Abstract
Viral attachment to the host cell is critical for tissue and species specificity of virus infections. Recently, pattern of viral attachment (PVA) in human respiratory tract was determined for highly pathogenic avian influenza virus of subtype H5N1. However, PVA of human influenza viruses and other avian influenza viruses in either humans or experimental animals is unknown. Therefore, we compared PVA of two human influenza viruses (H1N1 and H3N2) and two low pathogenic avian influenza viruses (H5N9 and H6N1) with that of H5N1 virus in respiratory tract tissues of humans, mice, ferrets, cynomolgus macaques, cats, and pigs by virus histochemistry. We found that human influenza viruses attached more strongly to human trachea and bronchi than H5N1 virus and attached to different cell types than H5N1 virus. These differences correspond to primary diagnoses of tracheobronchitis for human influenza viruses and diffuse alveolar damage for H5N1 virus. The PVA of low pathogenic avian influenza viruses in human respiratory tract resembled that of H5N1 virus, demonstrating that other properties determine its pathogenicity for humans. The PVA in human respiratory tract most closely mirrored that in ferrets and pigs for human influenza viruses and that in ferrets, pigs, and cats for avian influenza viruses.
Infections with human influenza A viruses of the subtypes H1N1 and H3N2 are important causes of respiratory tract disease. The most common lesion in immunocompetent individuals is tracheobronchitis.1 Uncommonly, human influenza A virus infection causes severe pneumonia, which requires hospitalization and may be fatal. This pattern of disease contrasts with the ongoing outbreak of highly pathogenic avian influenza A virus infection of the subtype H5N1. In this outbreak, severe pneumonia is the most common lesion in the >300 patients with confirmed H5N1 virus infection, and the case fatality rate is over 50% (World Health Organization http://www.who.int/csr/disease/avian_influenza/country/cases_table_2007_06_04/en/index.html). Until now, there is no evidence that this avian virus has become efficiently transmissible among humans, which could result in a new pandemic.2
The increased interest in H5N1 virus infection has highlighted large gaps in our knowledge of the pathogenesis of influenza A virus infections in humans. An important factor in this pathogenesis is tissue tropism, which depends largely on the ability of the virus to attach to the host cell.3 Influenza A viruses attach to host cells by binding of the hemagglutinin (HA) protein to sialosaccharides on the host cell surface. The HAs of influenza A viruses from different host species differ in their specificity of binding. For example, HAs of human influenza A viruses preferentially recognize sialic acid (SA)-α-2,6-Gal-terminated saccharides (α-2,6-SA), whereas HAs of avian influenza viruses preferentially recognize SA-α-2,3-Gal-terminated saccharides (α-2,3-SA).4,5,6 These differences generally correspond with the variation in the type of SAs expressed at important sites for influenza A virus replication in the respective host species. For example, human tracheal epithelium expresses mainly α-2,6-SA,7 whereas duck intestinal epithelium expresses mainly α-2,3-SA.8 Therefore, the type and distribution of SA is considered to be an important factor in the susceptibility of different host species to influenza A viruses.9 The SA recognized by influenza A virus is not only important in the host species range but also in its transmissibility. The latter was demonstrated in experimental infections of ferrets with the 1918 pandemic influenza virus. In this study, horizontal transmission was abolished by two amino acid mutations in the HA that caused a switch in binding preference from human α-2,6-SA to avian α-2,3-SA.10
A traditional method for studying the tissue tropism of different influenza A viruses is to measure the distribution of SAs by use of lectin histochemistry. Lectin histochemistry makes use of the property of a wide variety of lectins to specifically bind to SAs.11 Studies on receptors for influenza virus have made use of the plant lectins Sambucus nigra agglutinin, which has a major specificity for α-2,6-SA, and Maackia amurensis agglutinin, which has a major specificity for α-2,3-SA.12,13 Although useful for determining the distribution of SAs in tissues, these lectin histochemistry techniques are only an indirect measure of influenza A virus attachment to host tissues. They do not account for other variables that influence the binding specificity. For HA, these include glycosylation and sialylation close to the receptor binding site5; for the receptor, these include type of SA, alternative linkages,14 and sulfation and fucosylation of the saccharide residues.15
To circumvent these problems, we made use of virus histochemistry to study the pattern of virus attachment (PVA) in respiratory tissues. This method, modified from Couceiro et al,7 directly displays the attachment of influenza virus to tissues. By use of this method, we recently determined that H5N1 virus attachment in the human respiratory tract is progressively more abundant toward the alveoli, where the virus attaches predominantly to type II pneumocytes and alveolar macrophages.16 This attachment pattern fits with the limited pathology data on H5N1 virus infection in humans, which show diffuse alveolar damage as the primary lesion.17,18,19 Our results on H5N1 virus were supported by recent experimental studies in human ex vivo lung cultures, which demonstrated H5N1 virus replication in the lower respiratory tract (LRT).13,20
Although limited studies have been done on the human trachea,7 the PVA for human influenza A viruses in the LRT is not known. This information is important to understand better the pathogenesis of influenza pneumonia, which is centered on the LRT. In addition, it is not clear whether the PVA of H5N1 virus that we observed is unique among avian influenza viruses and therefore may in part explain its ability to cause respiratory disease in humans. Finally, the PVA of human influenza A virus in experimental animals is not known. This information is important to help select the most appropriate animal model for influenza pneumonia. Of particular interest is the domestic pig, which is permissive for both human and avian influenza A virus infections and may thus act as a “mixing vessel” for the generation of reassortant viruses.21 Therefore, we here describe the PVA of two currently circulating subtypes of human influenza A virus (H3N2 and H1N1) and low pathogenic avian influenza viruses (H5N9 and H6N1) to compare these with the PVA of highly pathogenic avian influenza A virus H5N1 in human respiratory tract. Furthermore, we determined the PVA of these human and avian influenza A viruses in respiratory tract of known experimental animals.
Materials and Methods
Experimental Design
To determine the PVA of human and avian influenza A viruses in the trachea and LRT of humans, we used two low pathogenic avian influenza viruses (H5N9 and H6N1), a highly pathogenic avian influenza A virus H5N1 isolate, and two recently circulating human influenza viruses (H3N2 and H1N1). We determined whether attachment occurred to epithelial cells in the trachea or LRT (including bronchi, bronchioles, and alveoli) and to alveolar macrophages.
The PVA of all of the above viruses was also determined in mammalian species, which are used for experimental influenza A virus infections. Animals included were cynomolgus macaque (Macaca fascicularis), European shorthair cat, ferret, Yorkshire-Landrace pig, and C57/BL6 mouse.
Viruses
Influenza virus A/Netherlands/35/05 (H1N1) and A/Netherlands/213/03 (H3N2) are recent human isolates grown on Madin-Darby canine kidney cells and were kindly provided by the National Influenza Center (Rotterdam, The Netherlands). Influenza virus A/Mallard/Sweden/79/02 (H5N9) and A/Mallard/Sweden/81/02 (H6N1) were obtained from cloacal swabs of migratory mallard ducks (Anas platyrhynchos) during ongoing influenza virus surveillance of wild birds and were subsequently passaged twice in embryonated hens’ eggs.22 Influenza virus A/Vietnam/1194/04 (H5N1) was isolated from a fatal human case. The virus was kindly provided by Dr. W. Lim (Queen Mary Hospital, Hong Kong, People’s Republic of China), and propagated once in Madin-Darby canine kidney cells.
Virus Preparation, Inactivation, and Labeling
The H1N1, H3N2, and H5N1 viruses, isolated from humans, were grown in Madin-Darby canine kidney cells. The supernatant was harvested and cleared by low-speed centrifugation. The H5N9 and H6N1 viruses, isolated from mallards, were grown in the allantoic cavity of 11-day-old embryonated hens’ eggs. The allantoic fluid was harvested after 2 days and cleared by low-speed centrifugation. Cleared supernatants and allantoic fluid samples were subsequently centrifuged 2 hours at 85,000 × g in a SW28 rotor at 4°C. The virus pellet was resuspended in 2 ml of phosphate-buffered saline (PBS), loaded on a 20 to 60% sucrose (w/w) gradient, and centrifuged overnight at 300,000 × g in a SW41 rotor at 4°C. To deplete the sucrose, the viruses were additionally centrifuged 2 hours at 85,000 × g in a SW28 rotor at 4°C, and the virus was resuspended in PBS.
H5N1 virus was inactivated by dialyzing against 0.1% formalin for 3 days. All other viruses were inactivated by incubation with 1:1 (v/v) 10% formalin for 1 hour at room temperature. After inactivation, virus suspensions were dialyzed against PBS. Inactivation was confirmed by failure to passage on Madin-Darby canine kidney cells.
Viruses were labeled by mixing concentrated, inactivated viruses, suspended in PBS, with an equal volume of 0.1 mg/ml fluorescein isothiocyanate (FITC) (Sigma, St. Louis, MO) in 0.5 mol/L bicarbonate buffer (pH 9.5) for 1 hour with constant stirring. To lose all unbound FITC, labeled viruses were dialyzed against PBS. To check for the continued capacity for hemagglutination by the inactivated viruses, the hemagglutination titer of the viruses was determined after formalin inactivation and FITC labeling.23
Respiratory Tract Tissues from Humans and Animals
Archival paraffin-embedded human tissue sections were obtained from the Department of Pathology, Erasmus MC. Archival paraffin-embedded animal tissue sections were obtained from the Department of Virology, Erasmus MC, or from the Department of Pathobiology, Faculty of Veterinary Medicine, University of Utrecht (Utrecht, The Netherlands). All tissues selected were from individuals without histological lesions or evidence of respiratory tract infection at the time of death. Three individuals per species were analyzed.
Virus Histochemistry on Tissue Sections
Formalin-fixed paraffin-embedded tissues were deparaffinized with xylene and hydrated using graded alcohols. FITC-labeled influenza viruses were incubated overnight at 4°C at a titer of 50 to 100 hemagglutinating units/50 μl. For the visualization by light miscroscopy, FITC label was detected with a peroxidase-labeled rabbit-anti-FITC (Dako, Glostrup, Denmark). The signal was amplified with a tyramide signal amplification system (Perkin Elmer, Boston, MA) according to the instructions of the manufacturer. Peroxidase was revealed with 3-amino-9-ethyl-carbazole (Sigma), resulting in a bright red precipitate. Tissues were counterstained with hematoxylin and embedded in glycerol-gelatin (Merck, Darmstadt, Germany). Attachment of influenza virus to tissues was visible as granular to diffuse red staining on the apical surface of epithelial cells. Cytoplasmic staining in epithelial cells and staining of other cell types was seen occasionally. For each tissue tested, in each run, an omission control was included to check for nonspecific amplification.
To validate the method, we incubated labeled H5N1 virus and H3N2 virus with human trachea and mallard duck intestine. The pattern of attachment of both viruses to these tissues was as expected. H5N1 virus, which has retained a preference for α-2,3-SA,15 bound abundantly to duck intestinal epithelium, which expresses mainly α-2,3-SA,8 and bound rarely to human tracheal epithelium, which expresses mainly α-2,6-SA.7 In contrast, H3N2 virus, which has a preference for α-2,6-SA, bound abundantly to human tracheal epithelium and bound poorly to duck intestinal epithelium.
Double Staining for H5N1 Virus Attachment and Type II Pneumocytes
H5N1 virus attachment in human lung was detected as described above, but peroxidase was not yet revealed. Type II pneumocytes were detected by incubation with a monoclonal mouse anti-human surfactant apoprotein A (PSP-A) antibody (Dako) for 1 hour at room temperature, followed by incubation with an alkaline phosphatase-labeled goat anti-mouse IgG2b (Southern Biotechnology Associates, Inc., Birmingham, AL) for 1 hour at room temperature. Peroxidase was revealed as described above, and alkaline phosphatase was revealed with 5-bromo-4-chloro-3-indolyl phosphate/nitro blue tetrazolium substrate system (Dako), resulting in a dark blue precipitate. Sections were not counterstained. Omission of H5N1 virus or an IgG2b isotype control (instead of mouse anti-human PSP-A) was included as a negative control in each run.
Results
Attachment of Human Influenza A Viruses to Human Respiratory Tract
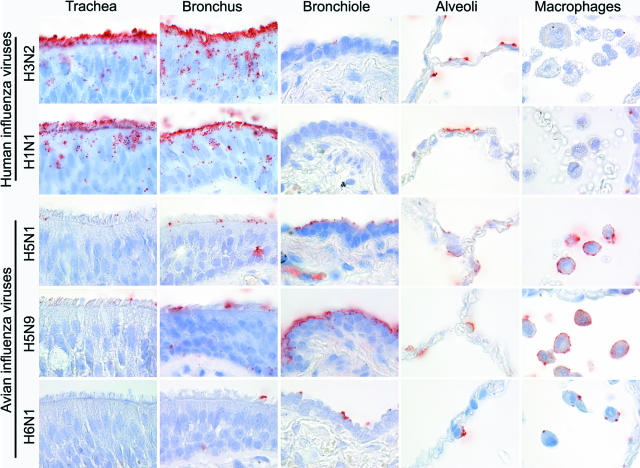
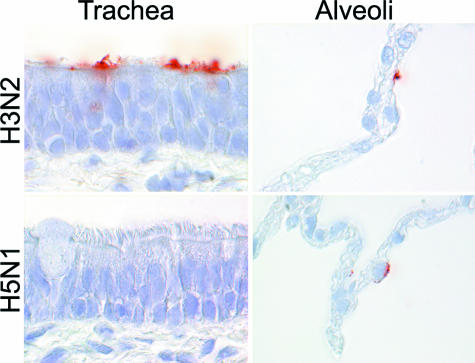
The human influenza A viruses H3N2 and H1N1 had a similar PVA to the human respiratory tract (Table 1; Figure 1). Virus attachment in the trachea and bronchi was more abundant than in the bronchioles. In trachea, bronchi, and bronchioles, virus predominantly attached to the surface of ciliated epithelial cells, occasionally attached to goblet cells, and rarely attached to bronchiolar nonciliated cuboidal cells. In alveoli, virus attached more to type I than to type II pneumocytes and very rarely to alveolar macrophages.
Table 1.
Virus Attachment in Respiratory Tract of Humans and Five Animal Species
| Virus | Species | Trachea
|
Bronchus
|
Bronchiole
|
Alveolus
|
||||
|---|---|---|---|---|---|---|---|---|---|
| Score | Predominant cell type | Score | Predominant cell type | Score | Predominant cell type | Score | Predominant cell type | ||
| H3N2 | Human | ++† | cil | ++† | cil | + | cil | + | Type I |
| Mouse | − | − | − | ± | |||||
| Ferret | ++* | cil | ±* | ± | + | Type I | |||
| Macaque | − | − | − | − | |||||
| Pig | ++† | cil | ++† | cil | ++ | cil | + | Type I | |
| Cat | − | − | − | − | |||||
| H1N1 | Human | ++† | cil | ++† | cil | + | cil | + | Type I |
| Mouse | − | − | − | ± | |||||
| Ferret | +* | cil | +* | cil | ± | + | Type I | ||
| Macaque | − | − | − | − | |||||
| Pig | ++† | cil | ++† | cil | ++ | cil | + | Type I | |
| Cat | − | − | − | − | |||||
| H5N1 | Human | −* | +* | cil | + | non-cil | +‡ | Type II | |
| Mouse | ++ | Both | + | non-cil | + | non-cil | + | Type II | |
| Ferret | − | − | ± | + | Type II | ||||
| Macaque | − | ± | ± | + | Type I | ||||
| Pig | − | − | − | + | Type II | ||||
| Cat | − | − | + | non-cil | +‡ | Type II | |||
| H5N9 | Human | −* | +* | cil | + | non-cil | +‡ | Type II | |
| Mouse | ++ | Both | ++ | non-cil | ++ | non-cil | + | Type II | |
| Ferret | − | − | − | ± | |||||
| Macaque | − | − | ± | ± | |||||
| Pig | − | − | ± | ± | |||||
| Cat | − | − | ± | +‡ | Type II | ||||
| H6N1 | Human | ±* | ±* | + | non-cil | +‡ | Type II | ||
| Mouse | ++ | Both | + | non-cil | + | non-cil | + | ||
| Ferret | − | − | − | ± | Type II | ||||
| Macaque | − | ± | ± | + | Type I | ||||
| Pig | − | − | ± | + | Type II | ||||
| Cat | − | − | ± | non-cil | +‡ | Type II | |||
The mean abundance of cells to which virus attached was scored as follows: −, no attachment; ±, attachment to rare or few cells; +, attachment to a moderate number of cells; ++, attachment to many cells. Where possible, the predominant cell type to which virus attached is indicated: ciliated cells (cil), nonciliated cuboidal cell (non-cil), type I pneumocytes (type I), or type II pneumocytes (type II).
Submucosal glands positive.
Goblet cells occasionally positive.
Alveolar macrophages positive.
Figure 1.
Attachment of human (H3N2 and H1N1) and avian (highly pathogenic H5N1 and low pathogenic H5N9 and H6N1) influenza viruses in human trachea, lower respiratory tract (bronchus, bronchiole, and alveoli), and alveolar macrophages.
Attachment of Avian Influenza Viruses to Human Respiratory Tract
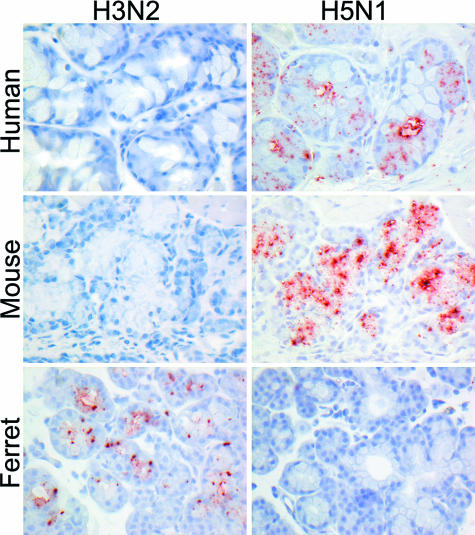
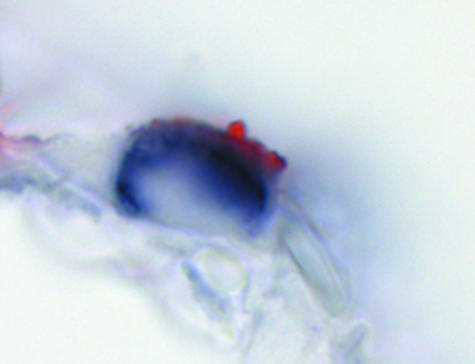
The avian influenza viruses H5N9 and H6N1 showed a similar PVA to tissues of the human respiratory tract that also resembled the PVA of H5N1 virus (Table 1; Figure 1). Attachment to the apical cell membrane was usually granular for H6N1 virus and more diffuse for H5N9 virus. In contrast to the human influenza A viruses, attachment of the avian influenza viruses was rare in the trachea and increased progressively toward the bronchioles. The avian influenza viruses also preferentially attached to different cell types than the human influenza A viruses: to acinar cells of the tracheal and bronchial submucosal glands (Figure 2) and to mucus at these sites, to nonciliated cuboidal cells in the bronchioles, and to type II pneumocytes and alveolar macrophages in the alveoli. To confirm that avian influenza viruses attached to type II pneumocytes, human lung tissue was double stained with H5N1 virus and PSP-A, which is a surfactant produced specifically by type II pneumocytes (Figure 3).
Figure 2.
Attachment of H3N2 virus and H5N1 virus to the submucosal glands in human, mouse, and ferret trachea.
Figure 3.
Confirmation of H5N1 virus attachment to type II pneumocytes in human alveoli by staining for human surfactant apoprotein A (PSP-A). H5N1 virus attachment is visible as red staining on the apical cell surface, whereas PSP-A expression, characteristic for type II pneumocytes, is visible as diffuse dark blue staining in the cytoplasm.
Attachment of Human Influenza A Viruses to Experimental Animal Respiratory Tract
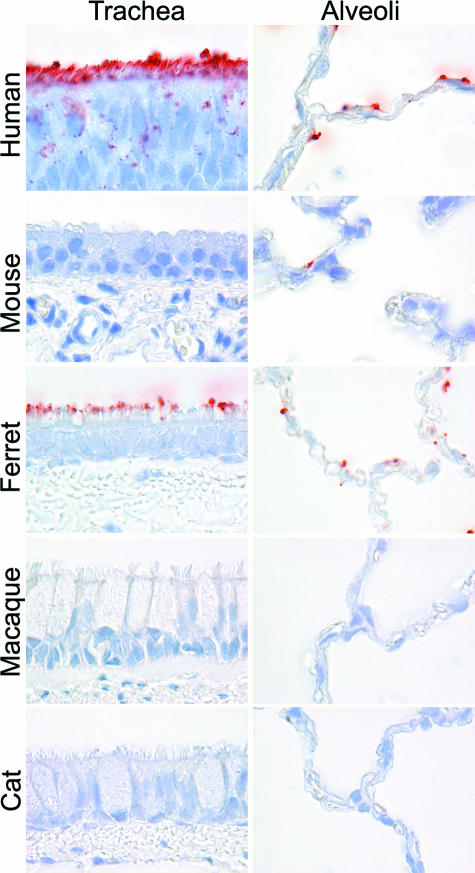
The PVA of human influenza A viruses to respiratory tract tissues of some experimental animal species resembled that of humans more than others (Table 1; Figures 2, 4, and 5). The PVA of ferrets and pigs resembled that of humans most closely, because they were the only two species in which the viruses attached to the surface of ciliated epithelial cells in the airways and to type I pneumocytes in alveoli. In ferrets, there also was virus attachment to submucosal glands and mucus (Figure 2). In mice, there was no attachment to trachea, bronchi, or bronchioles and only occasional attachment to alveolar epithelial cells of indeterminate type. In macaques and cats, virus attachment was not observed or was rarely observed at any level of the respiratory tract.
Figure 4.
Attachment of H3N2 virus to the trachea and alveoli of human, mouse, ferret, macaque, and cat.
Figure 5.
Attachment of H3N2 virus and H5N1 virus to pig trachea and alveoli.
Attachment of Avian Influenza Viruses to Experimental Animal Respiratory Tract
The PVA of H5N9 and H6N1 viruses to respiratory tract tissues resembled that of H5N1 virus in each of the five animal species (Table 1). As for human influenza A viruses, the PVA of avian influenza viruses to respiratory tract tissues of some animal species resembled that of humans more than others. The PVA of cats, ferrets, and pigs (Figure 5) resembled that of humans most closely, with rare virus attachment in the trachea and bronchi, rare to occasional attachment to nonciliated cuboidal cells in the bronchioles, and predominant attachment to type II pneumocytes in the alveoli. In cats, there also was virus attachment to alveolar macrophages, as in humans. The PVA of macaques also resembled that of humans, except that virus attachment was predominantly to type I instead of type II pneumocytes, although attachment to type II pneumocytes was also observed. In mice, virus attachment was most abundant in the trachea and became progressively weaker toward the alveoli, which was opposite to the PVA in humans. However, virus attachment to submucosal glands in mice mirrored that in humans (Figure 2).
Discussion
This study shows for the first time the PVA of human influenza A viruses in the human respiratory tract, from the trachea down to the alveoli. This information is important to understand better the pathogenesis of influenza pneumonia. The PVA of these viruses differs markedly from that of an H5N1 strain.16 These differences may explain, at least in part, the contrasts in localization and severity of respiratory disease between these virus infections in humans.
The common presentation of human influenza A virus infection is tracheobronchitis, which fits with the abundant attachment of these viruses to tracheal and bronchial epithelium (Table 1; Figure 1). Although rarely, human influenza A viruses can cause severe pneumonia. This fits with the ability of human influenza A viruses to attach to the human LRT. The difference in disease outcome between human influenza A viruses and H5N1 virus infection, where the primary lesion is severe pneumonia,24 fits with differences in virus attachment in the alveoli. Human influenza A viruses attached primarily to type I pneumocytes (Table 1; Figure 1), which are less numerous than type II pneumocytes (40 versus 60% of alveolar epithelial cells)25 and have low metabolic activity.26 We therefore speculate that if attachment leads to infection, virus production by type I pneumocytes is relatively low and can more easily be controlled by the host innate immune response. In addition, a large proportion of the human population has serum IgG antibodies against human influenza A viruses because of prior exposure. Although IgA is important for the protection of the respiratory tract from nose to bronchi, IgG is the main antibody involved in protecting the lung from pneumonia. These IgG antibodies are known to protect the richly vascularized lung parenchyma from infection and thus from development of severe pneumonia.27,28,29
The two low pathogenic avian influenza viruses, H5N9 and H6N1, had a similar PVA to human respiratory tract to that of H5N1 virus (Table 1; Figure 1), even though low pathogenic avian influenza viruses rarely infect or cause disease in humans.30,31 This means that the ability of H5N1 virus to attach to human respiratory tract cells—although necessary—is not sufficient to explain the ability of this virus to induce severe disease in humans. Alternative explanations for the uniqueness of H5N1 virus among avian influenza viruses may be its ability to replicate efficiently in human respiratory tract cells,20,32 to induce pro-inflammatory cytokine production,32,33,34,35 or to inhibit the host’s innate immune response.36
The respiratory tract of ferrets and pigs most closely resembled that of humans with regard to PVA of human influenza A viruses (Table 1; Figures 4 and 5). This corresponds with the permissiveness of pig37,38 and ferret39 for infection with nonadapted isolates of human influenza A viruses. As in humans, ferrets infected with human H3N2 virus usually develop upper respiratory tract disease and occasionally bronchitis and pneumonia,39,40 with similar histological changes as in human disease and expression of influenza virus antigen in ciliated epithelial cells of bronchi and bronchioles.41 Attachment of human influenza A virus to ciliated epithelial cells in the ferret trachea has been observed before.42 The PVA of human influenza A viruses in respiratory tracts of mouse, cynomolgus macaque, and cat differed strongly from that in human respiratory tract, with no attachment to trachea, bronchus, or bronchioles (Table 1; Figure 4). This fits with the results of experimental infections. Mice are not permissive for nonadapted human influenza A viruses.43 Cynomolgus macaques could be infected experimentally with human H3N2 virus, but no clinical signs were observed.44 In a recent study, we were unable to show experimental infection in domestic cats with a human H3N2 virus, although the same protocol resulted in productive infection of H5N1 virus.45
The respiratory tract of cats, ferrets, and pigs most closely resembled that of humans with regards to PVA of avian influenza viruses (Table 1). For cats and ferrets, the PVA of H5N1 virus to respiratory tract tissues has been reported previously.16 However, a species not reported on previously is the pig. Although α-2,3-SA (the preferred receptors of avian influenza viruses) and α-2,6-SA (the preferred receptors of human influenza A viruses) have been detected in pig trachea by lectin histochemistry,8 only human influenza A viruses attached to pig trachea in our study (Figure 5; Table 1). Pigs can be infected by human and avian influenza A viruses, and reassortant viruses have been isolated from pigs.46 Therefore, pigs have traditionally been considered as potential mixing vessels for avian and human influenza A viruses, from which pandemic influenza viruses may develop.21 However, reassortment of two influenza A viruses requires infection of the same host cell. Because our study indicates that such a reassortant is unlikely to occur in tracheal epithelium, it raises the question of where in the porcine respiratory tract do such reassortments occur.
The PVA of avian influenza viruses, including H5N1 virus, in experimental animals corresponds overall to the localization of respiratory tract disease reported for experimental H5N1 virus infection in these species. In cats,45 ferrets,47 macaques,48 and pigs,49 the lesions are most severe in alveoli and bronchioles, whereas bronchi and trachea are less or not affected. This fits with the PVA of H5N1 virus in these species, which is predominantly in alveoli and bronchioles (Table 1). In contrast, mice infected with H5N1 virus show substantial necrotizing lesions in trachea and bronchi in addition to alveolar and bronchiolar involvement.50,51 This corresponds to the moderate to abundant attachment of H5N1 virus to mouse trachea and bronchus (Table 1).
Tracheobronchial submucosal glands play two potentially opposite roles in influenza virus infection. On the one hand, they produce mucus that can inactivate virus and thus inhibit viral infection. On the other hand, the glands themselves may become infected with influenza virus, thus enhancing viral infection. In humans, tracheal submucosal glands produce a different sialosaccharide (α-2,3-SA) from that predominantly expressed by tracheal epithelial cells (α-2,6-SA).52 This fits with our attachment study, where avian influenza viruses bound to human submucosal gland cells and their mucus, but human influenza viruses did not (Figure 2). This mucus could trap these avian viruses and result in their expulsion by the mucociliary pathway before they can reach the LRT.52 This fits with the low transmission rate of avian influenza viruses (both low and highly pathogenic) from birds to humans30 and of H5N1 virus among humans.24 However, it is inconsistent with the observation that human H3N2 virus can replicate in human submucosal glands.53 The only species in which we detected attachment of human H3N2 influenza virus to tracheobronchial submucosal glands was the ferret (Figure 2), in which human H3N2 virus also has been shown to replicate in these glands.54 A possible explanation is that attachment of H3N2 virus to tracheobronchial submucosal gland is below the detection limit of our test for humans and above the limit for ferrets.
Together, the results of this virus attachment study improve our understanding of the pathogenesis of human respiratory tract disease from both human and avian influenza A virus infection. This information on virus attachment needs to be combined with studies on other factors that may contribute to pathogenicity of influenza A virus infection, such as replication rate,32,55 escape mechanisms from innate immune response,56 and induction of cytokine production.32 Virus attachment studies also may help in the choice of animal model to study disease mechanisms and to test preventive and therapeutic strategies against both human and avian influenza. The viral histochemistry technique used here for influenza A virus also may be useful in the research of other viral diseases.
Acknowledgments
We thank W. Lim for providing the H5N1 virus isolate; F. van der Panne for technical assistance; and M. den Bakker, H. Sharma, M. Vermeij, and J. van den Brand for providing tissues.
Footnotes
Address reprint requests to Thijs Kuiken, Department of Virology, Erasmus MC, P.O. Box 2040, 3015 CA, Rotterdam, The Netherlands. E-mail: t.kuiken@erasmusmc.nl.
See related Commentary on page 1089
Supported by the VIRGO Consortium, an Innovative Cluster approved by the Netherlands Genomics Initiative, and partially funded by the Dutch Government (BSIK 03012), The Netherlands.
References
- Zambon MC. Epidemiology and pathogenesis of influenza. J Antimicrob Chemother. 1999;44(Suppl B):3–9. doi: 10.1093/jac/44.suppl_2.3. [DOI] [PubMed] [Google Scholar]
- Garcia-Sastre A, Whitley RJ. Lessons learned from reconstructing the 1918 influenza pandemic. J Infect Dis. 2006;194(Suppl 2):S127–S132. doi: 10.1086/507546. [DOI] [PubMed] [Google Scholar]
- Baigent SJ, McCauley JW. Influenza type A in humans, mammals and birds: determinants of virus virulence, host-range and interspecies transmission. Bioessays. 2003;25:657–671. doi: 10.1002/bies.10303. [DOI] [PubMed] [Google Scholar]
- Rogers GN, Paulson JC. Receptor determinants of human and animal influenza virus isolates: differences in receptor specificity of the H3 hemagglutinin based on species of origin. Virology. 1983;127:361–373. doi: 10.1016/0042-6822(83)90150-2. [DOI] [PubMed] [Google Scholar]
- Suzuki Y. Sialobiology of influenza: molecular mechanism of host range variation of influenza viruses. Biol Pharm Bull. 2005;28:399–408. doi: 10.1248/bpb.28.399. [DOI] [PubMed] [Google Scholar]
- Connor RJ, Kawaoka Y, Webster RG, Paulson JC. Receptor specificity in human, avian, and equine H2 and H3 influenza virus isolates. Virology. 1994;205:17–23. doi: 10.1006/viro.1994.1615. [DOI] [PubMed] [Google Scholar]
- Couceiro JN, Paulson JC, Baum LG. Influenza virus strains selectively recognize sialyloligosaccharides on human respiratory epithelium: the role of the host cell in selection of hemagglutinin receptor specificity. Virus Res. 1993;29:155–165. doi: 10.1016/0168-1702(93)90056-s. [DOI] [PubMed] [Google Scholar]
- Ito T, Couceiro JN, Kelm S, Baum LG, Krauss S, Castrucci MR, Donatelli I, Kida H, Paulson JC, Webster RG, Kawaoka Y. Molecular basis for the generation in pigs of influenza A viruses with pandemic potential. J Virol. 1998;72:7367–7373. doi: 10.1128/jvi.72.9.7367-7373.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Ito T, Suzuki T, Holland RE, Jr, Chambers TM, Kiso M, Ishida H, Kawaoka Y. Sialic acid species as a determinant of the host range of influenza A viruses. J Virol. 2000;74:11825–11831. doi: 10.1128/jvi.74.24.11825-11831.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumpey TM, Maines TR, Van Hoeven N, Glaser L, Solorzano A, Pappas C, Cox NJ, Swayne DE, Palese P, Katz JM, Garcia-Sastre A. A two-amino acid change in the hemagglutinin of the 1918 influenza virus abolishes transmission. Science. 2007;315:655–659. doi: 10.1126/science.1136212. [DOI] [PubMed] [Google Scholar]
- Varki A. Sialic acids as ligands in recognition phenomena. FASEB J. 1997;11:248–255. doi: 10.1096/fasebj.11.4.9068613. [DOI] [PubMed] [Google Scholar]
- Baum LG, Paulson JC. Sialyloligosaccharides of the respiratory epithelium in the selection of human influenza virus receptor specificity. Acta Histochem Suppl. 1990;40:35–38. [PubMed] [Google Scholar]
- Shinya K, Ebina M, Yamada S, Ono M, Kasai N, Kawaoka Y. Avian flu: influenza virus receptors in the human airway. Nature. 2006;440:435–436. doi: 10.1038/440435a. [DOI] [PubMed] [Google Scholar]
- Wu W, Air GM. Binding of influenza viruses to sialic acids: reassortant viruses with A/NWS/33 hemagglutinin bind to alpha2,8-linked sialic acid. Virology. 2004;325:340–350. doi: 10.1016/j.virol.2004.05.013. [DOI] [PubMed] [Google Scholar]
- Gambaryan A, Tuzikov A, Pazynina G, Bovin N, Balish A, Klimov A. Evolution of the receptor binding phenotype of influenza A (H5) viruses. Virology. 2006;344:432–438. doi: 10.1016/j.virol.2005.08.035. [DOI] [PubMed] [Google Scholar]
- van Riel D, Munster VJ, de Wit E, Rimmelzwaan GF, Fouchier RA, Osterhaus AD, Kuiken T. H5N1 virus attachment to lower respiratory tract. Science. 2006;312:399. doi: 10.1126/science.1125548. [DOI] [PubMed] [Google Scholar]
- Uiprasertkul M, Puthavathana P, Sangsiriwut K, Pooruk P, Srisook K, Peiris M, Nicholls JM, Chokephaibulkit K, Vanprapar N, Auewarakul P. Influenza A H5N1 replication sites in humans. Emerg Infect Dis. 2005;11:1036–1041. doi: 10.3201/eid1107.041313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- To KF, Chan PK, Chan KF, Lee WK, Lam WY, Wong KF, Tang NL, Tsang DN, Sung RY, Buckley TA, Tam JS, Cheng AF. Pathology of fatal human infection associated with avian influenza A H5N1 virus. J Med Virol. 2001;63:242–246. doi: 10.1002/1096-9071(200103)63:3<242::aid-jmv1007>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Ng WF, To KF, Lam WW, Ng TK, Lee KC. The comparative pathology of severe acute respiratory syndrome and avian influenza A subtype H5N1: a review. Hum Pathol. 2006;37:381–390. doi: 10.1016/j.humpath.2006.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls JM, Chan MC, Chan WY, Wong HK, Cheung CY, Kwong DL, Wong MP, Chui WH, Poon LL, Tsao SW, Guan Y, Peiris JS. Tropism of avian influenza A (H5N1) in the upper and lower respiratory tract. Nat Med. 2007;13:147–149. doi: 10.1038/nm1529. [DOI] [PubMed] [Google Scholar]
- Webster RG, Bean WJ, Gorman OT, Chambers TM, Kawaoka Y. Evolution and ecology of influenza A viruses. Microbiol Rev. 1992;56:152–179. doi: 10.1128/mr.56.1.152-179.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munster VJ, Wallensten A, Baas C, Rimmelzwaan GF, Schutten M, Olsen B, Osterhaus AD, Fouchier RA. Mallards and highly pathogenic avian influenza ancestral viruses, northern Europe. Emerg Infect Dis. 2005;11:1545–1551. doi: 10.3201/eid1110.050546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmelzwaan GF, Baars M, Claas EC, Osterhaus AD. Comparison of RNA hybridization, hemagglutination assay, titration of infectious virus and immunofluorescence as methods for monitoring influenza virus replication in vitro. J Virol Methods. 1998;74:57–66. doi: 10.1016/s0166-0934(98)00071-8. [DOI] [PubMed] [Google Scholar]
- Beigel JH, Farrar J, Han AM, Hayden FG, Hyer R, de Jong MD, Lochindarat S, Nguyen TK, Nguyen TH, Hien TT, Nicoll A, Touch S, Yuen KY. Avian influenza A (H5N1) infection in humans. N Engl J Med. 2005;353:1374–1385. doi: 10.1056/NEJMra052211. [DOI] [PubMed] [Google Scholar]
- Castranova V, Rabovsky J, Tucker JH, Miles PR. The alveolar type II epithelial cell: a multifunctional pneumocyte. Toxicol Appl Pharmacol. 1988;93:472–483. doi: 10.1016/0041-008x(88)90051-8. [DOI] [PubMed] [Google Scholar]
- Stevens A, Lowe J. St. Louis: MO, Mosby,; Histology. 1993:pp 124–142. [Google Scholar]
- Bender BS, Small PA., Jr Influenza: pathogenesis and host defense. Semin Respir Infect. 1992;7:38–45. [PubMed] [Google Scholar]
- Cox RJ, Hovden AO, Brokstad KA, Szyszko E, Madhun AS, Haaheim LR. The humoral immune response and protective efficacy of vaccination with inactivated split and whole influenza virus vaccines in BALB/c mice. Vaccine. 2006;24:6585–6587. doi: 10.1016/j.vaccine.2006.05.040. [DOI] [PubMed] [Google Scholar]
- Riberdy JM, Flynn KJ, Stech J, Webster RG, Altman JD, Doherty PC. Protection against a lethal avian influenza A virus in a mammalian system. J Virol. 1999;73:1453–1459. doi: 10.1128/jvi.73.2.1453-1459.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beare AS, Webster RG. Replication of avian influenza viruses in humans. Arch Virol. 1991;119:37–42. doi: 10.1007/BF01314321. [DOI] [PubMed] [Google Scholar]
- Webby RJ, Webster RG. Emergence of influenza A viruses. Philos Trans R Soc Lond B Biol Sci. 2001;356:1817–1828. doi: 10.1098/rstb.2001.0997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Jong MD, Simmons CP, Thanh TT, Hien VM, Smith GJ, Chau TN, Hoang DM, Chau NV, Khanh TH, Dong VC, Qui PT, Cam BV, Ha do Q, Guan Y, Peiris JS, Chinh NT, Hien TT, Farrar J. Fatal outcome of human influenza A (H5N1) is associated with high viral load and hypercytokinemia. Nat Med. 2006;12:1203–1207. doi: 10.1038/nm1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szretter KJ, Gangappa S, Lu X, Smith C, Shieh WJ, Zaki SR, Sambhara S, Tumpey TM, Katz JM. Role of host cytokine responses in the pathogenesis of avian H5N1 influenza viruses in mice. J Virol. 2007;81:2736–2744. doi: 10.1128/JVI.02336-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MC, Cheung CY, Chui WH, Tsao SW, Nicholls JM, Chan YO, Chan RW, Long HT, Poon LL, Guan Y, Peiris JS. Proinflammatory cytokine responses induced by influenza A (H5N1) viruses in primary human alveolar and bronchial epithelial cells. Respir Res. 2005;6:135. doi: 10.1186/1465-9921-6-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung CY, Poon LL, Lau AS, Luk W, Lau YL, Shortridge KF, Gordon S, Guan Y, Peiris JS. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: a mechanism for the unusual severity of human disease? Lancet. 2002;360:1831–1837. doi: 10.1016/s0140-6736(02)11772-7. [DOI] [PubMed] [Google Scholar]
- Seo SH, Hoffmann E, Webster RG. The NS1 gene of H5N1 influenza viruses circumvents the host anti-viral cytokine responses. Virus Res. 2004;103:107–113. doi: 10.1016/j.virusres.2004.02.022. [DOI] [PubMed] [Google Scholar]
- Hinshaw VS, Bean WJ, Jr, Webster RG, Easterday BC. The prevalence of influenza viruses in swine and the antigenic and genetic relatedness of influenza viruses from man and swine. Virology. 1978;84:51–62. doi: 10.1016/0042-6822(78)90217-9. [DOI] [PubMed] [Google Scholar]
- Karasin AI, Schutten MM, Cooper LA, Smith CB, Subbarao K, Anderson GA, Carman S, Olsen CW. Genetic characterization of H3N2 influenza viruses isolated from pigs in North America, 1977–1999: evidence for wholly human and reassortant virus genotypes. Virus Res. 2000;68:71–85. doi: 10.1016/s0168-1702(00)00154-4. [DOI] [PubMed] [Google Scholar]
- Renegar KB. Influenza virus infections and immunity: a review of human and animal models. Lab Anim Sci. 1992;42:222–232. [PubMed] [Google Scholar]
- Smith H, Sweet C. Lessons for human influenza from pathogenicity studies with ferrets. Rev Infect Dis. 1988;10:56–75. doi: 10.1093/clinids/10.1.56. [DOI] [PubMed] [Google Scholar]
- Husseini RH, Sweet C, Bird RA, Collie MH, Smith H. Distribution of viral antigen with the lower respiratory tract of ferrets infected with a virulent influenza virus: production and release of virus from corresponding organ cultures. J Gen Virol. 1983;64:589–598. doi: 10.1099/0022-1317-64-3-589. [DOI] [PubMed] [Google Scholar]
- Piazza FM, Carson JL, Hu SC, Leigh MW. Attachment of influenza A virus to ferret tracheal epithelium at different maturational stages. Am J Respir Cell Mol Biol. 1991;4:82–87. doi: 10.1165/ajrcmb/4.1.82. [DOI] [PubMed] [Google Scholar]
- Tumpey TM, Garcia-Sastre A, Taubenberger JK, Palese P, Swayne DE, Pantin-Jackwood MJ, Schultz-Cherry S, Solorzano A, Van Rooijen N, Katz JM, Basler CF. Pathogenicity of influenza viruses with genes from the 1918 pandemic virus: functional roles of alveolar macrophages and neutrophils in limiting virus replication and mortality in mice. J Virol. 2005;79:14933–14944. doi: 10.1128/JVI.79.23.14933-14944.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimmelzwaan GF, Baars M, van Beek R, Van Amerongen G, Lovgren-Bengtsson K, Claas EC, Osterhaus AD. Induction of protective immunity against influenza virus in a macaque model: comparison of conventional and iscom vaccines. J Gen Virol. 1997;78:757–765. doi: 10.1099/0022-1317-78-4-757. [DOI] [PubMed] [Google Scholar]
- Kuiken T, Rimmelzwaan G, van Riel D, Van Amerongen G, Baars M, Fouchier R, Osterhaus A. Avian H5N1 influenza in cats. Science. 2004;306:241. doi: 10.1126/science.1102287. [DOI] [PubMed] [Google Scholar]
- Castrucci MR, Donatelli I, Sidoli L, Barigazzi G, Kawaoka Y, Webster RG. Genetic reassortment between avian and human influenza A viruses in Italian pigs. Virology. 1993;193:503–506. doi: 10.1006/viro.1993.1155. [DOI] [PubMed] [Google Scholar]
- Maines TR, Lu XH, Erb SM, Edwards L, Guarner J, Greer PW, Nguyen DC, Szretter KJ, Chen LM, Thawatsupha P, Chittaganpitch M, Waicharoen S, Nguyen DT, Nguyen T, Nguyen HH, Kim JH, Hoang LT, Kang C, Phuong LS, Lim W, Zaki S, Donis RO, Cox NJ, Katz JM, Tumpey TM. Avian influenza (H5N1) viruses isolated from humans in Asia in 2004 exhibit increased virulence in mammals. J Virol. 2005;79:11788–11800. doi: 10.1128/JVI.79.18.11788-11800.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiken T, Rimmelzwaan GF, Van Amerongen G, Osterhaus AD. Pathology of human influenza A (H5N1) virus infection in cynomolgus macaques (Macaca fascicularis). Vet Pathol. 2003;40:304–310. doi: 10.1354/vp.40-3-304. [DOI] [PubMed] [Google Scholar]
- Choi YK, Nguyen TD, Ozaki H, Webby RJ, Puthavathana P, Buranathal C, Chaisingh A, Auewarakul P, Hanh NT, Ma SK, Hui PY, Guan Y, Peiris JS, Webster RG. Studies of H5N1 influenza virus infection of pigs by using viruses isolated in Vietnam and Thailand in 2004. J Virol. 2005;79:10821–10825. doi: 10.1128/JVI.79.16.10821-10825.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dybing JK, Schultz-Cherry S, Swayne DE, Suarez DL, Perdue ML. Distinct pathogenesis of Hong Kong-origin H5N1 viruses in mice compared to that of other highly pathogenic H5 avian influenza viruses. J Virol. 2000;74:1443–1450. doi: 10.1128/jvi.74.3.1443-1450.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CW, Suarez DL, Tumpey TM, Sung HW, Kwon YK, Lee YJ, Choi JG, Joh SJ, Kim MC, Lee EK, Park JM, Lu X, Katz JM, Spackman E, Swayne DE, Kim JH. Characterization of highly pathogenic H5N1 avian influenza A viruses isolated from South Korea. J Virol. 2005;79:3692–3702. doi: 10.1128/JVI.79.6.3692-3702.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olofsson S, Kumlin U, Dimock K, Arnberg N. Avian influenza and sialic acid receptors: more than meets the eye? Lancet Infect Dis. 2005;5:184–188. doi: 10.1016/S1473-3099(05)01311-3. [DOI] [PubMed] [Google Scholar]
- Guarner J, Paddock CD, Shieh WJ, Packard MM, Patel M, Montague JL, Uyeki TM, Bhat N, Balish A, Lindstrom S, Klimov A, Zaki SR. Histopathologic and immunohistochemical features of fatal influenza virus infection in children during the 2003–2004 season. Clin Infect Dis. 2006;43:132–140. doi: 10.1086/505122. [DOI] [PubMed] [Google Scholar]
- Gentry SE, Culp DJ, Roberts NJ, Jr, Marin MG, Simons RL, Latchney LR. Influenza virus infection of tracheal gland cells in culture. J Virol. 1988;62:1524–1529. doi: 10.1128/jvi.62.5.1524-1529.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen GW, Chang SC, Mok CK, Lo YL, Kung YN, Huang JH, Shih YH, Wang JY, Chiang C, Chen CJ, Shih SR. Genomic signatures of human versus avian influenza A viruses. Emerg Infect Dis. 2006;12:1353–1360. doi: 10.3201/eid1209.060276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobasa D, Jones SM, Shinya K, Kash JC, Copps J, Ebihara H, Hatta Y, Kim JH, Halfmann P, Hatta M, Feldmann F, Alimonti JB, Fernando L, Li Y, Katze MG, Feldmann H, Kawaoka Y. Aberrant innate immune response in lethal infection of macaques with the 1918 influenza virus. Nature. 2007;445:319–323. doi: 10.1038/nature05495. [DOI] [PubMed] [Google Scholar]