− HT animals (magnification, ×400). Arrows indicate cells positive for Mac3 and/or F4/80 staining. D: Quantification of the number of Mac3 (left) and F4/80 (right) positive staining cells per field. *P < 0.05 versus SP+/+ HT.
Discussion
Activation of the renin-angiotensin system has been reported to play a major pathophysiological role in renal injury and ischemic cardiovascular events independent of effects on blood pressure.16 Angiotensin II has been implicated as a major contributor to renal and cardiovascular injury by inducing reactive oxygen species formation and inflammation and vascular fibrosis and remodeling.17,18,19,20 Studies have shown that doses of ANG II responsible for moderate increases in blood pressure result in vascular, glomerular, and tubulointerstitial injury, marked by cellular proliferation, inflammation, and fibrosis.21 Glomerular and tubulointerstitial expression of SPARC has been reported to be markedly elevated in passive and nephrotoxic Heymann nephritis and in 5/6 nephrectomy and ANG II-dependent hypertension.5,6,22,23 Whereas SPARC is constitutively expressed at low levels in podocytes (glomerular epithelial cells) under nonpathological conditions, all three glomerular cell types—podocytes, mesangial cells, and endothelial cells—produce SPARC under pathological conditions.23 We therefore tested the hypothesis that genetic deficiency of SPARC attenuates renal damage in ANG II-induced hypertension. Using a model involving ANG II infusion, SP−/− mice were shown to have significant ameliorations in i) the extent of urinary and renal reactive oxygen species formation, ii) the levels of urinary and renal inflammatory markers, and iii) the extent of renal matrix deposition. These renal changes in the SP−/− mice occurred independently of changes in blood pressure.
Despite establishing an in vitro link between ANG II and SPARC expression in cultured primary mesangial cells, our ANG II infusion studies did not reveal significant increases in either glomerular damage or expression of SPARC in SP+/+ HT animals, relative to NT controls (data not shown). However, the observed amelioration of fibrosis associated with tubulointerstitium and renal vessels in SP−/− HT mice, relative to SP+/+ HT counterparts is consistent with findings of Pichler et al5 and can, in part, be the result of a transient increase in SPARC expression after ANG II infusion. Interestingly, analysis of kidney functional data (proteinuria and microalbuminuria) did not reveal a statistically significant difference between HT SP+/+ and SP−/− animals (data not shown). A plausible hypothesis that could, at least in part, account for these outcomes is that overt differences between HT SP+/+ and SP−/− mice in renal function can only be observed if animals are subjected to a greater insult, such as when ANG II is combined with high salt. Concordant with this hypothesis, we have been able to detect about a fivefold amelioration of microalbuminuria in HT SP−/− animals subjected to a 14-day ANG II infusion combined with a high dietary salt (4% NaCl) intake relative to HT SP+/+ controls (M.J. Socha, M. Mahiani, J.D. Imig, K. Motamed, unpublished results).
Previous work has established a link between ANG II infusion and increases in vascular oxidative stress.24 This pro-oxidative effect of ANG II is reported in endothelial and vascular smooth muscle cells and is a result of the hormonal actions of ANG II rather than its effects on blood pressure.24 Reports indicate that ANG II, through the AT-1 receptor, increases the expression of the NAD(P)H oxidase subunits p22phox and Nox-1.13 Our findings demonstrated that ANG II-infused SPARC-null hypertensive mice display a significant diminution in renal oxidative stress, independent of significant alterations in the expression of NAD(P)H oxidase subunits and superoxide dismutases. Changes in the activity levels of NAD(P)H oxidases and the SODs in the absence of regulation of their expression is a possibility that cannot be excluded. Another possibility is that the alteration of oxidative stress could come from other sources of excess superoxide anions besides NAD(P)H oxidase. These include the mitochondrial electron transport chain,25 xanthine oxidase,26 and uncoupled nitric oxide synthase.27 These sources, along with the activity levels of NAD(P)H oxidases and SOD isoforms, are currently under investigation to elucidate fully the role of SPARC in renal oxidative stress during hypertension (M.J. Socha, M. Mahiani, J.D. Imig, K. Motamed, unpublished data).
Vascular and renal remodeling during hypertension has been shown to be partially mediated by inflammatory cytokines.28 This makes the inflammatory response a key step in renal and vascular injury resulting from ANG II hypertension. In animal and human studies, the pro-inflammatory properties of ANG II have been reported in large conduit and small arteries, in the heart, and in the kidney.29,30,31 Infiltration of monocytes/macrophages in the renal perivascular space and ANG II-mediated inflammatory damage in small renal vessels have been shown to be ameliorated by inhibition of nuclear factor-κB, the main mediator of ANG II-induced inflammatory injury.32,33 Our present findings illustrate a diminished inflammatory response in the SP−/− hypertensive mice. A decrease in the levels of urinary MCP-1 in the angiotensin hypertensive SP−/− mice correlated with a diminution of the number of infiltrating monocytes. This evidence implicates SPARC as a potential regulator of the inflammatory response in ANG II-induced hypertension.
It has been reported that addition of exogenous SPARC to cultured human monocytes increases their expression of proteinases MMP-1 and MMP-9.4 Matrix metalloproteinases are key players in vascular and renal remodeling and have been shown to be up-regulated by ANG II.34 On the other hand, it is known that glomerular and tubular fibrosis are hallmarks of renal injury resulting from hypertension.35 TGF-β1 is well established as a potent stimulator of extracellular matrix production during renal injury,36 and its induction by SPARC has been reported in mouse glomerular mesangial cells in vitro and in a rat model of glomerulonephritis.37,38 Herein, we have demonstrated that SPARC deficiency is associated with significant attenuations in urinary TGF-β1 levels, MMP-2 activity, and deposition of extracellular matrix in angiotensin-dependent hypertension. Therefore, it can be hypothesized that SPARC expression in response to ANG II hypertension plays a dual role in mediating renal matrix remodeling through i) stimulation of the infiltration of monocytes and their expression of MMPs, and ii) augmentation of fibrosis via up-regulation of TGF-β1.
In summary, we have shown in this study that the kidney damage resulting from ANG II-induced hypertension is ameliorated in SPARC-null mice. This is evidenced by a decrease in matrix deposition, reactive oxygen species, and the inflammatory response that occurs during ANG II-induced hypertension. Taken together, these findings suggest that SPARC can play a critical role in the progression of renal injury in response to ANG II and could prove to be an important therapeutic target for patients with hypertension.
Supplementary Material
Acknowledgments
We thank Dr. E. Helene Sage for providing SP−/− and SP+/+ mice.
Footnotes
Address reprint requests to Kouros Motamed, Ph.D., Vascular Biology Center, Medical College of Georgia,1459 Laney Walker Blvd., CB-3306, Augusta, GA 30912. E-mail: kmotamed@mcg.edu.
Supported in part by National Institute of Health grants K01-CA-089689 (to K.M.) and HL59699 (to J.D.I.).
Supplemental material for this article can be found on http://ajp. amjpathol.org.
M.J.S. and M.M. contributed equally to this work.
References
- Brekken RA, Sage EH. SPARC, a matricellular protein: at the crossroads of cell-matrix. Matrix Biol. 2000;19:569–580. doi: 10.1016/s0945-053x(00)00105-0. [DOI] [PubMed] [Google Scholar]
- Lane TF, Sage EH. The biology of SPARC, a protein that modulates cell-matrix interactions. FASEB J. 1994;8:163–173. [PubMed] [Google Scholar]
- Francki A, Bradshaw AD, Bassuk JA, Howe CC, Couser WG, Sage EH. SPARC regulates the expression of collagen type I and transforming growth factor-β1 in mesangial cells. J Biol Chem. 1999;274:32145–32152. doi: 10.1074/jbc.274.45.32145. [DOI] [PubMed] [Google Scholar]
- Shankavaram UT, DeWitt DL, Funk SE, Sage EH, Wahl LM. Regulation of human monocyte matrix metalloproteinases by SPARC. J Cell Physiol. 1997;173:327–334. doi: 10.1002/(SICI)1097-4652(199712)173:3<327::AID-JCP4>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Pichler RH, Hugo C, Shankland SJ, Reed MJ, Bassuk JA, Andoh TF, Lombardi DM, Schwartz SM, Bennett WM, Alpers CE, Sage EH, Johnson RJ, Couser WG. SPARC is expressed in renal interstitial fibrosis and in renal vascular injury. Kidney Int. 1996;50:1978–1989. doi: 10.1038/ki.1996.520. [DOI] [PubMed] [Google Scholar]
- Wu LL, Cox A, Roe CJ, Dziadek M, Cooper ME, Gilbert RE. Secreted protein acidic and rich in cysteine expression after subtotal nephrectomy and blockade of the renin-angiotensin system. J Am Soc Nephrol. 1997;8:1373–1382. doi: 10.1681/ASN.V891373. [DOI] [PubMed] [Google Scholar]
- Kanauchi M, Nishioka M, Dohi K. Secreted protein acidic and rich in cysteine (SPARC) in patients with diabetic nephropathy and tubulointerstitial injury. Diabetologia. 2000;43:1076–1077. doi: 10.1007/s001250051493. [DOI] [PubMed] [Google Scholar]
- Taneda S, Pippin JW, Sage EH, Hudkins KL, Takeuchi Y, Couser WG, Alpers CE. Amelioration of diabetic nephropathy in SPARC-null mice. J Am Soc Nephrol. 2003;14:968–980. doi: 10.1097/01.asn.0000054498.83125.90. [DOI] [PubMed] [Google Scholar]
- Elmarakby AA, Quigley JE, Pollock DM, Imig JD. Tumor necrosis factor α blockade increases renal Cyp2c23 expression and slows the progression of renal damage in salt-sensitive hypertension. Hypertension. 2006;47:557–562. doi: 10.1161/01.HYP.0000198545.01860.90. [DOI] [PubMed] [Google Scholar]
- Naito T, Masaki T, Nikolic-Paterson DJ, Tanji C, Yorioka N, Kohno N. Angiotensin II induces thrombospondin-1 production in human mesangial cells via p38 MAPK and JNK: a mechanism for activation of latent TGF-β1. Am J Physiol. 2004;286:F278–F287. doi: 10.1152/ajprenal.00139.2003. [DOI] [PubMed] [Google Scholar]
- Bolbrinker J, Markovic S, Wehland M, Melenhorst WB, van Goor H, Kreutz R. Expression and response to angiotensin-converting enzyme inhibition of matrix metalloproteinases 2 and 9 in renal glomerular damage in young transgenic rats with renin-dependent hypertension. J Pharmacol Exp Ther. 2006;316:8–16. doi: 10.1124/jpet.105.093112. [DOI] [PubMed] [Google Scholar]
- Arenas IA, Xu Y, Lopez-Jaramillo P, Davidge ST. Angiotensin II-induced MMP-2 release from endothelial cells is mediated by TNF-alpha. Am J Physiol. 2004;286:C779–C784. doi: 10.1152/ajpcell.00398.2003. [DOI] [PubMed] [Google Scholar]
- Chabrashvili T, Kitiyakara C, Blau J, Karber A, Aslam S, Welch WJ, Wilcox CS. Effects of ANG II type 1 and 2 receptors on oxidative stress, renal NADPH oxidase, and SOD expression. Am J Physiol. 2003;285:R117–R124. doi: 10.1152/ajpregu.00476.2002. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Ruiz-Ortega M, Lorenzo O, Ruperez M, Esteban V, Egido J. Inflammation and angiotensin II. Int J Biochem Cell Biol. 2003;35:881–900. doi: 10.1016/s1357-2725(02)00271-6. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ortega M, Esteban V, Ruperez M, Sanchez-Lopez E, Rodriguez-Vita J, Carvajal G, Egido J. Renal and vascular hypertension-induced inflammation: role of angiotensin II. Curr Opin Nephrol Hypertens. 2006;15:159–166. doi: 10.1097/01.mnh.0000203190.34643.d4. [DOI] [PubMed] [Google Scholar]
- Wolf G, Butzmann U, Wenzel UO. The renin-angiotensin system and progression of renal disease: from hemodynamics to cell biology. Nephron Physiol. 2003;93:P3–P13. doi: 10.1159/000066656. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ortega M, Ruperez M, Esteban V, Rodriguez-Vita J, Sanchez-Lopez E, Carvajal G, Egido J. Angiotensin II: a key factor in the inflammatory and fibrotic response in kidney diseases. Nephrol Dial Transplant. 2006;21:16–20. doi: 10.1093/ndt/gfi265. [DOI] [PubMed] [Google Scholar]
- Touyz RM, Schiffrin EL. Reactive oxygen species in vascular biology: implications in hypertension. Histochem Cell Biol. 2004;122:339–352. doi: 10.1007/s00418-004-0696-7. [DOI] [PubMed] [Google Scholar]
- Virdis A, Schiffrin EL. Vascular inflammation: a role in vascular disease in hypertension? Curr Opin Nephrol Hypertens. 2003;12:181–187. doi: 10.1097/00041552-200303000-00009. [DOI] [PubMed] [Google Scholar]
- Mezzano SA, Ruiz-Ortega M, Egido J. Angiotensin II and renal fibrosis. Hypertension. 2001;38:635–638. doi: 10.1161/hy09t1.094234. [DOI] [PubMed] [Google Scholar]
- Johnson RJ, Alpers CE, Yoshimura A, Lombardi D, Pritzl P, Floege J, Schwartz SM. Renal injury from angiotensin II-mediated hypertension. Hypertension. 1992;19:464–474. doi: 10.1161/01.hyp.19.5.464. [DOI] [PubMed] [Google Scholar]
- Floege J, Johnson RJ, Alpers CE, Fatemi-Nainie S, Richardson CA, Gordon K, Couser WG. Visceral glomerular epithelial cells can proliferate in vivo and synthesize platelet-derived growth factor B-chain. Am J Pathol. 1993;142:637–650. [PMC free article] [PubMed] [Google Scholar]
- Floege J, Alpers CE, Sage EH, Pritzl P, Gordon K, Johnson RJ, Couser WG. Markers of complement-dependent and complement-independent glomerular visceral epithelial cell injury in vivo: expression of antiadhesive proteins and cytoskeletal changes. Lab Invest. 1992;67:486–497. [PubMed] [Google Scholar]
- Seshiah PN, Weber DS, Rocic P, Valppu L, Taniyama Y, Griendling KK. Angiotensin II stimulation of NAD(P)H oxidase activity: upstream mediators. Circ Res. 2002;91:406–413. doi: 10.1161/01.res.0000033523.08033.16. [DOI] [PubMed] [Google Scholar]
- Liu Y, Fiskum G, Schubert D. Generation of reactive oxygen species by the mitochondrial electron transport chain. J Neurochem. 2002;80:780–787. doi: 10.1046/j.0022-3042.2002.00744.x. [DOI] [PubMed] [Google Scholar]
- McNally JS, Davis ME, Giddens DP, Saha A, Hwang J, Dikalov S, Jo H, Harrison DG. Role of xanthine oxidoreductase and NAD(P)H oxidase in endothelial superoxide production in response to oscillatory shear stress. Am J Physiol. 2003;285:H2290–H2297. doi: 10.1152/ajpheart.00515.2003. [DOI] [PubMed] [Google Scholar]
- Andrew PJ, Mayer B. Enzymatic function of nitric oxide synthases. Cardiovasc Res. 1999;43:521–531. doi: 10.1016/s0008-6363(99)00115-7. [DOI] [PubMed] [Google Scholar]
- Savoia C, Schiffrin EL. Inflammation in hypertension. Curr Opin Nephrol Hypertens. 2006;15:152–158. doi: 10.1097/01.mnh.0000203189.57513.76. [DOI] [PubMed] [Google Scholar]
- Diep QN, El Mabrouk M, Cohn JS, Endemann D, Amiri F, Virdis A, Neves MF, Schiffrin EL. Structure, endothelial function, cell growth, and inflammation in blood vessels of angiotensin II-infused rats: role of peroxisome proliferator-activated receptor-γ. Circulation. 2002;105:2296–2302. doi: 10.1161/01.cir.0000016049.86468.23. [DOI] [PubMed] [Google Scholar]
- Kiarash A, Pagano PJ, Tayeh M, Rhaleb NE, Carretero OA. Upregulated expression of rat heart intercellular adhesion molecule-1 in angiotensin II- but not phenylephrine-induced hypertension. Hypertension. 2001;37:58–65. doi: 10.1161/01.hyp.37.1.58. [DOI] [PubMed] [Google Scholar]
- Ruiz-Ortega M, Bustos C, Hernandez-Presa MA, Lorenzo O, Plaza JJ, Egido J. Angiotensin II participates in mononuclear cell recruitment in experimental immune complex nephritis through nuclear factor-κB activation and monocyte chemoattractant protein-1 synthesis. J Immunol. 1998;161:430–439. [PubMed] [Google Scholar]
- Barnes PJ, Karin M. Nuclear factor-κB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. 1997;336:1066–1071. doi: 10.1056/NEJM199704103361506. [DOI] [PubMed] [Google Scholar]
- Hernández-Presa M, Bustos C, Ortego M, Tunon J, Renedo G, Ruiz-Ortega M, Egido J. Angiotensin-converting enzyme inhibition prevents arterial nuclear factor-κB activation, monocyte chemoattractant protein-1 expression, and macrophage infiltration in a rabbit model of early accelerated atherosclerosis. Circulation. 1997;95:1532–1541. doi: 10.1161/01.cir.95.6.1532. [DOI] [PubMed] [Google Scholar]
- Luchtefeld M, Grote K, Grothusen C, Bley S, Bandlow N, Selle T, Struber M, Haverich A, Bavendiek U, Drexler H, Schieffer B. Angiotensin II induces MMP-2 in a p47phox-dependent manner. Biochem Biophys Res Commun. 2005;328:183–188. doi: 10.1016/j.bbrc.2004.12.152. [DOI] [PubMed] [Google Scholar]
- Yoshioka K, Tohda M, Takemura T, Akano N, Matsubara K, Ooshima A, Maki S. Distribution of type I collagen in human kidney diseases in comparison with type III collagen. J Pathol. 1990;162:141–148. doi: 10.1002/path.1711620207. [DOI] [PubMed] [Google Scholar]
- Border WA, Noble NA. Transforming growth factor β in tissue fibrosis. N Engl J Med. 1994;331:1286–1292. doi: 10.1056/NEJM199411103311907. [DOI] [PubMed] [Google Scholar]
- Francki A, Motamed K, McClure TD, Kaya M, Murri C, Blake DJ, Carbon JG, Sage EH. SPARC regulates cell cycle progression in mesangial cells via its inhibition of IGF-dependent signaling. J Cell Biochem. 2003;88:802–811. doi: 10.1002/jcb.10424. [DOI] [PubMed] [Google Scholar]
- Bassuk JA, Pichler R, Rothmier JD, Pippen J, Gordon K, Meek RL, Bradshaw AD, Lombardi D, Strandjord TP, Reed M, Sage EH, Couser WG, Johnson R. Induction of TGF-β1 by the matricellular protein SPARC in a rat model of glomerulonephritis. Kidney Int. 2000;57:117–128. doi: 10.1046/j.1523-1755.2000.00811.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.