Abstract
Stromal-epithelial interactions play a critical role in development of benign prostatic hyperplasia. We have previously shown that stromal cells associated with prostatic carcinoma can potentiate proliferation and reduce cell death of prostatic epithelial cells. Genetic alterations in stromal cells affect stromal-epithelial interactions and modulate epithelial growth. The c-Jun proteins that are early transcription factor molecules have been shown to regulate stromal-epithelial interactions via paracrine signals. Moreover, the Jun-family member proteins have been shown to play an important role in proper development of the genitourinary organs. In this study, we show that c-Jun protein in fibroblasts regulates production and paracrine signals of insulin-like growth factor-1 (IGF-1). c-jun+/+ fibroblasts secrete higher levels of IGF-1 and stimulate benign prostatic hyperplasia-1 cellular proliferation. In addition, stromally produced IGF-1 up-regulates epithelial mitogen-activated protein kinase, Akt, and cyclin D protein levels while down-regulating the cyclin-dependent kinase inhibitor p27. These data suggest that stromally expressed c-Jun may promote prostatic epithelial proliferation through IGF-1 as a paracrine signal that, in turn, can promote prostate epithelial proliferation. Identification of the signal transduction pathways between prostate epithelial cells and the surrounding stromal cells will improve our understanding of the normal and abnormal biology in prostatic diseases.
Benign prostatic hyperplasia (BPH) is the most common age-related proliferative abnormality of the human prostate affecting elderly men throughout the world. Half of all men have BPH identifiable histologically by age 60, and by age 85 the prevalence is ∼90%.1 The excessive cell proliferation associated with BPH causes benign prostatic enlargement, bladder outlet obstruction, and lower urinary tract symptoms, which afflict the patients.2 BPH is a histological diagnosis associated with both epithelial and stromal (fibroblasts and smooth muscle cells of prostate) hyperplasia.3 Cellular alterations that include changes in proliferation, differentiation, apoptosis, and senescence in the epithelium and stroma are implicated in the pathogenesis of BPH. Medical treatments available to patients with BPH target both prostatic stromal and epithelial cells.
Watchful waiting, pharmacological therapy, and surgery are three treatment modalities for BPH patients. Medical therapy is a long-term commitment for the patient. Surveys show that a large number of patients discontinue therapy, often because they perceive lack of efficacy and/or experience side effects.4,5 We can accordingly improve our treatment of BPH by better targeted medical therapy to decrease the number of invasive therapies for this benign disease. Better alternative and improved therapies can be achieved by studying the normal and abnormal reciprocal cross talk between prostatic stromal and epithelial cells.
Stromal-epithelial interactions play a critical role in the development and growth of the prostate gland and BPH. Cunha and colleagues6,7,8 have demonstrated that the stromal embryonic tissue is responsible for instructing the proper development of prostate epithelial cells. Tissue recombinants between embryonic urogenital sinus mesenchyme and adult prostatic tissue have demonstrated that the fetal mesenchyme can drive differentiation and growth of adult prostatic epithelial cells.9 It has been proposed that BPH may be caused by reactivation of dormant embryonic growth in the adult stroma.10 The proliferation of the stromal elements can stimulate the ingrowth of epithelial cells to produce a benign hyperplastic growth that is recognized histologically as BPH. Paracrine pathways through stromal cells can activate epithelial budding and subsequent BPH nodule formation.
Several families of growth factors, which have been shown to act as paracrine mediators of stromal-epithelial interactions, are involved in the development of prostate cancer and BPH. These include the following: fibroblast growth factor, insulin-like growth factor (IGF), epithelial growth factor, hepatocyte growth factor, and transforming growth factor-β. Although most of these factors are predominantly stimulators of proliferation,11,12,13,14,15,16 some can have dual functions as both stimulators of proliferation and promoters of cell death.17,18
Differential expression of some genes in the stroma has been shown to produce signals that affect epithelial cell growth. For example, Szabowski and colleagues19,20 demonstrated that in the presence of stromally expressed c-Jun, KGF [keratinocyte growth factor (also known as fibroblast growth factor-7)] and granulocyte macrophage colony-stimulating factor (GM-CSF) affect development of epithelial cells. Recently, the activator protein-1 (AP-1) family of proteins has been postulated to play a critical role in stromal-epithelial interactions and regulation of epithelial proliferation and differentiation.19,20
AP-1 family transcription factor is a dimeric protein complex composed of heterodimers between Jun (c-Jun, JunB, and JunD), Fos (c-Fos, FosB, Fra-1, and Fra-2), and ATF family gene products. The predominant forms of AP-1 in most cells are Fos/Jun heterodimers, which have a high affinity for binding to an AP-1 site. Extracellular or intracellular signals, including growth factors, transforming oncoproteins, and UV irradiation, stimulate c-Jun activation and regulate c-Jun-dependent transcription.21,22 Potentially, therefore, activated c-Jun could play an important role in stromal-epithelial interactions.20,23 The Jun-family of proteins function as critical transcription factors, regulating the expression of many genes such as IGFs, fibroblast growth factors, epithelial growth factor, hepatocyte growth factor, and transforming growth factor-β. It is not surprising then that they may also regulate downstream paracrine effectors between stromal and epithelial cells.19,24,25 Because development of BPH is closely linked to the interplay between stromal and epithelial cells, we suspect that the relative expression of different Jun-family proteins in the prostatic stroma can have a paracrine effect on epithelial hyperplasia. Further, other groups have suggested that IGF-1 and IGF binding protein-3 (IGFBP-3) are associated with BPH development and prostatic cancer.11,14,26,27
In the present study, we demonstrate that expression of c-Jun in the fibroblastic stroma can promote secretion of IGF-1. We show IGF-1 acts as a paracrine molecule that stimulates prostate epithelial cell proliferation through activating specific target genes. Therefore, paracrine signals from stromal cells with genetically modified c-Jun can regulate prostatic epithelial proliferation. These signaling pathways may play an important role in development of BPH in elderly men.
Materials and Methods
Cell Culture and Treatment
Human BPH-1 cells that were immortalized with SV-40 large T-antigen cells were kindly provided by Dr. Simon Hayward (Vanderbilt University, Nashville, TN). c-Jun wild-type (c-jun+/+) and c-Jun knockout (c-jun−/−) mouse embryonic fibroblasts were obtained from Dr. Timothy C. Chambers (University of Arkansas for Medical Sciences, Little Rock, AR). Prostate-specific c-jun−/− cells were not possible to obtain because this strain of mice is embryonically lethal at mid gestation (∼12.5 days of gestation).28 The hormone-sensitive human prostate cancer cell line CWR22-Rv1 was obtained from American Type Culture Collection (Manassas, VA). Cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) tissue culture medium supplemented with 2 mmol/L l-glutamine, 10% fetal bovine serum (FBS), and 1% penicillin-streptomycin at 37°C with 5% CO2.
In our co-culture experiment, BPH-1 cells were cultured in 0.4-μm-pore size permeable membrane transwell insert (Corning Inc., Corning, NY) in the upper co-culture compartment system, and mouse fibroblasts were cultured in the lower co-culture compartment using 24-well plates. BPH-1 cells (5 × 103) were seeded in transwell in each well. When 50% confluent, BPH-1 cells were serum-starved (FBS-free DMEM containing 50 μg/ml penicillin-streptomycin and 2 mmol/L l-glutamine) for 24 hours. At the same time, 5 × 104 fibroblasts were plated onto 24-well plates. When 90% confluent, fibroblasts were cultured using 1% FBS DMEM medium for 24 hours. Transwells containing BPH-1 cells were subsequently transferred to 24-well plates with fibroblasts or no cells for the controls. The stromal-epithelial co-cultures were maintained in 1% FBS DMEM medium. Co-cultures were performed for 72 hours.
Cell Proliferation Assays
Cell proliferation was determined by the MTS method in accordance with the manufacturer’s instructions (Cell Titer 96 aqueous assay; Promega, Madison, WI). In brief, MTS substrates were added and incubated for 2 hours at 37°C. Absorbance was measured at 490 nm using a microtiter plate reader. Proliferation of control cells was set at 100%, and absorbance of wells with medium and without cells was set at zero. Each measurement was performed in triplicate.
Chemicals and Antibodies
Recombinant mouse IGF-1, monoclonal anti-mouse IGF-1 neutralizing antibody, and anti-mouse IGF-1 enzyme-linked immunosorbent assay (ELISA) kit were obtained from R&D Systems Inc. (Minneapolis, MN). IGF-1 receptor inhibitor I-OMe-AG538 was obtained from EMD Calbiochem (La Jolla, CA) and dissolved in dimethyl sulfoxide at a stock concentration of 2 mmol/L at −20°C. Fresh dilutions in medium were made for each experiment. PI3K inhibitor LY294002 was obtained from Cell Signaling (Beverly, MA). The following antibodies were obtained: horseradish peroxidase-conjugated secondary antibody (goat anti-mouse, goat anti-rabbit), IGF-IRα, c-Jun (for immunohistochemical analysis), cyclin D2, cyclin-dependent kinase (CDK4 and CDK6), and p27 from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA); c-Jun (for Western blot), phospho-IGF-1Rβ (Tyr1131), phospho-IRS-1 (Ser636/639), Akt, phospho-Akt (Ser473), extracellular signal-regulated kinase (ERK), phospho-ERK (Thr202/Tyr204), p38, phospho-p38 (Thr180/Tyr182), c-Jun NH2-terminal kinase (JNK), phospho-JNK (Thr183/Tyr185), cyclin D1, and cyclin D3 from Cell Signaling; and GAPDH antibody from Abcam, Inc. (Cambridge, MA).
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue blocks and frozen tissue sections embedded in OCT were cut into 5-μm sections and mounted on positively charged slides. Tissue sections were deparaffinized with xylene and rehydrated with graded alcohol solutions. After antigen retrieval in citrate buffer (pH 6.0), endogenous peroxidase was quenched in 3% hydrogen peroxide/phosphate-buffered saline (PBS) for 5 minutes. Slides were washed with PBS and water. Tissue sections were incubated with the primary antibody of interest per the manufacturer’s recommendations. After incubation with primary antibody, slides were washed with PBS and incubated with biotinylated streptavidin-horseradish peroxidase secondary antibody. Slides were then counterstained with hematoxylin. Positive and negative controls were used throughout all immunostaining protocols.
Cell Extracts and Western Blot Analysis
Cells were harvested for total cell lysates with RIPA buffer (1% Nonidet P-40, 50 mmol/L Tris-HCl, pH 8.0, 150 mmol/L NaCl, 0.5% deoxycholate, and 0.1% sodium dodecyl sulfate) containing a mixture of protease inhibitors (cocktail 1×, 1 mmol/L phenylmethyl sulfonyl fluoride, 20 mmol/L β-glycerophosphate, 40 mmol/L NaF, and 3 mmol/L Na3VO4). After sonication for 15 seconds, cell debris was discarded by centrifugation at 12,000 × g for 10 minutes at 4°C, and the protein concentration was determined by BCA protein assay reagent (Pierce, Rockford, IL). The lysate was boiled for 10 minutes and frozen at −80°C. Western blot was performed as previously described.29
Semiquantitative Reverse Transcriptase-Polymerase Chain Reaction (RT-PCR) Analysis
Total RNA was isolated with the RNeasy mini kit (Qiagen, Valencia, CA) according to the manufacturer’s instructions. The RNA yield and purity were evaluated by measuring A260/A280 and agarose gel electrophoresis. RT-PCR was performed using a Superscript One-Step RT-PCR kit (Invitrogen Life Technologies, Carlsbad, CA). Of the total RNA, 0.4 μg was used for 25 μl of the reaction system. cDNA synthesis was performed at 50°C for 30 minutes using the following temperature cycles and times: denaturation at 94°C for 50 seconds, annealing at 56°C for 50 seconds, and polymerization at 72°C for 1 minute (total number of cycles = 30), final extension at 72°C for 10 minutes. In each reaction, the same amount of GAPDH was used as an internal control. The primers used for PCR were as follows: IGF-1, 5′-ATGTCGTCTTCACACCT-3′ (forward) and 5′-ACTTGTGTTCTTCAAATGTACTTCC-3′ (reverse); GM-CSF, 5′-ATCAAAGAAGCCCTAAACCTCCTG-3′ (forward) and 5′-CTGGCCTGGGCTTCCTCATT-3′ (reverse); KGF, 5′-CTGGCCTTGTCACGACCTGTTTCT-3′ (forward); and 5′-CCCTTTCACTTTGCCTCGTTTGTC-3′ (reverse); GAPDH, 5′-TCCACCACCCTGTTGCTGTA-3′ (forward) and 5′-ACCACAGTCCATGCCATCAC-3′ (reverse). The PCR products were resolved on 1% agarose gels, stained with ethidium bromide, and then photographed.
ELISA
A commercially available kit for ELISA for IGF-1, obtained from R&D Systems Inc. (catalog no. MG100), was used throughout the experiments. In the transwell co-culture system, the media that had been co-cultured for 72 hours were collected. The assays were performed following the instructions of the manufacturer. After completion of the ELISA assay, luminescence was assayed by a plate-reader at 450 nm.
Results
Expression of c-Jun Protein in the Stromal and Epithelial Cells
To determine differentially expressed genes between prostatic stroma and epithelial cells, microdissected human prostate stromal and human epithelial cells were evaluated by microarray gene-chip analysis. We found that the Jun family of genes was differentially expressed in the prostatic stroma seven times higher than in prostate epithelial cells.30
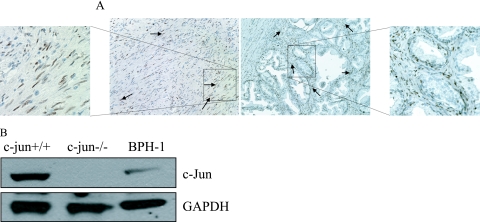
To determine whether stromal expression of Jun-family proteins is important in human BPH samples, surgical specimens from 15 patients who had undergone transurethral resection of prostate for BPH or radical prostatectomy for prostate cancer were evaluated by immunohistochemistry. Prostatic tissues with histological confirmation of BPH without any evidence of prostate cancer were evaluated for immunoreactivity to the c-Jun, JunD, and JunB proteins. We found that c-Jun (Figure 1A) and JunD (data not shown) were expressed heavily in the prostatic stroma but not in the epithelium. Moreover, c-Jun was expressed in high levels in the BPH nodules enriched with stroma.
Figure 1.
Expression of c-Jun protein in stromal and epithelial cells. A: Immunoreactivity of c-Jun protein in human BPH. Prostate stroma demonstrates high reactivity with the c-Jun antibody, whereas prostate epithelial cells show no reactivity. Left (low-power) and inset (high-power) represent a portion of nodular BPH, whereas right and inset represent glandular BPH. Arrows represent areas of immunoreactivity with the c-Jun antibody. B: c-Jun protein is expressed in the c-jun+/+ fibroblasts and to a small degree in the BPH-1 cells, but not expressed in the c-jun−/− fibroblasts. GAPDH is used as loading control.
To examine the role of stromally expressed c-Jun protein in the stromal-epithelial interaction, c-jun+/+ and c-jun−/− mouse fibroblasts were used in our in vitro co-culture systems. We used mouse fibroblastic stroma to directly examine the potential effect of stromally expressed c-Jun on prostate epithelial cell proliferation and growth. Western blot using the c-Jun antibody demonstrated that c-Jun is expressed in c-jun wild type but not in the c-jun−/− cells. Immortalized but nontumorigenic prostate epithelial cells, BPH-1,31 expressed low levels of c-Jun protein compared with wild-type fibroblasts (Figure 1B).
Stromally Expressed c-Jun Affects Epithelial Cell Proliferation
To explore the effects of stromally expressed c-Jun on BPH-1 prostate epithelial cells, we used a co-culture system between stromal and epithelial cells. Co-cultures of BPH-1 cells with fibroblasts promoted proliferation of BPH-1 cells compared to BPH-1 cells in monocultures (Figure 2A). Moreover, c-jun+/+ fibroblasts had a stronger effect in promoting BPH-1 cell proliferation than c-jun−/− fibroblasts. Because we used a co-culture transwell system, we avoided cell-to-cell direct contacts between fibroblasts and BPH-1 cells. We suspect, therefore, that specific soluble factors secreted from the fibroblasts play a crucial role in on BPH-1 cell proliferation. Conditioned media from fibroblasts promoted BPH-1 cellular proliferation. This demonstrated that specific soluble factors secreted from c-jun+/+ cells will affect BPH-1 epithelial cell proliferation (Figure 2B), a condition that is less prominent in the c-jun−/− fibroblasts.
Figure 2.
Fibroblasts promote BPH-1 cellular proliferation. A: Proliferation of BPH-1 cells in co-culture with c-jun+/+ or c-jun−/− fibroblasts, or in monoculture. c-jun+/+ fibroblasts promote proliferation of prostate epithelial cells. B: Proliferation of BPH-1 cells cultured with conditioned media (CM) from c-jun+/+ or c-jun−/− fibroblasts. CM from c-jun+/+ promote proliferation of BPH-1 cells. Control sample is cultured in DMEM with the same FBS concentration in the CM.
c-Jun Potentiates Secretions of Specific Paracrine Growth Factors
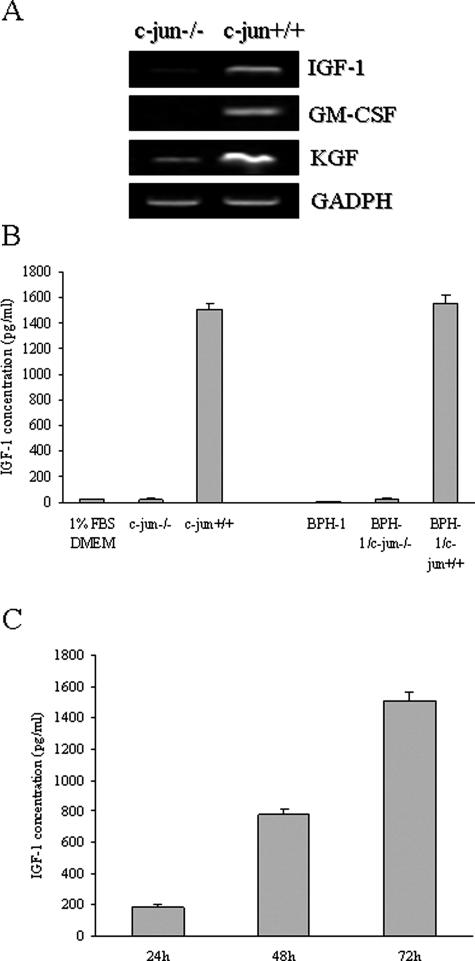
To investigate further the effect of c-Jun expression in the fibroblasts, we examined the mRNA expression of known target genes for c-Jun. We found that c-jun+/+ fibroblasts expressed higher levels of IGF-1, GM-CSF, and KGF than did c-jun−/− fibroblasts (Figure 3A). Szabowski and colleagues19,20 have shown that stromally expressed GM-CSF and KGF play an important role in epithelial cell organogenesis and tissue homeostasis. In our system, relative mRNA levels of GM-CSF in c-jun+/+ fibroblasts are significantly higher than in c-jun−/− fibroblasts. Nevertheless, the basal protein levels of GM-CSF are low in both c-jun−/− and c-jun+/+ fibroblasts (data not shown). Recent studies have suggested that men with elevated plasma levels of IGF-1 and IGF binding protein-3 (IGFBP-3) may have an increased risk for future development of BPH and prostate cancer.14,27,32,33 Next, we examined the paracrine stromal-epithelial interactions that involve the IGF-1 pathway responsible for development of BPH. We examined the quantity of IGF-1 by ELISA assay in our co-culture system. We found that IGF-1 was predominantly secreted from the c-jun+/+ fibroblasts and not from the c-jun−/− or the BPH-1 monocultures. Co-cultures of c-jun+/+ and BPH-1 cells demonstrated an equivalent level of IGF-1 secretion, suggesting that all of the IGF-1 in our systems is from the c-jun+/+ fibroblasts (Figure 3B). Further, production of IGF-1 is time-dependent in c-jun+/+ fibroblasts (Figure 3C). We suspect, therefore, that production of IGF-1 by the c-jun+/+ stromal cells plays a crucial role in promoting proliferation of prostate epithelial cells.
Figure 3.
c-jun+/+ fibroblasts express and excrete more growth factors than c-jun−/− fibroblasts. A: RT-PCR semiquantitative analysis of fibroblasts with different c-jun genotypes. IGF-1, KGF, and GM-CSF are expressed at higher levels in c-jun+/+ cells than c-jun−/− cells. B: ELISA for IGF-1 using mouse-specific IGF-1 antibody in monocultures or co-cultures after 3 days. IGF-1 is preferentially expressed from c-jun+/+ cells. C: ELISA assay for IGF-1 in c-jun+/+ fibroblasts in monoculture suggests increased levels of IGF-1 in a time-dependent manner. Data represent the means and SDs from three independent experiments.
IGF-1 Promotes BPH-1 Cellular Proliferation through the IGF-1 Receptor
To confirm that IGF-1 is a factor that acts as a paracrine signal to promote prostate epithelial cell growth, we examined the effect of recombinant IGF-1 on BPH-1 prostate epithelial cells. We found that IGF-1 stimulated BPH-1 cellular proliferation in a concentration-dependent manner (Figure 4A). We also found that the IGF-1 receptor is expressed at an abundant level in BPH-1 cells (Figure 4B). The IGF-1 receptor inhibitor I-OMe-AG538 partly abrogated the stimulation of c-jun+/+ fibroblasts on BPH-1 proliferation in co-culture (Figure 4C), suggesting that there are other paracrine factors capable of affecting prostate epithelial cell proliferation. For example, we found that IGF-1 neutralizing antibodies also abrogated proliferation of BPH-1 cells when co-cultured with c-jun+/+ fibroblasts (Figure 4D). These results suggest that IGF-1 may be an important paracrine factor in the interaction between stromal and epithelial cells in BPH.
Figure 4.
IGF-1 promotes BPH-1 cellular proliferation through the IGF-1 receptor. A: Increased exogenous concentration of IGF-1 promotes proliferation of BPH-1 cells. B: Immunofluoroscopy using IGF-1 receptor antibody indicated that there are abundant IGF-1 receptors on the surface of BPH-1 cells. IGF-1 receptor inhibitor I-OMe-AG538 (C) and IGF-1 neutralizing antibody (D) partly abrogate the ability of c-jun+/+ fibroblasts to promote BPH-1 proliferation in co-culture. Data represent the means and SDs from three independent experiments (*P < 0.05).
IGF-1 and Stromally Expressed c-Jun Activate Mitogen-Activated Protein Kinase (MAPK) and Akt in Prostate Epithelial Cells
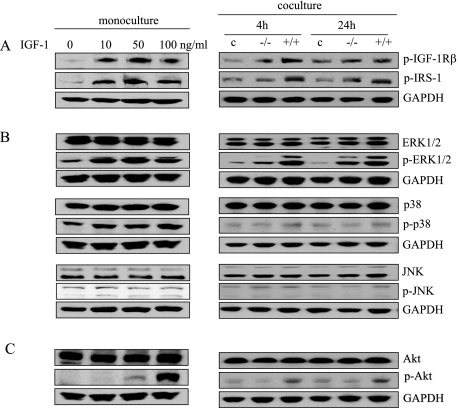
To compare the effects of IGF-1 and stromally expressed c-Jun on prostate epithelial downstream signaling pathways, we examined the BPH-1 cells in mono- and co-culture systems, respectively. As expected, IGF-1 activated IGF-1R tyrosine kinase and IRS-1, one of the primary substrates of the IGF-1R (Figure 5A, left). Similar results obtained in the co-culture system (Figure 5A, right) suggest that IGF-1 is a key activator of stromal-epithelial interactions.
Figure 5.
IGF-1 and stromally expressed c-Jun activate regulators of cell proliferation in prostate epithelial cells. IGF-1 activates IGF-1R tyrosine kinase (A), MAPK (B), and Akt (C) in BPH-1 cells. The left panels represent levels of indicated proteins in BPH-1 cells with the addition of exogenous recombined IGF-1 (24-hour exposure), whereas the right panels show the levels of the indicated proteins in BPH-1 cells that were co-cultured with either c-jun+/+ or c-jun−/− fibroblasts for 24 hours.
The MAPK (including ERK, p38, and JNK) pathway is a major regulator of cell proliferation, apoptosis, and differentiation.34,35 Other groups have shown that BPH can be stimulated by activation of MAPKs.36 To determine the role of MAPKs in our model, we evaluated MAPK-related proteins after exogenous stimulation with IGF-1 in our co-culture system in the presence or absence of stromally expressed c-Jun. We found that the total protein levels for ERK 1/2, p38, and JNK remained the same, yet the phosphorylated levels of ERK 1/2 and p38 were increased in the presence of exogenous IGF-1 (Figure 5B, left). Likewise, we observed increased levels of p-ERK 1/2 in the BPH-1 cells when co-cultured with fibroblasts expressing c-Jun (Figure 5B, right). Expression of phospho-p38 in the co-cultures was less pronounced than in the monocultures, but we saw no significant change in JNK protein levels in the mono- and co-culture systems (Figure 5B).
IGF-1 has been shown to be a key activator of the PI3K/Akt pathway and a key regulator of cellular proliferation, cell cycle, apoptosis, and tumor progression.37,38 We found that exogenous IGF-1 (monocultures) and stromally expressed c-Jun (co-cultures) both promoted increased levels of p-Akt but not total Akt levels (Figure 5C). These results suggest that stromal fibroblasts are capable of activating MAPKs and Akt in the BPH-1 prostate epithelial cells by secreting IGF-1 as a paracrine mediator. The expression of c-Jun in the stromal cells, therefore, plays an important role in mediating secretion of IGF-1 and epithelial cell proliferation.
IGF-1 and Stromally Expressed c-Jun Regulate Cyclin D Levels in Prostate Epithelial Cells
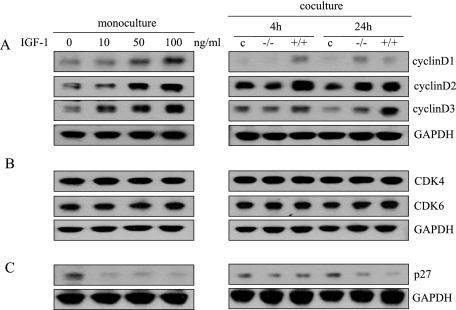
Both MAPKs and Akt are critical kinases that regulate a variety of biological processes including survival, proliferation, apoptosis, and differentiation through downstream targets.34,35,37,38 One family of these downstream targets is the D cyclins (cyclins D1, D2, and D3) and their associated cyclin-dependent kinases (CDK4 or CDK6), which are essential components of the core cell-cycle machinery.39,40,41 Dysregulated expression of cyclins induces abnormal cellular proliferation that can lead to hyperplastic and malignant conditions.42,43 We wished to determine whether cyclins and CDKs are up-regulated by IGF-1 and stromal-epithelial interactions in BPH. We found that cyclins D1, D2, and D3 protein levels in BPH-1 cells are increased after treatment with IGF-1. In addition, BPH-1 cells co-cultured with c-jun+/+ fibroblasts showed a higher expression of cyclin D1, D2, and D3 protein levels than when either BPH-1 cells were in monoculture or when BPH-1 cells were co-cultured with c-jun−/− cells. Increased levels of cyclin D2 and D3 were particularly pronounced in the BPH-1 cells that were co-cultured with c-jun+/+ cells (Figure 6A). We found that exogenous IGF-1 and stromally expressed c-Jun promote expression of cyclin D levels. But we did not observe any change in CDK4 or CDK6 levels in BPH-1 cells in our mono- or co-culture systems (Figure 6B).
Figure 6.
IGF-1 and stromally expressed c-Jun regulate cell cycle proteins in prostate epithelial cells. A: Cyclin D1, D2, and D3 protein levels increased in BPH-1 cells with exogenous IGF-1 (24-hour exposure) and with c-jun+/+ co-cultures. B: CDK4 and CDK6 protein levels in BPH-1 cells did not change significantly with either exogenous IGF-1 or with c-jun+/+ co-cultures. C: CDK inhibitor p27 protein levels are decreased with exogenous IGF-1 or with c-jun+/+ co-cultures.
CDK inhibitor p27 (Kip1) is an important regulator of the cell cycle. Degradation of p27 is critical for re-entry of cells into the cell cycle and proliferation. Alterations of p27 gene expression have been found in BPH and prostatic carcinoma.44 In this study, we found that exogenous IGF-1 decreased the p27 protein level in BPH-1 cells. Similarly, intracellular p27 protein levels of BPH-1 cells decreased significantly when co-cultured with c-jun+/+ fibroblasts, particularly after 24 hours of co-culture (Figure 6C). Therefore, these data indicate that D cyclins and the CDK inhibitor p27 are target genes that promote proliferation of epithelial cells in stromal-epithelial interactions, and IGF-1 acts as a paracrine molecule between stromal-epithelial cross talk.
IGF-1 Increases Cyclin D Levels and Stimulates BPH-1 Proliferation in a PI3K/Akt-Dependent Manner
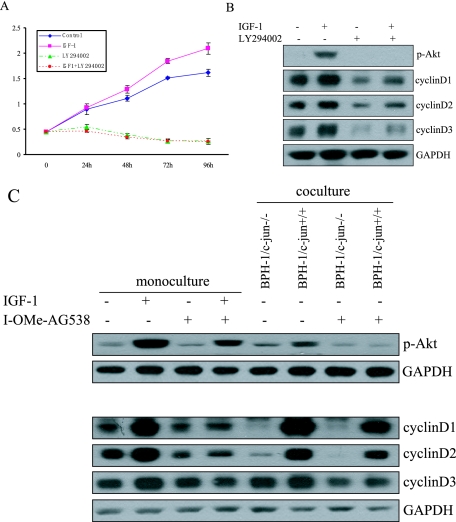
As shown in Figures 4, 5, and 6, IGF-1 promoted proliferation of BPH-1 cells and activated p-Akt and up-regulated cyclin D levels. We then investigated if proliferation of BPH-1 cells by IGF-1 could be blocked in an Akt-dependent manner. We found that cellular proliferation of BPH-1 cells was increased in the presence of exogenous IGF-1. We also found that the PI3 kinase inhibitor (LY294002) inhibited proliferation of BPH-1 cells, which was not rescued by exogenous IGF-1 exposure (Figure 7A).
Figure 7.
IGF-1 and stromally expressed c-Jun up-regulate cyclin D levels and stimulate BPH-1 proliferation in a PI3K/Akt-dependent manner. Cell proliferation assay (A) and Western blot analysis (B) in the presence or absence of the PI3K/Akt inhibitor LY294002. C: Phospho-Akt and cyclin D protein levels in the presence of IGF-1 receptor inhibitor I-OMe-AG538 in monocultures and co-cultures.
Next, we determined if cyclin D expression was mediated through activation of Akt. We found that in the presence of PI3K inhibitor (LY294002) there was no increase in Akt phosphorylation after IGF-1 simulation. Similarly, activation of cyclin D was abrogated in the presence of PI3 kinase inhibitor, LY294002 (Figure 7B). To confirm the IGF-1/Akt/cyclin D signaling axis in the prostate stromal-epithelial interactions, we used the IGF-1R inhibitor I-OMe-AG538 to inhibit IGF-1’s ability to stimulate BPH-1 cells. We found that the IGF-1R inhibitor eliminated IGF-1’s ability to promote p-Akt in both monoculture and co-culture systems. Neither exogenous IGF-1 (monoculture) nor stromally expressed c-Jun (co-culture) could promote p-Akt in BPH-1 cells in the presence of the IGF-1R inhibitor (Figure 7C). In addition, in the presence of the IGF-1R inhibitor, we observed much lower levels of cyclin D1 and cyclin D2 in both mono- and co-culture experiments. And in the presence of the IGF-1R inhibitor, the cyclin D levels in BPH-1 cells that were co-cultured were higher than cyclin D levels that were in monoculture suggesting there are alternate pathways to increase cyclin D levels other than the IGF-1 axis. These results then indicate that stromal-epithelial interactions via IGF-1 stimulate cyclin D levels and BPH-1 cellular proliferation through an Akt-dependent pathway. Therefore, Akt acts as an important regulator of signal transduction between stromal and epithelial cells.
Exogenous IGF-1 and c-jun+/+ Fibroblasts Promote Proliferation of Prostate Cancer Cells
To examine whether stromal expression of c-Jun is specific to nontumorigenic cells, we used the androgen-dependent CWR22-Rv1 prostate cancer cells45,46 in our monoculture and co-culture experiments. CWR22-Rv1 prostate cancer cells had increased proliferation rate when co-cultured with c-jun+/+ fibroblasts (see Supplemental Figure S1A, available online at http://ajp.amjpathol. org) or when exposed to exogenous IGF-1 (see Supplemental Figure S1B, available online at http://ajp.amjpathol. org). Similar to our findings for BPH-1 cells, p-Akt levels and cyclin D levels were also increased in the CWR22-Rv1 cells when co-cultured with c-jun+/+ cells or when exposed to IGF-1 in monocultures (see Supplemental Figures S2A and S2B, available online at http://ajp. amjpathol.org). However, one important difference between the tumorigenic CWR22-Rv1 cells and the nontumorigenic BPH-1 cells in our systems is that p-ERK 1/2 levels are not increased from the baseline in the CWR22-Rv1 cells when the cells are exposed to exogenous IGF-1 or when the cells are in co-culture with c-jun+/+ fibroblasts (see Supplemental Figure S3, available online at http://ajp.amjpathol.org). One potential explanation could be that the baseline expression level of p-ERK 1/2 in the tumorigenic CWR22-Rv1 cells is very high; therefore, neither exogenous IGF-1 nor co-cultures with c-jun+/+ cells has any added effect on the level of p-ERK 1/2.
Discussion
BPH is defined as hyperplasia and nonmalignant growth associated with both stromal and epithelial compartments of the prostate.10,47 Molecular cross talk between the stromal and epithelial cells is important in maintaining proper homeostasis. Stromal-epithelial interactions not only play a critical role in development and growth of the normal prostate gland but also play a role in such abnormalities of the prostate gland as BPH and prostate carcinoma. Stromal cells can affect BPH progression by regulating epithelial proliferation, apoptosis, and angiogenesis.48,49 This mutual cross talk is mediated by cell surface protein, extracellular matrix, and soluble factors.19,50
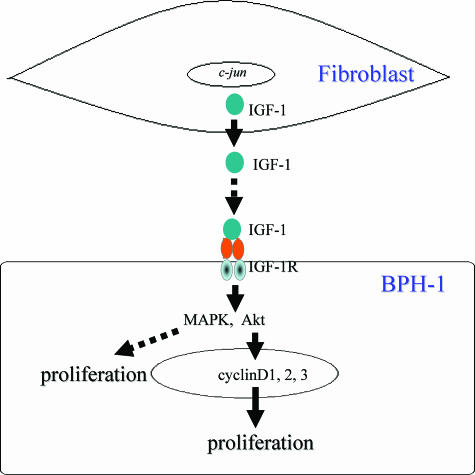
Aging and androgens are the two established risk factors for development of BPH.51,52,53,54 Recent studies suggest that androgens alone may not be the sole contributing factor to progression and development of clinically significant BPH.33 Moreover, androgens may act indirectly on the prostate through production of growth factors.55 The prostatic stroma contains many cellular components including fibroblasts, smooth muscle cells, blood vessels, and nerve fibers. Numerous studies have demonstrated that stromal cells are important producers of multiple soluble growth factors, which are important for normal tissue development and growth.33,56 Accordingly, stromal-epithelial interactions and stromal-derived soluble factors can play a pivotal role in promoting development of BPH. In this study, we report that human prostatic stroma differentially expresses c-Jun. We show that IGF-1 functions as a paracrine stromally secreted molecule that promotes BPH-1 cellular proliferation via the IGF-1R/Akt/cyclin D axis (Figure 8).
Figure 8.
The role of stromally expressed c-Jun protein in stromal-epithelial interactions in BPH. Stromally expressed c-Jun promotes IGF-1 expression and paracrine signaling to enhance prostate epithelial proliferation.
The fibroblast is a major cell type of the stromal compartment and as such is involved intimately in orchestrating the stromal half of the dialogue in tissue homeostasis. In the past, we have shown that the stromal fibroblasts within and surrounding a tumor (described as the carcinoma-associated fibroblasts) can alter epithelial morphology, potentiate cell proliferation, reduce cell death, and direct tumor progression of initiated human prostatic epithelium. Notably this effect is not detected when normal human prostatic fibroblasts were grown with the prostatic epithelium.57,58 When we compared fibroblasts cultured from carcinoma-associated fibroblasts to normal human prostatic fibroblasts, we found that transforming growth factor-β1 was expressed in higher concentrations in carcinoma-associated fibroblasts than in normal human prostatic fibroblasts.59 These results suggest that stromal cells are critical for regulating epithelial activities including hyperplasia, transformation, apoptosis, and differentiation through paracrine factors.
During tumorigenesis, the prevailing models suggest that epithelial cells acquire multiple genetic mutations.60,61 Modification of fibroblasts in the stroma immediately adjacent to transformed epithelial cells has been documented in several tumor systems.58,62,63 We demonstrate herein that stromally expressed c-Jun plays a crucial function in regulating proliferation of prostate epithelial cells.
c-Jun is an important member of the AP-1 family of transcription factors that regulates cellular proliferation, apoptosis, and differentiation.19,21,22 Embryos lacking a functional c-jun gene die during mid-gestation.28,64 Although c-Jun, as an oncogene, has been extensively studied in single cell systems, very little is known, however, about c-Jun’s function in stromal-epithelial cross-talks. We have found that c-Jun is predominantly expressed in the prostatic stroma as demonstrated by microarray and immunohistochemical studies. Moreover, Szabowski and colleagues19,20 have shown that the c-Jun protein expressed in fibroblasts plays an important role in directing epithelial cell organogenesis and tissue homeostasis in skin. Based on these observations, we hypothesized that stromally expressed c-Jun may play a role in the stromal-epithelial interaction through paracrine signals in BPH. We found that c-jun+/+ fibroblasts produced and secreted predominantly IGF-1, whereas c-jun−/− fibroblasts lacked this ability. Recent studies have suggested that IGF-1 and IGF-binding protein-3 (IGFBP-3) are associated with BPH development.14,27,33,65 Here, we report stromally expressed c-Jun can regulate IGF-1 levels. Although it is established that IGF-1 is an activator of Akt and can promote cell proliferation,37,38 the precise mechanism is still unclear. Our data suggest that IGF-1 promotes BPH-1 proliferation by increasing cyclin D protein levels in monoculture and co-culture systems. Moreover, the increase of cyclin D in our stromal-epithelial model system is increased in an Akt-dependent manner. As a result, stromal expression of c-jun can affect expression of IGF-1 and prostate epithelial cellular proliferation (Figure 8).
The availability of suitable model systems is one of the major limitations in elucidating the molecular mechanism associated with development of BPH, a medical condition that affects more than 90% of men age 85 years or older worldwide.1 In this study, we used genetically modified c-Jun stromal cells based on initial results that suggested compartmentalization and expression of c-Jun in human prostatic stroma tissue samples in microarray and immunohistochemical studies. Using genetically modified stromal cells enabled us to examine if stromal expression of c-Jun affects prostate epithelial proliferation. We conclude that stromal expression of c-Jun promotes expression of IGF-1, which can function as a paracrine molecule between stromal and epithelial cells. IGF-1 promotes prostate epithelial proliferation via an Akt-dependent pathway leading to up-regulation of the cyclin D family proteins. Identification of the signal transduction pathways between prostate epithelial cells and the surrounding stromal cells will, therefore, greatly improve our understanding of the normal and abnormal biology in prostatic diseases.
Supplementary Material
Footnotes
Address reprint requests to Aria F. Olumi, Massachusetts General Hospital, Yawkey Bdlg., Suite 7E, 55 Fruit St., Boston, MA 02114-2354. E-mail: aolumi@partners.org.
Supported by the National Institutes of Health (National Institute of Diabetes and Digestive and Kidney Diseases grant DK64062 to A.F.O.).
Supplemental material for this article can be found on http://ajp. amjpathol.org.
References
- Gong EM, Gerber GS. Saw palmetto and benign prostatic hyperplasia. Am J Chin Med. 2004;32:331–338. doi: 10.1142/S0192415X04001989. [DOI] [PubMed] [Google Scholar]
- Burnett AL, Wein AJ. Benign prostatic hyperplasia in primary care: what you need to know. J Urol. 2006;175:S19–S24. doi: 10.1016/S0022-5347(05)00310-1. [DOI] [PubMed] [Google Scholar]
- Shapiro E, Hartanto V, Lepor H. Quantifying the smooth muscle content of the prostate using double-immunoenzymatic staining and color assisted image analysis. J Urol. 1992;147:1167–1170. doi: 10.1016/s0022-5347(17)37508-0. [DOI] [PubMed] [Google Scholar]
- Chapple CR. Pharmacological therapy of benign prostatic hyperplasia/lower urinary tract symptoms: an overview for the practicing clinician. BJU Int. 2004;94:738–744. doi: 10.1111/j.1464-410X.2004.05022.x. [DOI] [PubMed] [Google Scholar]
- Sarma AV, Jacobson DJ, McGree ME, Roberts RO, Lieber MM, Jacobsen SJ. A population based study of incidence and treatment of benign prostatic hyperplasia among residents of Olmsted County, Minnesota: 1987 to 1997. J Urol. 2005;173:2048–2053. doi: 10.1097/01.ju.0000158443.13918.d6. [DOI] [PubMed] [Google Scholar]
- Cunha GR. Stromal induction and specification of morphogenesis and cytodifferentiation of the epithelia of the Mullerian ducts and urogenital sinus during development of the uterus and vagina in mice. J Exp Zool. 1976;196:361–370. doi: 10.1002/jez.1401960310. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Hayward SW, Wang YZ, Ricke WA. Role of the stromal microenvironment in carcinogenesis of the prostate. Int J Cancer. 2003;107:1–10. doi: 10.1002/ijc.11335. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Chung LW. Stromal-epithelial interactions–I. Induction of prostatic phenotype in urothelium of testicular feminized (Tfm/y) mice. J Steroid Biochem. 1981;14:1317–1324. doi: 10.1016/0022-4731(81)90338-1. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Lung B. The possible influence of temporal factors in androgenic responsiveness of urogenital tissue recombinants from wild-type and androgen-insensitive (Tfm) mice. J Exp Zool. 1978;205:181–193. doi: 10.1002/jez.1402050203. [DOI] [PubMed] [Google Scholar]
- McNeal JE. Origin and evolution of benign prostatic enlargement. Invest Urol. 1978;15:340–345. [PubMed] [Google Scholar]
- Cohen P, Peehl DM, Lamson G, Rosenfeld RG. Insulin-like growth factors (IGFs), IGF receptors, and IGF-binding proteins in primary cultures of prostate epithelial cells. J Clin Endocrinol Metab. 1991;73:401–407. doi: 10.1210/jcem-73-2-401. [DOI] [PubMed] [Google Scholar]
- Yan G, Fukabori Y, Nikolaropoulos S, Wang F, McKeehan WL. Heparin-binding keratinocyte growth factor is a candidate stromal-to-epithelial-cell andromedin. Mol Endocrinol. 1992;6:2123–2128. doi: 10.1210/mend.6.12.1491693. [DOI] [PubMed] [Google Scholar]
- Finch PW, Rubin JS, Miki T, Ron D, Aaronson SA. Human KGF is FGF-related with properties of a paracrine effector of epithelial cell growth. Science. 1989;245:752–755. doi: 10.1126/science.2475908. [DOI] [PubMed] [Google Scholar]
- Kawada M, Inoue H, Masuda T, Ikeda D. Insulin-like growth factor I secreted from prostate stromal cells mediates tumor-stromal cell interactions of prostate cancer. Cancer Res. 2006;66:4419–4425. doi: 10.1158/0008-5472.CAN-05-4239. [DOI] [PubMed] [Google Scholar]
- Yang F, Tuxhorn JA, Ressler SJ, McAlhany SJ, Dang TD, Rowley DR. Stromal expression of connective tissue growth factor promotes angiogenesis and prostate cancer tumorigenesis. Cancer Res. 2005;65:8887–8895. doi: 10.1158/0008-5472.CAN-05-1702. [DOI] [PubMed] [Google Scholar]
- Ayala GE, Dai H, Tahir SA, Li R, Timme T, Ittmann M, Frolov A, Wheeler TM, Rowley D, Thompson TC. Stromal antiapoptotic paracrine loop in perineural invasion of prostatic carcinoma. Cancer Res. 2006;66:5159–5164. doi: 10.1158/0008-5472.CAN-05-1847. [DOI] [PubMed] [Google Scholar]
- Trapani JA. The dual adverse effects of TGF-β secretion on tumor progression. Cancer Cell. 2005;8:349–350. doi: 10.1016/j.ccr.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Zhou W, Park I, Pins M, Kozlowski JM, Jovanovic B, Zhang J, Lee C, Ilio K. Dual regulation of proliferation and growth arrest in prostatic stromal cells by transforming growth factor-β1. Endocrinology. 2003;144:4280–4284. doi: 10.1210/en.2003-0554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabowski A, Maas-Szabowski N, Andrecht S, Kolbus A, Schorpp-Kistner M, Fusenig NE, Angel P. c-Jun and JunB antagonistically control cytokine-regulated mesenchymal-epidermal interaction in skin. Cell. 2000;103:745–755. doi: 10.1016/s0092-8674(00)00178-1. [DOI] [PubMed] [Google Scholar]
- Angel P, Szabowski A. Function of AP-1 target genes in mesenchymal-epithelial cross-talk in skin. Biochem Pharmacol. 2002;64:949–956. doi: 10.1016/s0006-2952(02)01158-9. [DOI] [PubMed] [Google Scholar]
- Eferl R, Wagner EF. AP-1: a double-edged sword in tumorigenesis. Nat Rev Cancer. 2003;3:859–868. doi: 10.1038/nrc1209. [DOI] [PubMed] [Google Scholar]
- Wagner EF. AP-1—introductory remarks. Oncogene. 2001;20:2334–2335. doi: 10.1038/sj.onc.1204416. [DOI] [PubMed] [Google Scholar]
- Angel P, Szabowski A, Schorpp-Kistner M. Function and regulation of AP-1 subunits in skin physiology and pathology. Oncogene. 2001;20:2413–2423. doi: 10.1038/sj.onc.1204380. [DOI] [PubMed] [Google Scholar]
- Thomson AA, Foster BA, Cunha GR. Analysis of growth factor and receptor mRNA levels during development of the rat seminal vesicle and prostate. Development. 1997;124:2431–2439. doi: 10.1242/dev.124.12.2431. [DOI] [PubMed] [Google Scholar]
- Singer C, Rasmussen A, Smith HS, Lippman ME, Lynch HT, Cullen KJ. Malignant breast epithelium selects for insulin-like growth factor II expression in breast stroma: evidence for paracrine function. Cancer Res. 1995;55:2448–2454. [PubMed] [Google Scholar]
- Chen BK, Overgaard MT, Bale LK, Resch ZT, Christiansen M, Oxvig C, Conover CA. Molecular regulation of the IGF-binding protein-4 protease system in human fibroblasts: identification of a novel inducible inhibitor. Endocrinology. 2002;143:1199–1205. doi: 10.1210/endo.143.4.8729. [DOI] [PubMed] [Google Scholar]
- Wolk A, Andersson SO, Mantzoros CS, Trichopoulos D, Adami HO. Can measurements of IGF-1 and IGFBP-3 improve the sensitivity of prostate-cancer screening? Lancet. 2000;356:1902–1903. doi: 10.1016/S0140-6736(00)03266-9. [DOI] [PubMed] [Google Scholar]
- Johnson RS, van Lingen B, Papaioannou VE, Spiegelman BM. A null mutation at the c-jun locus causes embryonic lethality and retarded cell growth in culture. Genes Dev. 1993;7:1309–1317. doi: 10.1101/gad.7.7b.1309. [DOI] [PubMed] [Google Scholar]
- Zhang X, Jin TG, Yang H, DeWolf WC, Khosravi-Far R, Olumi AF. Persistent c-FLIP(L) expression is necessary and sufficient to maintain resistance to tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in prostate cancer. Cancer Res. 2004;64:7086–7091. doi: 10.1158/0008-5472.CAN-04-1498. [DOI] [PubMed] [Google Scholar]
- Singh D, Febbo PG, Ross K, Jackson DG, Manola J, Ladd C, Tamayo P, Renshaw AA, D’Amico AV, Richie JP, Lander ES, Loda M, Kantoff PW, Golub TR, Sellers WR. Gene expression correlates of clinical prostate cancer behavior. Cancer Cell. 2002;1:203–209. doi: 10.1016/s1535-6108(02)00030-2. [DOI] [PubMed] [Google Scholar]
- Hayward SW, Dahiya R, Cunha GR, Bartek J, Deshpande N, Narayan P. Establishment and characterization of an immortalized but non-transformed human prostate epithelial cell line: BPH-1. In Vitro Cell Dev Biol Anim. 1995;31:14–24. doi: 10.1007/BF02631333. [DOI] [PubMed] [Google Scholar]
- Krueckl SL, Sikes RA, Edlund NM, Bell RH, Hurtado-Coll A, Fazli L, Gleave ME, Cox ME. Increased insulin-like growth factor I receptor expression and signaling are components of androgen-independent progression in a lineage-derived prostate cancer progression model. Cancer Res. 2004;64:8620–8629. doi: 10.1158/0008-5472.CAN-04-2446. [DOI] [PubMed] [Google Scholar]
- Wong YC, Wang YZ. Growth factors and epithelial-stromal interactions in prostate cancer development. Int Rev Cytol. 2000;199:65–116. doi: 10.1016/s0074-7696(00)99002-8. [DOI] [PubMed] [Google Scholar]
- Bradham C, McClay DR. p38 MAPK in development and cancer. Cell Cycle. 2006;5:824–828. doi: 10.4161/cc.5.8.2685. [DOI] [PubMed] [Google Scholar]
- MacCorkle RA, Tan TH. Mitogen-activated protein kinases in cell-cycle control. Cell Biochem Biophys. 2005;43:451–461. doi: 10.1385/CBB:43:3:451. [DOI] [PubMed] [Google Scholar]
- Papatsoris AG, Papavassiliou AG. Molecular ‘palpation’ of BPH: a tale of MAPK signalling? Trends Mol Med. 2001;7:288–292. doi: 10.1016/s1471-4914(01)02015-9. [DOI] [PubMed] [Google Scholar]
- Larue L, Bellacosa A. Epithelial-mesenchymal transition in development and cancer: role of phosphatidylinositol 3′ kinase/AKT pathways. Oncogene. 2005;24:7443–7454. doi: 10.1038/sj.onc.1209091. [DOI] [PubMed] [Google Scholar]
- Song G, Ouyang G, Bao S. The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 2005;9:59–71. doi: 10.1111/j.1582-4934.2005.tb00337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dekanty A, Sauane M, Cadenas B, Coluccio F, Barrio M, Casala J, Paciencia M, Rogers F, Coso OA, Piwien-Pilipuk G, Rudland PS, de Asua LJ. Leukemia inhibitory factor induces DNA synthesis in Swiss mouse 3T3 cells independently of cyclin D1 expression through a mechanism involving MEK/ERK1/2 activation. J Biol Chem. 2006;281:6136–6143. doi: 10.1074/jbc.M505839200. [DOI] [PubMed] [Google Scholar]
- Marderosian M, Sharma A, Funk AP, Vartanian R, Masri J, Jo OD, Gera JF. Tristetraprolin regulates cyclin D1 and c-Myc mRNA stability in response to rapamycin in an Akt-dependent manner via p38 MAPK signaling. Oncogene. 2006;25:6277–6290. doi: 10.1038/sj.onc.1209645. [DOI] [PubMed] [Google Scholar]
- Yu C, Rahmani M, Dai Y, Conrad D, Krystal G, Dent P, Grant S. The lethal effects of pharmacological cyclin-dependent kinase inhibitors in human leukemia cells proceed through a phosphatidylinositol 3-kinase/Akt-dependent process. Cancer Res. 2003;63:1822–1833. [PubMed] [Google Scholar]
- Yu Q, Ciemerych MA, Sicinski P. Ras and Myc can drive oncogenic cell proliferation through individual D-cyclins. Oncogene. 2005;24:7114–7119. doi: 10.1038/sj.onc.1208853. [DOI] [PubMed] [Google Scholar]
- Ciemerych MA, Kenney AM, Sicinska E, Kalaszczynska I, Bronson RT, Rowitch DH, Gardner H, Sicinski P. Development of mice expressing a single D-type cyclin. Genes Dev. 2002;16:3277–3289. doi: 10.1101/gad.1023602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordon-Cardo C, Koff A, Drobnjak M, Capodieci P, Osman I, Millard SS, Gaudin PB, Fazzari M, Zhang ZF, Massague J, Scher HI. Distinct altered patterns of p27KIP1 gene expression in benign prostatic hyperplasia and prostatic carcinoma. J Natl Cancer Inst. 1998;90:1284–1291. doi: 10.1093/jnci/90.17.1284. [DOI] [PubMed] [Google Scholar]
- Chesire DR, Isaacs WB. Ligand-dependent inhibition of beta-catenin/TCF signaling by androgen receptor. Oncogene. 2002;21:8453–8469. doi: 10.1038/sj.onc.1206049. [DOI] [PubMed] [Google Scholar]
- Sramkoski RM, Pretlow TG, II, Giaconia JM, Pretlow TP, Schwartz S, Sy MS, Marengo SR, Rhim JS, Zhang D, Jacobberger JW. A new human prostate carcinoma cell line, 22Rv1. In Vitro Cell Dev Biol. 1999;35:403–409. doi: 10.1007/s11626-999-0115-4. [DOI] [PubMed] [Google Scholar]
- Untergasser G, Madersbacher S, Berger P. Benign prostatic hyperplasia: age-related tissue-remodeling. Exp Gerontol. 2005;40:121–128. doi: 10.1016/j.exger.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Bilalović N, Vranic S, Serdarevic F, Foco F. The role of the stroma in carcinogenesis. Bosn J Basic Med Sci. 2006;6:33–38. doi: 10.17305/bjbms.2006.3207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha GR, Hayward SW, Wang YZ. Role of stroma in carcinogenesis of the prostate. Differentiation. 2002;70:473–485. doi: 10.1046/j.1432-0436.2002.700902.x. [DOI] [PubMed] [Google Scholar]
- Bonkhoff H, Remberger K. Differentiation pathways and histogenetic aspects of normal and abnormal prostatic growth: a stem cell model. Prostate. 1996;28:98–106. doi: 10.1002/(SICI)1097-0045(199602)28:2<98::AID-PROS4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Berry SJ, Coffey DS, Walsh PC, Ewing LL. The development of human benign prostatic hyperplasia with age. J Urol. 1984;132:474–479. doi: 10.1016/s0022-5347(17)49698-4. [DOI] [PubMed] [Google Scholar]
- Collins MM, Stafford RS, O’Leary MP, Barry MJ. Distinguishing chronic prostatitis and benign prostatic hyperplasia symptoms: results of a national survey of physician visits. Urology. 1999;53:921–925. doi: 10.1016/s0090-4295(98)00636-0. [DOI] [PubMed] [Google Scholar]
- Montie JE, Pienta KJ. Review of the role of androgenic hormones in the epidemiology of benign prostatic hyperplasia and prostate cancer. Urology. 1994;43:892–899. doi: 10.1016/0090-4295(94)90163-5. [DOI] [PubMed] [Google Scholar]
- Thomas JA. Diet, micronutrients, and the prostate gland. Nutr Rev. 1999;57:95–103. doi: 10.1111/j.1753-4887.1999.tb06932.x. [DOI] [PubMed] [Google Scholar]
- Salm SN, Koikawa Y, Ogilvie V, Tsujimura A, Coetzee S, Moscatelli D, Moore E, Lepor H, Shapiro E, Sun TT, Wilson EL. Transforming growth factor-beta is an autocrine mitogen for a novel androgen-responsive murine prostatic smooth muscle cell line, PSMC1. J Cell Physiol. 2000;185:416–424. doi: 10.1002/1097-4652(200012)185:3<416::AID-JCP12>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432:332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olumi AF, Dazin P, Tlsty TD. A novel coculture technique demonstrates that normal human prostatic fibroblasts contribute to tumor formation of LNCaP cells by retarding cell death. Cancer Res. 1998;58:4525–4530. [PubMed] [Google Scholar]
- Olumi AF, Grossfeld GD, Hayward SW, Carroll PR, Tlsty TD, Cunha GR. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- San Francisco IF, DeWolf WC, Peehl DM, Olumi AF. Expression of transforming growth factor-beta 1 and growth in soft agar differentiate prostate carcinoma-associated fibroblasts from normal prostate fibroblasts. Int J Cancer. 2004;112:213–218. doi: 10.1002/ijc.20388. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Sekiya T. Accumulation of genetic alterations and their significance in each primary human cancer and cell line. Mutat Res. 1998;400:421–437. doi: 10.1016/s0027-5107(98)00031-1. [DOI] [PubMed] [Google Scholar]
- Hill R, Song Y, Cardiff RD, Van Dyke T. Selective evolution of stromal mesenchyme with p53 loss in response to epithelial tumorigenesis. Cell. 2005;123:1001–1011. doi: 10.1016/j.cell.2005.09.030. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R, Mackie EJ, Pearson CA, Sakakura T. Tenascin: an extracellular matrix protein involved in tissue interactions during fetal development and oncogenesis. Cell. 1986;47:131–139. doi: 10.1016/0092-8674(86)90374-0. [DOI] [PubMed] [Google Scholar]
- Basset P, Bellocq JP, Wolf C, Stoll I, Hutin P, Limacher JM, Podhajcer OL, Chenard MP, Rio MC, Chambon P. A novel metalloproteinase gene specifically expressed in stromal cells of breast carcinomas. Nature. 1990;348:699–704. doi: 10.1038/348699a0. [DOI] [PubMed] [Google Scholar]
- Hilberg F, Aguzzi A, Howells N, Wagner EF. c-jun is essential for normal mouse development and hepatogenesis. Nature. 1993;365:179–181. doi: 10.1038/365179a0. [DOI] [PubMed] [Google Scholar]
- Culig Z, Hobisch A, Cronauer MV, Radmayr C, Trapman J, Hittmair A, Bartsch G, Klocker H. Androgen receptor activation in prostatic tumor cell lines by insulin-like growth factor-I, keratinocyte growth factor, and epidermal growth factor. Cancer Res. 1994;54:5474–5478. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.