Abstract
Oxidative and nitrosative stress have been implicated in prostate carcinogenesis, but the cause(s) of redox imbalance in the gland remains poorly defined. We and others have reported that administration of testosterone plus 17β-estradiol to Noble rats for 16 weeks induces dysplasia and stromal inflammation of the lateral prostate (LP) but not the ventral prostate. Here, using laser capture microdissected specimens, we found that the combined hormone regimen increased the expression of mRNA of specific members of NAD(P)H oxidase (NOX-1, NOX-2, and NOX4), nitric-oxide synthase [NOS; inducible NOS and endothelial NOS], and cyclooxygenase (COX-2) in the LP epithelium and/or its adjacent inflammatory stroma. Accompanying these changes was the accumulation of 8-hydroxy-2′-deoxyguanosine, 4-hydroxynonenal protein adducts, and nitrotyrosine, primarily in the LP epithelium, suggesting that NOX, NOS, and COX may mediate hormone-induced oxidative/nitrosative stress in epithelium. We concluded that the oxidative/nitrosative damage resulting from the testosterone-plus-17β-estradiol treatment is not solely derived from stromal inflammatory lesions but likely also originates from the epithelium per se. In this context, the up-regulation of COX-2 from epithelium represents a potential mechanism by which the hormone-initiated epithelium might induce inflammatory responses. Thus, we link alterations in the hormonal milieu with oxidative/nitrosative/inflammatory damage to the prostate epithelium that promotes carcinogenesis.
Oxidative stress (OS) and nitrosative stress (NS) refer to a state of redox imbalance leading to excessive production of reactive oxygen species (ROS) and reactive nitrogen species (RNS), respectively, that overwhelms the antioxidant defenses of a cell. Such imbalances have recently been implicated in the genesis of prostate cancer (PCa). Thus, biomarkers of OS and NS have been reported in human prostatic intraepithelial neoplasia, also termed dysplasia purported PCa precursor, and in cancer of the prostate.1,2
Reactive oxygen species are normally generated as byproducts of aerobic respiration in the mitochondria, as well as a variety of inflammatory cells, producing large quantities of ROS and RNS.3 In addition to these two sources, superoxide-generating homologues of phagocytic NAD(P)H oxidase catalytic subunit gp91phox (NOX-1 and NOX-3, NOX-4, and NOX-5) and nitric-oxide synthase (NOS) family members, including inducible NOS (iNOS), endothelial NOS (eNOS), and neuronal NOS (nNOS), are primary O2⨪ sources of superoxide (O2–·) and nitric oxide (·NO), respectively, in nonphagocytic cells, as well as in various epithelial cell types.4 With regard to carcinogenesis, NOX-1 and NOX-5 have been reported to exert major effects on prostate tumor growth and angiogenesis.5,6 ·NO is also an important signaling molecule for inducing inflammation, including those of the prostate.7 Moreover, the conversion of arachidonic acid to prostaglandins via cyclooxygenases (COX-1 and COX-2) are potent inducers of inflammation.8 In addition to their proinflammatory actions, COXs also produce peroxyl radicals as a byproduct of prostaglandin synthesis.9,10 Apropos to carcinogenesis of the prostate, consistent overexpression of COX-2 was found in proliferative inflammatory atrophy (PIA)11 and prostatic intraepithelial neoplasia lesions,10 but its expression level in PCa remains controversial.10
Damage to cellular DNA and protein, produced directly in tissues or indirectly by ROS and RNS, as a consequence of inflammation, has been implicated as causative factors for a variety of cancers.9,12,13,14 In this regard, PIA, an inflammation-associated proliferative lesion in the human prostate, has been proposed as a precursor of prostatic intraepithelial neoplasia and PCa.7,15 It has been postulated that the genesis of PIA results from ROS/RNS produced by inflammatory cells that induce epithelial atrophy and subsequent regenerative proliferation, which has been considered to represent early changes in the carcinogenic process.15
Although androgens and estrogens have major influences on the regulation of cell growth and differentiation, as well as on malignant transformation of the prostate,16,17 information is limited concerning the role that these hormones may play in the regulation of redox balance in this organ.18,19 We previously demonstrated that androgens and estrogens can influence redox status and antioxidant enzyme activity in the prostates of Noble (NBL) rats.19 Exposure of NBL rats to treatment with a combination of testosterone (T) and 17β-estradiol (E2) for 16 weeks induces dysplasia accompanied by inflammation selectively in the lateral prostates (LPs), and longer exposure of these steroids induces a high incidence of adenocarcinomas in the LP.20,21 However, neither of these lesions are induced in the ventral prostates (VPs) of all treated animals.20,21,22,23,24 Using the NBL experimental model, our current results provide evidence that this steroid treatment selectively causes major disruptive effects in the OS/NS of the LP that affects damage to DNA, protein, and lipids. Thus, for the first time, we provide a causative link to sex hormone-mediated alterations in redox imbalance and cellular damage in the prostate, which strongly implicates this mechanism in the development of cancer in the LP of the gland.
Materials and Methods
Animals and Hormone Treatments
Protocols of animal usage were approved by the University of Massachusetts Medical School Animal Care and Usage Committee. NBL rats were purchased from Charles River Laboratories (Kingston, NY), kept under standard conditions, and treated as previously reported.23,24 In brief, control rats (n = 8) were implanted with empty capsules. Hormone-treated rats (n = 8) were implanted with 2-cm lengths of Silastic tubing (1.0-mm inner diameter × 2.2-mm outer diameter; Dow Corning, Midland, MI) that were tightly packed with 14.4 ± 2.1 mg of T (Sigma, St. Louis, MO), and 1-cm lengths of the same tubing were packed with 14.8 ± 2.6 mg of E2 (Sigma). At the end of a 16-week treatment period, animals were sacrificed with an overdose of isoflurane, and VPs and LPs were excised. One half of each lobe was processed for histological examination, and the other half was snap frozen for RNA extraction or laser capture microdissection (LCM).
Histopathology and LCM
Formalin-fixed samples were processed for light microscopy. Twelve step-sections sampled serially through an entire half-LP/ventral prostate (VP), in a single-blinded manner, were subjected to histological examination for inflammatory, dysplastic, and malignant lesions by I.L.22 The other half of the LP was embedded in Tissue-Tek O.C.T. compound (Sakura Finetek USA, Torrance, CA) and then snap frozen in liquid nitrogen. Serial frozen sections were cryocut from each specimen. The first section of a replicate series was fixed, stained with hematoxylin, and used as a guide for the LCM (Arcturus, Mountain View, CA) conducted on the next serial sections, as previously described.25
Radioimmunoassay (RIA) of Serum T and E2 Levels
Serum total T and E2 levels from at least five animals in the control and in the T+E2-treated groups were measured by RIA, which was conducted in the ILAT Steroid RIA Laboratory, University of Massachusetts Medical School (Worcester, MA). The RIA kits for T and E2 assays were provided by Diagnostic Products (Los Angeles, CA) and Diagnostic Systems Laboratories (Webster, TX), respectively. The sensitivities of the T and E2 assays are 0.04 ng/ml and 2.2 pg/ml, respectively.
RNA Isolation, cDNA Synthesis, and Real-Time Quantitative Polymerase Chain Reaction
Total RNA was isolated and reverse-transcribed into cDNA as previously described.18 Real-time quantitative polymerase chain reaction (PCR) was performed with the iCycler IQ Real-Time PCR detection system (Bio-Rad Laboratories, Hercules, CA) as reported.24 Intron-spanning, gene-specific primers for nNOS (sense: 5′-CCCTGGCCAATGTGAGGTTC-3′, antisense: 5′-TCCTCTCCCCTCCCAGTTCC-3′), iNOS (sense: 5′-CACCTTGGAGTTCACCCAGT-3′, antisense: 5′-ACCACTCGTACTTGGGATGC-3′), eNOS (sense: 5′-CCAGCCAGGGGACCACATAG-3′, antisense: 5′-CAGTTGTTCCACGGCCACAG-3′), COX-1 (sense: 5′-GCCTCGACCACTACCAATGT-3′, antisense: 5′-AGGTGGCATTCACAAA CTCC-3′), and COX-2 (sense: 5′-GCTTTTCAACCAGCAGTTCC-3′, antisense: 5′-CTGCTTGTACAGCGATTGGA-3′) were designed with PRIMER 3 software, purchased from MWG Biotech (High Point, NC), and optimized for real-time PCR. Primers and PCR conditions for NOX-1, NOX-2, NOX-4, superoxide dismutase 1 (SOD-1), superoxide dismutase 2 (SOD-2), and ribosomal protein L19 (RPL19) have been documented previously.18
PCR products generated by each primer pair were column-purified by the Wizard SV gel and PCR clean-up system (Promega, Madison, WI) and quantified spectrophotometrically at 260 nm. The molecular mass of target cDNA was calculated on the following basis (Real-time PCR Instruction Manual; Applied Biosystems, Foster City, CA): mass of double-stranded DNA = number of bp × 1.096 × 10−21 g. Stock solutions of 300,000 copies of each standard cDNA/5 μl were prepared and serially diluted (1:10) with nuclease-free water. The target-gene transcript copy numbers in tissues/LCM samples were determined with a standard curve generated by plotting cycles at threshold against the logarithmic values of the standard copy number. The loading control was normalized by using the RPL19 level.
Immunohistochemical Analysis of OS/NS Biomarkers
Immunohistochemical staining for 8-hydroxy-2′-deoxy-guanosine (8-OHdG), 4-hydroxynonenal (4-HNE), and nitrotyrosine was conducted on paraffin slides as previously described.18,26 For controls, the primary antibodies were preincubated with either excess antigens [1.5 mg/ml 8-OHdG] (Sigma) or 10 mmol/L nitrotyrosine (Calbiochem, San Diego, CA) or were replaced with the corresponding normal isotype serum (Zymed, South San Francisco, CA).
Statistical Analyses
The statistical significance of the difference in expression levels between treatment groups was determined with Systat software (Student version 6.0.1) (SPSS, Chicago, IL) for one-way analysis of variance, followed by Tukey’s post hoc analyses or Student’s t-test. A P value of ≤0.05 was taken as a statistically significant difference between two groups.
Results
T+E2 Regimen Maintained Physiological T Levels and Elevated Levels of E2
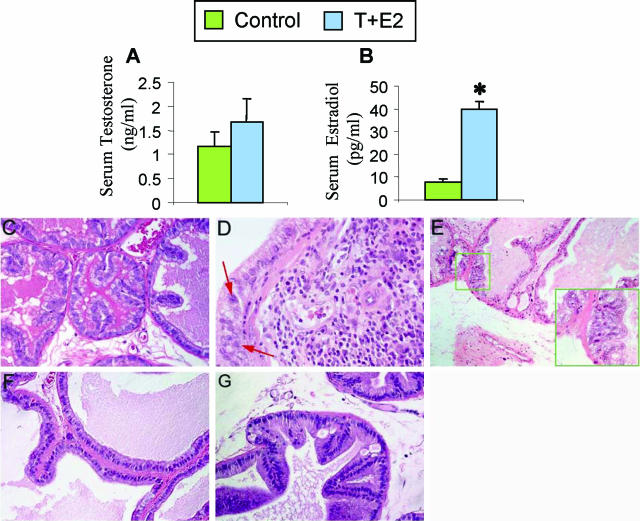
RIA analyses of serum T and E2 levels (Figure 1, A and B, respectively) revealed that the number and the size of Silastic capsules used maintained the serum T at physiological levels (untreated control rats = 1.17 ± 0.28 ng/ml versus T+E2-treated rats = 1.66 ± 0.49 ng/ml; P > 0.05) and increased E2 levels four- to fivefold (39.6 ± 3.5 pg/ml, P < 0.05) when compared with the untreated control rats (8.0 ± 1.14 pg/ml). The elevated E2 levels are within the physiological range of cyclic fluctuation of E2 (from 17 ± 2 to 88 ± 2 pg/ml) in female rats during their 4-day estrous cycle.27
Figure 1.
T+E2 co-treatment results in changes in the sex hormone milieu (A and B) and a lobe-specific induction of stromal inflammatory response and focal epithelial dysplastic lesions in LPs (C–E), but not in VPs (F and G), of treated rats. After a 16-week implantation of Silastic capsules containing T and E2, serum levels of T and E2 were determined by radioimmunoassay. A: Serum T levels of treated rats were not significantly different from those of untreated control rats. B: Serum E2 levels of treated rats were significantly (*P < 0.05) increased by four- to fivefold compared with levels in untreated control rats. Data represent the mean ± SEM of values for at least five animals. C: Normal control LP. Epithelial tubules are lined by a single layer of cuboidal secretory cells; the stroma comprises primarily a periacinar layer of smooth muscle cells and interstitial fibroblasts. Note the scarcity of inflammatory cells in the interstitial stroma. D: T+E2-treated LP. Dysplastic epithelium associated with stromal inflammation is shown. Infiltrating inflammatory cells accumulate in the stroma juxtaposed to the prostatic epithelium exhibiting a continuum of normal morphology to dysplasia, as evidenced by the presence of pleomorphic nuclei (red arrows), the earliest recognizable sign of preneoplasia. E: T+E2-treated LP. Epithelial dysplasia with no inflammatory cell infiltration in the adjacent stroma. The inset illustrates a dysplastic lesion characterized by deranged epithelial cells with pleomorphic nuclei and loss of cell polarity. F: Normal control VP. Glandular acini are composed of columnar epithelial cells surrounded by a thin layer of smooth muscles and fibroblasts. Inflammatory cells are rarely detected in the interstitial stroma. G: T+E2-treated VP. A representative region of the VP shows no induction of epithelial dysplasia and stromal inflammatory response.
T+E2 Co-Treatment Selectively Induced Inflammation and Premalignant Dysplasia in the LPs, but Not in the VPs, of Treated Rats
The T+E2-induced histopathological changes in the NBL rat model have been reported previously.22,24 In this article, we give only the morphological alterations directly relevant to the present study. The LPs and VPs of untreated rats were histologically normal (Figure 1, C and F, respectively). Dysplasia and inflammation developed specifically in the LPs (Figure 1D), but not in the VPs (Figure 1G), of all treated rats. A spectrum of focal dysplastic epithelial lesions associated with variable degrees of chronic inflammation in the stroma was observed in the LPs of treated rats, as has been reported previously.23 We found, however, that focal dysplastic lesions were sometimes evident in the LP region in the absence of inflammation (Figure 1, E and inset).
T+E2 Co-Treatment Selectively Increased the Expression of NOXs, NOSs, and COXs in the LPs, but Not in the VPs, of Treated Animals
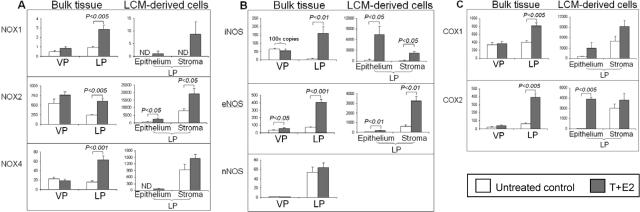
In the VP, only the expression of eNOS mRNA was significantly increased following T+E2 treatment (Figure 2B). In contrast, in the LP, T+E2 treatment significantly increased mRNA levels of all three NOX isoforms [NOX-1 (threefold, P < 0.005), NOX-2 (2.5-fold, P < 0.005), and NOX-4 (fourfold, P < 0.001)] (Figure 2A, left: bulk tissue), two NOS isoforms [iNOS (28-fold, P < 0.01) and eNOS (5.5-fold, P < 0.001)] (Figure 2B, left: bulk tissue), and two COX isoforms [COX-1 (twofold, P < 0.005) and COX-2 (sixfold, P < 0.005)] (Figure 2C, left: bulk tissue) compared with those of untreated controls. However, the mRNA levels of nNOS (Figure 2B, left: bulk tissue), SOD-1, and SOD-2 (data not shown) remained unchanged in the LPs and VPs following T+E2 exposure.
Figure 2.
Aberrant up-regulation of (A) O2–·-generating NOX, (B) ·NO-producing NOS, and (C) proinflammatory COX genes in the LP in which epithelial and stromal compartments show differential response to dual exposure to T + E2. Total RNA was extracted from each half of VP or LP lobes, which are referred to as the “bulk tissues” panel (left) or from the microdissected epithelial and stromal cells in LPs’ cryosections, which represent the “LCM-derived cells” panel (right). The results of real-time quantitative PCR analyses of target-gene abundance are expressed in mRNA copy numbers that have been normalized for 1 million copies of RPL19, an endogenous housekeeping gene. “ND” denotes an undetectable gene. In the iNOS panel of B, the copy numbers represented by both columns of VPs (control and T+E2 treatment) are multiplied by 100 for the sake of visualization. Each column represents the average value from four or five animals, and error bars represent SEM.
T+E2 Co-Treatment Increased Expression of Specific NOXs, NOSs, and COXs in the Stromal and Epithelial Compartments of the LPs of Treated Animals
Because most of the changes in ROS/RNS-generating enzymes induced by the combined hormone treatment occurred in the LP, we next used LCM to determine which tissue compartment, epithelium or stroma, was the major source of these alterations (Figure 2, A to C, right: LCM-derived cells). After the T+E2 treatment, marked increases in NOX-1 (no mRNA was detected in the epithelium of control LPs), NOX-2 (threefold, P < 0.05), NOX-4 (no mRNA was detected in the epithelium of control LPs), iNOS (30-fold, P < 0.05), eNOS (threefold, P < 0.01), COX-1 (fivefold, P > 0.05), and COX-2 (110-fold, P < 0.005) mRNA were observed in the dysplastic epithelium. Comparably increased levels of expression of these genes also were found in the nondysplastic epithelium of treated rats (data not shown). The combined hormone treatment also elicited pronounced up-regulation of NOX-1 mRNA expression (no mRNA was detected in the stroma of control LPs), NOX-2 (twofold, P < 0.05), iNOS (16-fold, P < 0.05), and eNOS (fivefold, P < 0.01) in the stroma associated with inflammatory cells.
T+E2 Co-Treatment Induces Lobe-Specific Elevation of 8-OHdG, 4-HNE Protein Adducts, and Nitrotyrosine in the LPs, but Not in the VPs, of Treated Rats
For oxidative DNA and protein damage, the best recognized biomarkers are 8-OHdG bases in DNA13 and 4-HNE protein adducts,28 respectively. Regarding the nitrative protein damage, nitrotyrosine, the stable modification of tyrosine residues, is formed.29 Extensive oxidative and nitrosative damage, as reflected by an accumulation of these surrogate markers, may lead to the genomic and proteomic alterations considered critical for neoplastic transformation.9,12
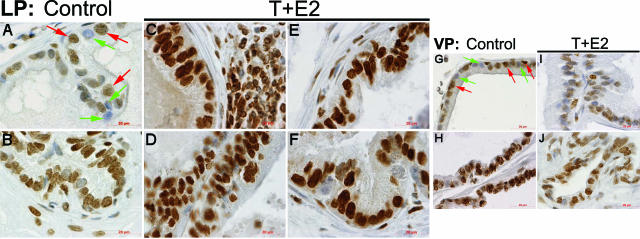
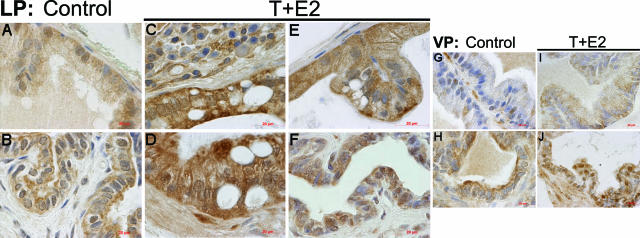
In LPs and VPs of untreated control animals, modest levels of heterogeneous immunostaining for 8-OHdG (Figure 3, A and G, respectively), 4-HNE protein adducts (Figure 4, A and G, respectively), and nitrotyrosine (Figure 5, A and G, respectively) were seen frequently in the glandular epithelium and occasionally in the adjacent stroma. Nuclear staining of 8-OHdG was always relatively stronger in the ducts of LP (Figure 3B) and VP (Figure 3H) than in the acini of corresponding lobes. The ducts of both LP (Figure 4B) and VP (Figure 4H) were more intensely immunostained for 4-HNE than were the acini of respective lobes. However, the immunostaining of nitrotyrosine in the LP ducts (Figure 5B) was generally comparable with that observed in the acini, whereas staining of nitrotyrosine in the ducts of VP (Figure 5H) was more conspicuous than that in the acinar region.
Figure 3.
Immunolocalization of 8-OHdG in the LP and VP after T+E2 co-treatment. A and B: Untreated control LP. Negatively stained nuclei (green arrows) are often found interspersed with positive nuclei (red arrows) in the glandular epithelium (A). Relatively strong nuclear staining in the ductal epithelium and stroma is shown (B). C: T+E2-treated LP. A representative mild dysplastic tubule closely associated with stromal inflammatory infiltration is shown. Both epithelial cells and inflammatory cells exhibit a strong nuclear immunoreactivity. D: T+E2-treated LP. High-grade dysplastic epithelia exhibit intense staining in nuclei. Note that inflammatory infiltration is not evident in the area shown. E: T+E2-treated LP. A remarkable increase in nuclear 8-OHdG immunostaining is seen both in a nondysplastic (apparently morphologically normal) acinus. F: T+E2-treated LP. A representative prostatic duct shows strong and uniform 8-OHdG staining in the epithelium. G and H: Untreated control VP. Numerous negatively stained nuclei (green arrows) are mingled with weakly positive nuclei (red arrows) in the acinar epithelium (G), whereas strong nuclear staining is found in the ductal epithelium (H). I and J: T+E2-treated VP. No remarkable increase of the 8-OHdG staining in acini (I) and ducts is seen (J). Scale bars = 20 μm.
Figure 4.
Immunolocalization of 4-HNE protein adducts in the LP and VP after T+E2 co-treatment. A and B: Untreated control LP. Positive immunostaining is observed in the acinar (A) and ductal epithelium (B). C: T+E2-treated LP. A noticeable increase in immunoreactivity of 4-HNE protein adducts in a dysplastic gland closely associated with stromal inflammatory infiltration is shown. D: T+E2-treated LP. A representative high-grade dysplastic gland, which lacks the induction of stromal inflammatory response, exhibits intense immunostaining in the epithelium. E: T+E2-treated LP. A morphologically normal gland shows intense staining in the epithelium. Note the sparse staining in the adjacent stroma with no association with stromal inflammation. F: T+E2-treated LP. A modest increase in immunostaining is seen in the ductal epithelium. G and H: Untreated control VP. Weak and sporadic immunostaining is detected in acini (G), whereas relatively strong staining is found in ducts (H). I and J: T+E2-treated VP. No apparent increase of the staining in the glandular (I) and ductal (J) regions is seen. Scale bars = 20 μm.
Figure 5.
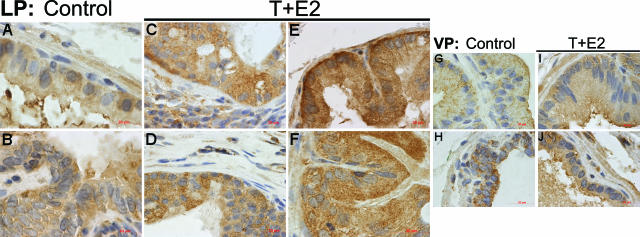
Immunolocalization of nitrotyrosine in the LP and the VP after T+E2 co-treatment. A and B: Untreated control LP. Positive immunostaining is localized predominantly to the glandular (A) and ductal (B) epithelium. C: T+E2-treated LP. A representative dysplastic acinar region shows a remarkable increase in nitrotyrosine staining in the epithelium and the interstitial inflammatory stroma. D: T+E2-treated LP. High-grade dysplastic epithelium with minimal association with stromal inflammation shows a strong and uniform nitrotyrosine staining. Note the generally weak staining in the interstitial stroma. E: T+E2-treated LP. Intense nitrotyrosine immunoreactivity in a nondysplastic gland is shown. Note the sparse staining in the adjacent stroma without inflammatory infiltration. F: T+E2-treated LP. There is a modest increase of nitrotyrosine staining in the ductal epithelium. G and H: Untreated control VP. Immunostaining for nitrotyrosine in the acinar epithelium (G) is generally weak or negative. A representative duct (H) shows conspicuous staining in the epithelium. I and J: T+E2-treated VP. There is only a mild elevation of nitrotyrosine staining in acini (I) and ducts (J). Scale bars = 20 μm.
After T+E2 exposure, the LPs showed a widespread increase in 8-OHdG (Figure 3, C–F), 4-HNE (Figure 4, C–F), and nitrotyrosine (Figure 5, C–F) staining in ducts, dysplastic epithelia, and nondysplastic (apparently morphologically normal) glands. This dramatic increase in staining occurred whether inflammatory foci were immediately adjacent or distant to the involved ducts and glands. In most instances, vascular endothelium and inflammatory cells in the stroma also showed consistently high levels of nitrotyrosine immunostaining (data not shown). In marked contrast, glands and ducts in the VPs of the T+E2-treated animals exhibited no changes in 8-OHdG (Figure 3, I and J) and 4-HNE protein (Figure 4, I and J) immunostaining compared with that of untreated controls; however, a slight increase in nitrotyrosine accumulation (Figure 5, I and J) was evident in the epithelium. Immunopositivity was eliminated by preabsorption with counterpart antigens or replacement with isotype normal serum (data not shown).
Discussion
In the current study, we demonstrated that androgen-supported estrogen action induced oxidative/nitrosative damage to DNA (8-hydroxy-2′-deoxyguanosine bases) and proteins (4-hydroxynonenal adducts and nitrotyrosine), primarily in prostatic epithelia and, to a lesser extent, in the stroma of the cancer-susceptible LP of the NBL rat compared with values in untreated controls. These changes were associated with the elevated expression of mRNA of specific ROS/RNS-generating enzymes (NOX, NOS, and COX) in LCM-captured epithelial or stromal cells. In contrast, however, the administration of T and E2 together did not induce OS/NS-related changes in the VP, where this treatment does not cause dysplasia/carcinoma. Our findings suggest that hormone-induced OS/NS is closely correlated with the lobe-specific development for prostate cancer in this animal model.
Our data indicate that the sex hormone-induced OS/NS can arise both directly from prostatic epithelium and indirectly from inflammatory cells. This may occur through the dramatic elevation of steady-state levels of mRNA of ROS/RNS-generating enzymes (NOX-1, NOX-2, iNOS, eNOS, and COX-2) in either/both tissue components of LP following hormonal treatment. In contrast to LPs, inflammation, redox disruption, and elevation of the pro-oxidant/-pro-nitrosant gene expression was virtually absent in VPs of treated rats. In this context, it has been proposed that OS/NS of an inflammatory source, in particular, contribute to prostate carcinogenesis.30 As observed in the current study, however, the weak topographic association between epithelial OS/NS and inflammatory cell infiltrates corroborates the concept that altered hormonal milieu can directly induce redox disruption in epithelium per se, which is independent from the indirect effects of stromal inflammatory cells.
COX-2 up-regulation in epithelium represents a potential mechanism by which the hormone-initiated epithelium might induce and dictate inflammatory responses in underlying stroma. The T+E2-treated epithelium, which exhibits high levels of COX-2, may then release prostaglandins, which promote an inflammatory cell infiltration into the adjacent stroma that can augment the epithelial OS/NS-related injuries (Figure 6). Up-regulation of COX-2 has been shown in human PIA that is proposed as an inflammation-associated prostate cancer precursor.11 Although specific factors or agents causing COX-2 elevation in PIA remain obscure, chronic inflammatory lesions and PCa are common in elderly men. In this regard, elderly men frequently exhibit an increase in the ratio of estrogen to androgen17,31 similar to that produced in treated NBL rats.
Figure 6.
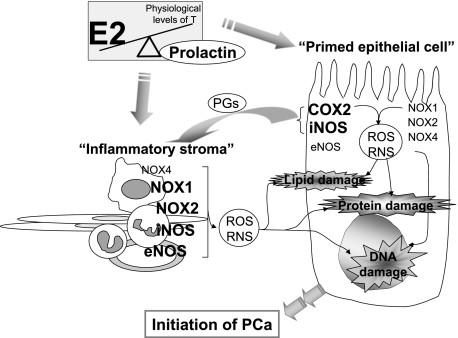
Proposed working model of sex hormone-induced oxidative/nitrosative stress that favors initiation of prostate cancer. An altered circulating hormone milieu (ie, increased ratio of E2 to T, and the E2-induced hyperprolactinemia) can directly induce oxidative/nitrosative damages to macromolecules (DNA, lipids, and proteins) of prostatic epithelial cells by the in situ induction of iNOS and COX-2 expression. In parallel, the “hormone-primed” epithelium, with elevated COX-2 expression, can promote inflammatory cell recruitment and infiltration to the stroma by the release of proinflammatory prostaglandins. The resulting reactive inflammatory stroma, which expresses high levels of NOX1, NOX2, iNOS, and eNOS, could in turn exacerbate the epithelial OS/NS-related injury in a reciprocal manner. Thus, we propose that these multifaceted, hormone-driven cellular events may create a vicious double-feed forward cycles of dysregulated ROS/RNS metabolism and inflammatory activation between the initiated epithelium and the inflammatory stroma that could ultimately lead to a pro-cancer microenvironment in the tissue. The hormonal actions on OS/NS are discussed in detail in the Discussion. The “inflammatory stroma” comprises primarily inflammatory cell infiltrates, fibroblasts, smooth muscle cells, and endothelium. PG, prostaglandins.
Evidence of hormone-induced OS/NS-related damage in both dysplastic and nondysplastic LP epithelium suggests that this injury precedes dysplasia and/or carcinoma development in some LP glands. This finding may indicate that, despite the presence of OS/NS injury, subsequent genetic and/or epigenetic changes in susceptible cells may be required for tumor progression. Alternatively, a subset of cells may be particularly susceptible to redox injury, whereas others may be totally resistant to development of carcinoma. In this context, previous studies20,21 have suggested that carcinoma develops from periurethral ducts of the LP in the NBL rat model. It is important to note that we now also find evidence of OS/NS damage in these ducts.
Our previous findings in castrated and T-supplemented rats suggest that androgens play a role in regulation of the NOX gene in the prostate.18 In addition, studies have demonstrated that E2 modulates the expression of NOS and COX genes in a cell/tissue-specific manner.32,33,34,35,36 In the NBL rat model, the action of E2 can be indirect and mediated by an elevation in prolactin level from the pituitary gland.23,37,38 Thus, it is likely that elevated levels of prolactin also contribute to the up-regulation of pro-oxidant/pro-nitrosant/proinflammatory genes. Interestingly, prolactin has been implicated as a modulator of immune functions and inflammation, and some of its actions have been linked to the regulation of ·NO synthesis.39,40,41 The combined action of sex hormones may therefore directly or indirectly enhance NOX, COX, and NOS expression in the epithelium/stroma and initiate aberrant production of ROS/RNS. Moreover, it has been proposed that E2 metabolic activation by cytochrome P450 enzymes and coupled redox cycling reactions between catechol estrogens and their corresponding quinine metabolites generate oxidative stress in an estrogen receptor-independent manner.42 This estrogen-mediated redox mechanism has been proposed to play a role in renal carcinogenesis43,44 as well as in prostate45 and breast cancer.46
In summary, for the first time we show evidence that the circulating hormonal milieu is a critical determinant of NOX, NOS, and COX expression in the prostate epithelium and/or infiltrating inflammatory cells. The resulting oxidative and nitrosative DNA and protein damage, as a direct consequence of ROS/RNS overproduction by the epithelium and/or mediated indirectly by stromal inflammation, may subsequently give rise to dysplasia and carcinoma. Notably, the hormone-initiated epithelium may also provoke a local inflammatory response through the up-regulation of COX2. Thus, we propose that these multifaceted, hormone-driven cellular events may create vicious “double-feed forward cycles” of dysregulated ROS/RNS metabolism and inflammatory activation between the initiated epithelial cells and the reactive inflammatory stroma (Figure 6) that could ultimately create a pro-oxidant/pro-nitrosant microenvironment that favors the initiation of prostate cancer.47
Acknowledgments
We thank Dr. Ricky Leung of the University of Cincinnati for his technical advice on the quantitative real-time PCR.
Footnotes
Address reprint requests to Dr. Shuk-Mei Ho, Department of Environmental Health, Kettering Complex, Room 130, 3223 Eden Ave., University of Cincinnati Medical Center, PO Box 670056, Cincinnati, OH 45267-0056. E-mail: shuk-mei.Ho@uc.edu.
Supported in part by National Institutes of Health grants CA112532, CA15776, and CA62269 awarded to S.M.H.
References
- Bostwick DG, Alexander EE, Singh R, Shan A, Qian J, Santella RM, Oberley LW, Yan T, Zhong W, Jiang X, Oberley TD. Antioxidant enzyme expression and reactive oxygen species damage in prostatic intraepithelial neoplasia and cancer. Cancer. 2000;89:123–134. [PubMed] [Google Scholar]
- Oberley TD, Zhong W, Szweda LI, Oberley LW. Localization of antioxidant enzymes and oxidative damage products in normal and malignant prostate epithelium. Prostate. 2000;44:144–155. doi: 10.1002/1097-0045(20000701)44:2<144::aid-pros7>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Fang FC. Antimicrobial reactive oxygen and nitrogen species: concepts and controversies. Nat Rev Microbiol. 2004;2:820–832. doi: 10.1038/nrmicro1004. [DOI] [PubMed] [Google Scholar]
- Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- Arbiser JL, Petros J, Klafter R, Govindajaran B, McLaughlin ER, Brown LF, Cohen C, Moses M, Kilroy S, Arnold RS, Lambeth JD. Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proc Natl Acad Sci USA. 2002;99:715–720. doi: 10.1073/pnas.022630199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brar SS, Corbin Z, Kennedy TP, Hemendinger R, Thornton L, Bommarius B, Arnold RS, Whorton AR, Sturrock AB, Huecksteadt TP, Quinn MT, Krenitsky K, Ardie KG, Lambeth JD, Hoidal JR. NOX5 NAD(P)H oxidase regulates growth and apoptosis in DU 145 prostate cancer cells. Am J Physiol. 2003;285:C353–C369. doi: 10.1152/ajpcell.00525.2002. [DOI] [PubMed] [Google Scholar]
- Nelson WG, De Marzo AM, Deweese TL, Isaacs WB. The role of inflammation in the pathogenesis of prostate cancer. J Urol. 2004;172:S6–11. doi: 10.1097/01.ju.0000142058.99614.ff. [DOI] [PubMed] [Google Scholar]
- Funk CD. Prostaglandins and leukotrienes: advances in eicosanoid biology. Science. 2001;294:1871–1875. doi: 10.1126/science.294.5548.1871. [DOI] [PubMed] [Google Scholar]
- Hussain SP, Hofseth LJ, Harris CC. Radical causes of cancer. Nat Rev Cancer. 2003;3:276–285. doi: 10.1038/nrc1046. [DOI] [PubMed] [Google Scholar]
- Hussain T, Gupta S, Mukhtar H. Cyclooxygenase-2 and prostate carcinogenesis. Cancer Lett. 2003;191:125–135. doi: 10.1016/s0304-3835(02)00524-4. [DOI] [PubMed] [Google Scholar]
- Zha S, Gage WR, Sauvageot J, Saria EA, Putzi MJ, Ewing CM, Faith DA, Nelson WG, De Marzo AM, Isaacs WB. Cyclooxygenase-2 is up-regulated in proliferative inflammatory atrophy of the prostate, but not in prostate carcinoma. Cancer Res. 2001;61:8617–8623. [PubMed] [Google Scholar]
- Klaunig JE, Kamendulis LM. The role of oxidative stress in carcinogenesis. Annu Rev Pharmacol Toxicol. 2004;44:239–267. doi: 10.1146/annurev.pharmtox.44.101802.121851. [DOI] [PubMed] [Google Scholar]
- Kawanishi S, Hiraku Y, Pinlaor S, Ma N. Oxidative and nitrative DNA damage in animals and patients with inflammatory diseases in relation to inflammation-related carcinogenesis. Biol Chem. 2006;387:365–372. doi: 10.1515/BC.2006.049. [DOI] [PubMed] [Google Scholar]
- Nakabeppu Y, Sakumi K, Sakamoto K, Tsuchimoto D, Tsuzuki T, Nakatsu Y. Mutagenesis and carcinogenesis caused by the oxidation of nucleic acids. Biol Chem. 2006;387:373–379. doi: 10.1515/BC.2006.050. [DOI] [PubMed] [Google Scholar]
- De Marzo AM, Marchi VL, Epstein JI, Nelson WG. Proliferative inflammatory atrophy of the prostate: implications for prostatic carcinogenesis. Am J Pathol. 1999;155:1985–1992. doi: 10.1016/S0002-9440(10)65517-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taplin ME, Ho SM. Clinical review 134: the endocrinology of prostate cancer. J Clin Endocrinol Metab. 2001;86:3467–3477. doi: 10.1210/jcem.86.8.7782. [DOI] [PubMed] [Google Scholar]
- Bostwick DG, Burke HB, Djakiew D, Euling S, Ho SM, Landolph J, Morrison H, Sonawane B, Shifflett T, Waters DJ, Timms B. Human prostate cancer risk factors. Cancer. 2004;101:2371–2490. doi: 10.1002/cncr.20408. [DOI] [PubMed] [Google Scholar]
- Tam NN, Gao Y, Leung YK, Ho SM. Androgenic regulation of oxidative stress in the rat prostate: involvement of NAD(P)H oxidases and antioxidant defense machinery during prostatic involution and regrowth. Am J Pathol. 2003;163:2513–2522. doi: 10.1016/S0002-9440(10)63606-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam NN, Ghatak S, Ho SM. Sex hormone-induced alterations in the activities of antioxidant enzymes and lipid peroxidation status in the prostate of Noble rats. Prostate. 2003;55:1–8. doi: 10.1002/pros.10169. [DOI] [PubMed] [Google Scholar]
- Bosland MC, Ford H, Horton L. Induction at high incidence of ductal prostate adenocarcinomas in NBL/Cr and Sprague-Dawley Hsd:SD rats treated with a combination of testosterone and estradiol-17β or diethylstilbestrol. Carcinogenesis. 1995;16:1311–1317. doi: 10.1093/carcin/16.6.1311. [DOI] [PubMed] [Google Scholar]
- Tam NN, Chung SS, Lee DT, Wong YC. Aberrant expression of hepatocyte growth factor and its receptor, c-Met, during sex hormone-induced prostatic carcinogenesis in the Noble rat. Carcinogenesis. 2000;21:2183–2191. doi: 10.1093/carcin/21.12.2183. [DOI] [PubMed] [Google Scholar]
- Leav I, Ho SM, Ofner P, Merk FB, Kwan PW, Damassa D. Biochemical alterations in sex hormone-induced hyperplasia and dysplasia of the dorsolateral prostates of Noble rats. J Natl Cancer Inst. 1988;80:1045–1053. doi: 10.1093/jnci/80.13.1045. [DOI] [PubMed] [Google Scholar]
- Lane KE, Leav I, Ziar J, Bridges RS, Rand WM, Ho SM. Suppression of testosterone and estradiol-17beta-induced dysplasia in the dorsolateral prostate of Noble rats by bromocriptine. Carcinogenesis. 1997;18:1505–1510. doi: 10.1093/carcin/18.8.1505. [DOI] [PubMed] [Google Scholar]
- Thompson CJ, Tam NN, Joyce JM, Leav I, Ho SM. Gene expression profiling of testosterone and estradiol-17β-induced prostatic dysplasia in Noble rats and response to the antiestrogen ICI 182,780. Endocrinology. 2002;143:2093–2105. doi: 10.1210/endo.143.6.8846. [DOI] [PubMed] [Google Scholar]
- Zhu X, Leav I, Leung YK, Wu M, Liu Q, Gao Y, McNeal JE, Ho SM. Dynamic regulation of estrogen receptor-β expression by DNA methylation during prostate cancer development and metastasis. Am J Pathol. 2004;164:2003–2012. doi: 10.1016/s0002-9440(10)63760-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam NN, Nyska A, Maronpot RR, Kissling G, Lomnitski L, Suttie A, Bakshi S, Bergman M, Grossman S, Ho SM. Differential attenuation of oxidative/nitrosative injuries in early prostatic neoplastic lesions in TRAMP mice by dietary antioxidants. Prostate. 2006;66:57–69. doi: 10.1002/pros.20313. [DOI] [PubMed] [Google Scholar]
- Butcher RL, Collins WE, Fugo NW. Plasma concentration of LH, FSH, prolactin, progesterone and estradiol-17β throughout the 4-day estrous cycle of the rat. Endocrinology. 1974;94:1704–1708. doi: 10.1210/endo-94-6-1704. [DOI] [PubMed] [Google Scholar]
- Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- Beckman JS, Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- De Marzo AM, Platz EA, Sutcliffe S, Xu J, Gronberg H, Drake CG, Nakai Y, Isaacs WB, Nelson WG. Inflammation in prostate carcinogenesis. Nat Rev Cancer. 2007;7:256–269. doi: 10.1038/nrc2090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho SM. Estrogens and anti-estrogens: key mediators of prostate carcinogenesis and new therapeutic candidates. J Cell Biochem. 2004;91:491–503. doi: 10.1002/jcb.10759. [DOI] [PubMed] [Google Scholar]
- Doré M, Chevalier S, Sirois J. Estrogen-dependent induction of cyclooxygenase-2 in the canine prostate in vivo. Vet Pathol. 2005;42:100–103. doi: 10.1354/vp.42-1-100. [DOI] [PubMed] [Google Scholar]
- Hertrampf T, Schmidt S, Laudenbach-Leschowsky U, Seibel J, Diel P. Tissue-specific modulation of cyclooxygenase-2 (Cox-2) expression in the uterus and the v. cava by estrogens and phytoestrogens. Mol Cell Endocrinol. 2005;243:51–57. doi: 10.1016/j.mce.2005.08.007. [DOI] [PubMed] [Google Scholar]
- Harris MT, Feldberg RS, Lau KM, Lazarus NH, Cochrane DE. Expression of proinflammatory genes during estrogen-induced inflammation of the rat prostate. Prostate. 2000;44:19–25. doi: 10.1002/1097-0045(20000615)44:1<19::aid-pros3>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Grande M, Carlstrom K, Stege R, Pousette A, Faxen M. Estrogens increase the endothelial nitric oxide synthase (ecNOS) mRNA level in LNCaP human prostate carcinoma cells. Prostate. 2000;45:232–237. doi: 10.1002/1097-0045(20001101)45:3<232::aid-pros5>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Chamness SL, Ricker DD, Crone JK, Dembeck CL, Maguire MP, Burnett AL, Chang TS. The effect of androgen on nitric oxide synthase in the male reproductive tract of the rat. Fertil Steril. 1995;63:1101–1107. [PubMed] [Google Scholar]
- Tangbanluekal L, Robinette CL. Prolactin mediates estradiol-induced inflammation in the lateral prostate of Wistar rats. Endocrinology. 1993;132:2407–2416. doi: 10.1210/endo.132.6.8504745. [DOI] [PubMed] [Google Scholar]
- Gilleran JP, Putz O, DeJong M, DeJong S, Birch L, Pu Y, Huang L, Prins GS. The role of prolactin in the prostatic inflammatory response to neonatal estrogen. Endocrinology. 2003;144:2046–2054. doi: 10.1210/en.2002-0038. [DOI] [PubMed] [Google Scholar]
- Meli R, Raso GM, Gualillo O, Pacilio M, Di Carlo R. Prolactin modulation of nitric oxide and TNF-alpha production by peripheral neutrophils in rats. Life Sci. 1997;61:1395–1403. doi: 10.1016/s0024-3205(97)00685-1. [DOI] [PubMed] [Google Scholar]
- Bolander FF., Jr Prolactin activation of mammary nitric oxide synthase: molecular mechanisms. J Mol Endocrinol. 2002;28:45–51. doi: 10.1677/jme.0.0280045. [DOI] [PubMed] [Google Scholar]
- Lee SH, Nishino M, Mazumdar T, Garcia GE, Galfione M, Lee FL, Lee CL, Liang A, Kim J, Feng L, Eissa NT, Lin SH, Yu-Lee LY. 16-kDa prolactin down-regulates inducible nitric oxide synthase expression through inhibition of the signal transducer and activator of transcription 1/IFN regulatory factor-1 pathway. Cancer Res. 2005;65:7984–7992. doi: 10.1158/0008-5472.CAN-05-0631. [DOI] [PubMed] [Google Scholar]
- Yager JD. Endogenous estrogens as carcinogens through metabolic activation. J Natl Cancer Inst Monogr. 2000:67–73. doi: 10.1093/oxfordjournals.jncimonographs.a024245. [DOI] [PubMed] [Google Scholar]
- McCormick ML, Oberley TD, Elwell JH, Oberley LW, Sun Y, Li JJ. Superoxide dismutase and catalase levels during estrogen-induced renal tumorigenesis, in renal tumors and their autonomous variants in the Syrian hamster. Carcinogenesis. 1991;12:977–983. doi: 10.1093/carcin/12.6.977. [DOI] [PubMed] [Google Scholar]
- Bhat HK, Calaf G, Hei TK, Loya T, Vadgama JV. Critical role of oxidative stress in estrogen-induced carcinogenesis. Proc Natl Acad Sci USA. 2003;100:3913–3918. doi: 10.1073/pnas.0437929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri EL, Devanesan P, Bosland MC, Badawi AF, Rogan EG. Catechol estrogen metabolites and conjugates in different regions of the prostate of Noble rats treated with 4-hydroxyestradiol: implications for estrogen-induced initiation of prostate cancer. Carcinogenesis. 2002;23:329–333. doi: 10.1093/carcin/23.2.329. [DOI] [PubMed] [Google Scholar]
- Hurh YJ, Chen ZH, Na HK, Han SY, Surh YJ. 2-Hydroxyestradiol induces oxidative DNA damage and apoptosis in human mammary epithelial cells. J Toxicol Environ Health A. 2004;67:1939–1953. doi: 10.1080/15287390490514598. [DOI] [PubMed] [Google Scholar]
- Schwartsburd PM. Chronic inflammation as inductor of pro-cancer microenvironment: pathogenesis of dysregulated feedback control. Cancer Metastasis Rev. 2003;22:95–102. doi: 10.1023/a:1022220219975. [DOI] [PubMed] [Google Scholar]