Abstract
It is rarely considered that age-related common vascular co-morbidities may affect therapeutic outcomes of antiangiogenic therapy in cancer. Indeed, the accepted model of human disease consists of 4- to 8-week-old (young) tumor-bearing, but otherwise healthy, experimental mice, yet human cancers are diagnosed and treated in later decades of life when atherosclerosis and vascular diseases are highly prevalent. Here we present evidence that tumor growth and angiogenesis are profoundly altered in mice affected by natural aging and with genetically induced atherosclerosis (in ApoE−/− mice). Thus, transplantable tumors (Lewis lung carcinoma and B16F1) grew at higher rates in young (4 to 8 weeks old) ApoE+/+ and ApoE−/− nonatherosclerotic syngeneic recipients than in their old (12 to 18 months old) or atherosclerotic (old/ApoE−/−) counterparts. These age-related changes were paralleled by reduced tumor vascularity, lower expression of tumor endothelial marker 1, increased acute tumor hypoxia, depletion of circulating CD45−/VEGFR+ cells, and impaired endothelial sprouting ex vivo. Exposure of tumor-bearing mice to metronomic therapy with cyclophosphamide exerted antimitotic effects on tumors in young hosts, but this effect was reduced in atherosclerotic mice. Collectively, our results suggest that vascular aging and disease may affect tumor progression, angiogenesis, and responses to therapy.
In the Western world, the lifetime risk of developing cancer is in the range of 30 to 40%,1 whereas the risk of having some degree of atherosclerotic damage to the vasculature by the age of 80 approaches certainty.2 It follows that atherosclerosis becomes an increasingly common co-morbidity of cancer in the aging population. To date, this coincidence has attracted little attention, and most of the work in this area has concentrated around the possible anticancer activity of cholesterol-lowering drugs (statins)3 or more general aspects of cancer patient management, such as cardiovascular performance status or vascular complications (eg, thrombosis).4 Hence, it is currently unknown whether the course of cancer progression could be affected by atherosclerosis.
One possible point of pathobiological convergence between cancer and atherosclerosis is in the involvement of the vascular system. Thus, processes leading to atherosclerotic vascular damage typically begin during the first decades of life with initially silent accumulation of lipids, lipid-laden macrophages (foam cells), and fatty streaks detectable within walls of major arteries.2 With age, these lesions progress through a complex series of inflammatory, proliferative, and degenerative stages, leading to formation of atherosclerotic plaques composed of cellular debris, inflammatory infiltrates, and vascularized smooth muscle cell outgrowths.2 The fibrous cap of such plaques may eventually become unstable and rupture, an event associated with well-known thrombosis-related cardiovascular emergencies such as myocardial infarction and stroke.2 Although overt plaques originate in defined segments of larger vessels because of shear stress, endothelial injury, altered mechanosignaling, and damage to the vessel wall,2 the disease also possesses distinct systemic hallmarks. These may include metabolic complications such as hypercholesterolemia, diabetes, or hyperhomocysteinemia2 but also a systemic activation of inflammatory cells (eg, macrophages) and release of cytokines into the circulation. These inflammatory constituents of the atherogenic process are thought to participate in the diffuse damage to macro- and microvascular endothelium5 and changes affecting circulating endothelial (and other) progenitor cells.6
Although in humans, progression of vascular changes is closely associated with other aspects of aging, mice are genetically resistant to atherosclerosis. In these animals, a near phenocopy of this disease could be induced through experimental disruption (knockout) of genes encoding critical regulators of cholesterol uptake, such as apolipoprotein E (ApoE) or low-density lipoprotein receptor.2,7,8 ApoE-deficient (ApoE−/−) mice represent a particularly well-characterized model of atherosclerosis in which, similar to humans, early onset hypercholesterolemia is followed by formation of fatty streaks and progressive age-dependent growth of overt obturative macrovascular plaques.7 Interestingly, the related defects extend also to the microvasculature, which in ApoE−/− mice has been found to display impaired angiogenic responses to exogenously added growth factors.9
Angiogenesis is a hallmark and a critical determinant of cancer progression.10 The process is thought to be driven by cancer cells, in which activated oncogenic and hypoxic pathways induce changes that ultimately lead to mobilization of resident endothelial cells, circulating endothelial precursors, inflammatory cells (eg, macrophages), and activated fibroblasts.11,12 The resultant tumor microcirculation enables expansion of the tumor mass, metastasis, and consequently clinical progression of the disease.10
It is thought provoking that many cellular components of the angiogenic circuitry in cancer (eg, endothelial or inflammatory cells) are also profoundly affected by the atherosclerotic process and other aspects of vascular aging.13 This could be of considerable significance for the efficacy of anticancer therapies including traditional chemo- and radiotherapy, both dependent on the functional vascular compartment for drug and oxygen delivery, respectively.14 The status of the vasculature could be of even greater importance for the efficacy of antiangiogenic therapies, the increasing role of which is epitomized by the recent approval of bevacizumab and at least two other agents.15 It is noteworthy that the development of these new agents has been possible because of the availability of various sophisticated mouse models of physiological and pathological angiogenesis, including various types of transplantable or genetically induced tumors.16 It is also true, however, that despite their molecular precision these models have often failed to accurately predict the diversity of therapeutic outcomes encountered in the clinic, something that is usually ascribed to unspecified species-dependent biological differences.
To better understand some of the possible causes of these aforementioned discrepancies, we considered one aspect that fundamentally separates standard experimental conditions in tumor-bearing mice from those in corresponding human malignancies, namely the influence of vascular aging and atherosclerosis. Indeed, the majority of human cancers occur late in life and generally in the context of pre-existing and age-dependent atherosclerotic vascular alterations, ie, conditions that are primarily absent in the tumor-bearing experimental mice that are presently in use. Here, we provide evidence that tumor growth, angiogenesis, and at least some of the effects of metronomic (antiangiogenic) chemotherapy differ markedly between standard (ie, young, 4 to 8 weeks old, healthy) experimental mice and their 12- to 18-month-old and atherosclerotic (ApoE−/−) counterparts. We propose that aging and vascular co-morbidities are important modifiers of tumor progression in vivo, something that perhaps should be considered more widely when devising and testing new therapies.
Materials and Methods
Cells and Culture Conditions
Lewis lung carcinoma (LLC) and B16F1 melanoma cells were purchased from American Type Culture Collection (Manassas, VA). The cells were cultured in Dulbecco’s modified Eagle medium (HyClone, Logan, UT) supplemented with 10% fetal bovine serum (Invitrogen, Carlsbad, CA).
Mice, Tumor Cell Injections, and Metronomic Therapy
Both wild-type C57BL/6J mice and their syngeneic ApoE−/− counterparts were purchased from The Jackson Laboratory (Bar Harbor, ME) as 4- to 8-week-old females and males or as retired breeders 7 to 8 months old. The latter mice were then housed until they reached 12 to 18 months of age, at which point they were age-matched and used for experiments. Tumor growth was induced by a subcutaneous injection of 0.7 to 2.0 × 106 (the same number in a given experiment) of either LLC or B16F1 cells in 0.2 ml of phosphate-buffered saline. Single-cell suspension was obtained by brief trypsinization and used if >95% of cells excluded trypan blue. During injections, mice were under brief isoflurane anesthesia (1 to 3%) with oxygen or room air (0.5 L/minute). Tumor growth was monitored by biweekly measurements and calculated according to the formula: TV = (a2 × b) × 0.52 [mm3], where TV is the tumor volume and a and b are perpendicular tumor dimensions in mm.17 In experiments involving metronomic chemotherapy, mice were randomized to groups as indicated. The treatment arm received 150 mg/kg cyclophosphamide (CTX) (Procytox; ASTA Medica, Ltd., Frankfurt, Germany) on day 2, as recently described.18 After a 6-day break, mice were administered daily doses of 25 mg/kg i.p. CTX, or equivalent volume of the vehicle (saline). All in vivo experiments were conducted according to protocols approved by the institutional animal care committees at McMaster and McGill Universities and in accordance with the guidelines of the Canadian Council of Animal Care.
Monitoring Progression of Atherosclerosis by Necropsy and Corrosion Casting of Large Vessels
Mice were anesthetized, injected intravenously with 10,000 U/kg heparin (Wyeth-Ayerst, St. Laurent, QC, Canada), euthanized, and inspected visually for vascular lesions in aorta and large vessels. Corrosion casting was performed as described earlier.19 In brief, the vasculature was perfused with lactated Ringer’s solution (Baxter, Toronto, ON, Canada) and filled with Batson’s no. 17 casting polymer, a mixture of 6.5 ml of monomer base, 1.5 ml of catalyst, and 0.2 ml of promoter solution (Polysciences, Warrington, PA). After polymerization, the tissue was digested with 30% potassium hydroxide solution, followed by extensive washing. The gold-coated vessel casts were imaged by scanning electron microscopy (S-570; Hitachi, Tokyo, Japan). Data are available in Supplemental Figure 1 (available online at http://ajp. amjpathol.org).
Immunostaining
Microvascular density (MVD) of tumors was assessed by staining for PECAM1/PECAM/CD31 as described earlier.19 In brief, tissue samples were cryoprotected in 15% and 30% sucrose (O/N in 4°C) and frozen in Tissue Tek Cryo-Oct Compound (Sakura Finetek, Torrance, CA). Cryosections 10 μm in thickness were postfixed in ice-cold acetone and stained overnight with the rat anti-mouse CD31/PECAM antibody (Pharmingen, BD Biosciences, Toronto, ON, Canada) at 1:200 dilution. This was followed by incubation with biotinylated rabbit anti-rat secondary antibody (Jackson Laboratory) and Histostain kit (Zymed Laboratories Inc., South San Francisco, CA) containing aminoethylcarbazole chromophore. Blood vessels were counted from at least five vascular hot spots per tumor at ×400 magnification (Axioskop 2; Zeiss, Thornwood, NY). An in situ cell death detection kit (POD; Roche Diagnostics GmbH, Roche Applied Science, Mannheim, Germany) and a Zymed PCNA staining kit were used to highlight proliferating and apoptotic cells, respectively, as described by the suppliers. Areas of tumor necrosis were identified and measured morphometrically (Northern Eclipse, Mississauga, ON, Canada) on standard hematoxylin and eosin (H&E)-stained slides. To assess endothelial cell proliferation, double staining for PECAM and PCNA was performed as above except for inclusion of different secondary antibodies, namely: Alexa Fluor 488 goat anti-rat and streptavidin Alexa Fluor 594, respectively, both from Molecular Probes (Eugene, OR). At least five areas from each tumor (four to seven tumors per group) were analyzed under ×630 magnification. Images obtained with green (PECAM) and red (PCNA) fluorescence were merged, and the numbers of double-labeled cells (dividing endothelial cells) were quantified.
Assessment of Tumor Hypoxia
Pimonidazole diluted in 0.9% saline was injected intraperitoneally at 60 mg/kg to mice 60 minutes before euthanasia, as indicated by the manufacturer (Hypoxyprobe; Chemicon International Inc., Temecula, CA). Tumors were excised, fixed in 10% formalin, and 5-μm thick sections were incubated with the primary antibody directed against pimonidazole-protein adducts (mAb, HypoxyProbe-1 Kit; Chemicon International Inc.). Subsequent treatments with biotinylated goat anti-mouse secondary antibody (Zymed Laboratories Inc.) and streptavidin peroxidase (Zymed Laboratories Inc.) produced color reaction indicative of hypoxia, which was quantified morphometrically through computerized detection of the relative color saturation (versus internal control) by a blinded investigator.
Gene Expression Analysis by in Situ Hybridization and Northern Blotting
In situ hybridization was performed as described previously.20 DIG-labeled RNA probes (TEM-1 and VEGFR-2) were generated by PCR amplification of 500- to 600-bp products incorporating T7 promoters into antisense primers. Sense sequences were used as negative controls. Tumor sections were postfixed with 4% paraformaldehyde, permeabilized with pepsin, blocked with in situ hybridization solution (DAKO, Carpinteria, CA) and incubated with RNA probes (100 ng/ml, overnight at 55°C). The signal was amplified by sequential incubation with anti-DIG and anti-biotin antibodies and biotin tyramide (all from DAKO), and detected with FastRed TR/Naphtol AS-MX (Sigma Chemical Co., St. Louis, MO). The signal intensity was quantified morphometrically using the Zeiss Axioscope 2 and Northern Eclipse software. Northern blotting analysis was performed as described elsewhere.19
Aortic Ring Assay
This assay was conducted as previously described.21 In brief, abdominal and thoracic aortas were excised from the respective groups of mice and sectioned into 1-mm-long pieces. These fragments were embedded in Matrigel and fed with complete endothelial EGM-2 (Clonetics, Cambrex BioScience, Walkersville, MD) or Dulbecco’s modified Eagle medium supplemented with 10% fetal bovine serum. The extent of sprouting was monitored and quantified microscopically. This vascular reaction was expressed as the number of rings containing endothelial sprouts at different time points, and the number of sprouts per ring at the end of the assay, the latter designated as a sprouting index.
Detection of Circulating Endothelial-Like Cells (CCs)
These cells were defined as blood-borne nonleukocytic (CD45−) expressors of VEGFR-2/Flk-1 (VEGFR-2+) and assayed in LLC tumor-bearing mice (day 8) or tumor-free mice, essentially as described elsewhere.22 In brief, peripheral blood was collected into 2 mmol/L Na2EDTA and transferred to microtainer tubes containing K2EDTA (BD Diagnostics, Franklin Lakes, NJ). Cell counts for red blood cells, white blood cells, and the differential were followed by lysis of red blood cells and washing of samples in the staining buffer containing 2 μg/ml FcR Block (BD Biosciences, San Diego, CA). The cells were incubated for 30 minutes with fluorochrome-conjugated primary antibodies, or control IgGs, including CD45-PerCP, Flk-1-PE, CD117-APC, IgG2a-PE, and IgG2b-APC (all from BD Biosciences), washed, and fixed in 1% paraformaldehyde. The multicolor flow cytometry analysis was conducted on sequential gating of leukocytes, CD45− cells and quantification of events positive for VEGFR2. Because the incidence of double-positive (VEGFR2+/CD117+) cells was low and inconsistent, these cells were excluded from further analysis. The results were expressed as the number of CD45−/VEGFR-2+ cells/μl of blood.
Statistical Analysis
The data were presented as the mean value (±SD) derived from several independent data points. Each experimental group consisted of four to nine animals (three to five in experiments involving fluorescence-activated cell sorting). Experiments were reproduced at least twice (some up to nine times) with similar results. In most assays, analysis of variance was used to test the significance of numerical differences, except for experiments with repeated measurements across time, in which multivariate analysis of variance was applied (tumor volume). In addition, a least squares difference test for multiple comparisons was used and the sprouting assay was analyzed with Fisher’s exact test. Values are considered statistically different (*) when P is <0.05, with significance level α = 0.05.
Results
Differential Tumor Growth in Healthy versus Atherosclerotic Mice
Cancer models based on tumor generation in healthy 4- to 8-week-old experimental mice are a source of increasing concerns,16 because they do not account for the impact of aging and atherosclerosis, both common in adult cancer patients. To begin bridging this gap, we used as tumor recipients ApoE−/− mice, in which as in humans, a well-described sequence of events leads from hypercholesterolemia to progressive formation of intravascular plaques and other hallmarks of atherosclerosis, especially in older animals (Supplemental Figure 1, see http://ajp.amjpathol.org).7 Thus, B16F1 mouse melanoma or LLC cells were injected subcutaneously into either standard age, young and healthy 4- to 8-week-old C57BL/6 mice (designated as young throughout the text) or to their counterparts with overt atherosclerosis (Figure 1). The latter condition was found to culminate in 12- to 18-month-old C57BL/6/ApoE−/− mice (old/ApoE−/−) because of disrupted expression of the ApoE gene combined with age-dependent progression of overt vascular lesions (Supplemental Figure 1, see http://ajp.amjpathol.org).7 Such lesions are not apparent in age-matched (12 to 18 months old) C57BL/6/ApoE+/+ mice, or in young (4 to 8 weeks old) C57BL/6/ApoE−/− hypercholesterolemic animals, both of which were initially used as controls in our study and designated as either old or young/ApoE−/−, respectively.
Figure 1.
The impact of aging and atherosclerosis on tumor aggressiveness in mice. Robust growth of B16F1 melanoma (A) and LLC (B) in healthy 4- to 8-week-old/wild-type (young) C57BL/6 mice is markedly suppressed in 12- to 18-month-old C57BL/6/wild-type (old) mice and even more so in 12- to 18-month-old ApoE−/− mice (old/ApoE−/−) with developed atherosclerosis. C: Tumor growth is unaffected by the ApoE+/+ or ApoE−/− status in young mice, the latter of which are severely hyperlipidemic but have no overt vascular disease (*, statistically significant; NS, insignificant; details in Results). D: Comparison of tumor volumes in ApoE−/− versus ApoE+/+ recipients as a function of their age (because this is a compilation of independent experiments, analysis of variance tests were conducted on paired groups of interest, as indicated).
Interestingly, both aging and atherosclerosis exerted a pronounced, negative impact on tumor take and progression (Figure 1). Thus, B16F1 and LLC tumors exhibited significantly (P < 0.05) diminished growth rates in old/ApoE−/− (atherosclerotic) mice relative to their young/ApoE+/+ (atherosclerosis-free) counterparts (Figure 1, A and B). These effects could not be attributed to hypercholesterolemia or other obvious metabolic perturbations associated with the ApoE deficiency because tumor growth remained essentially unchanged in young (4 to 8 weeks old) mice regardless of their ApoE−/− (hyperlipidemic/nonatherosclerotic) or ApoE+/+ (normolipidemic/nonatherosclerotic) status (Figure 1C). Tumor growth retardation was also observed in aging ApoE+/+ (old) mice, but this effect was somewhat less robust than in the case of age-matched mice with overt atherosclerosis (Figure 1, A and B). These observations suggest that aging per se, but more so aging superimposed with atherosclerosis, contributes to modulation of the inherent aggressiveness of cancer cells in vivo (Figure 1D).
Impact of Host Aging and Atherosclerosis on the Properties of Cancer Cells in Situ
Because changes in tumor growth occurred only in old and atherosclerotic (old/ApoE−/−) mice, we chose to assess further the impact of these two host conditions on cancer cells. In this regard, staining of tumor sections for markers of cellular mitogenesis, such as proliferating cell nuclear antigen (PCNA) revealed a significant reduction in proliferative activity of cancer cells (LLC) in both old (ApoE+/+) and atherosclerotic (old/ApoE−/−) tumor recipients, as compared with their young (ApoE+/+) counterparts (Figure 2A and Supplemental Figure 2 at http://ajp.amjpathol.org). This effect was significantly more pronounced in old/ApoE−/− mice. In contrast, although staining for apoptotic cancer cells (TUNEL) documented their ubiquitous presence in all LLC tumors, their numbers did not visibly change in old and atherosclerotic hosts, but rather exhibited increasing tumor-to-tumor variability (Figure 2B). Such variability was also observed in expression of phosphorylated forms of MAPK and Akt, although in this case both activities were visibly reduced in cancer cells growing in old/atherosclerotic mice versus those in young animals (data not shown).
Figure 2.
Impact of host aging and atherosclerosis on changes within the tumor cell compartment. A: PCNA staining of LLC tumors reveals diminished mitogenic activity of cancer cells (LLC) growing in old and/or atherosclerotic (old/ApoE−/−) mice versus those in young mice; section on the left, PCNA quantification; section on the right, example of immunostaining (LLC in a young mouse). B: TUNEL staining of tumors (right) reveals increasing variability but no consistent change in apoptotic indices of LLC tumors in young, old, and old/ApoE−/− mice (left). C: Increase in the extent of severe hypoxia (left) in LLC tumors growing in old and/or atherosclerotic (old/ApoE−/−) mice versus those in young mice. Immunodetection of pimonidazole-protein adducts (right, red staining) was used as a measure to assess the degree of hypoxia (*, statistically significant; NS, insignificant; details in Results).
One possible reason for regional variation in the behavior of cancer cells may be their exposure to different microenvironmental stresses, such as hypoxia. To examine this possibility we stained LLC tumors for protein adducts of pimonidazole (HypoxyProbe), which are produced by cells deprived of oxygen. Essentially all tissue specimens contained regions of pimonidazole staining (hypoxia), but we observed a significant shift from moderate to severe hypoxia in LLC tumors growing in atherosclerotic (old/ApoE−/−) mice relative to their young nonatherosclerotic counterparts. An increase in severe hypoxia also occurred in old/ApoE+/+ mice, albeit to a somewhat lesser extent (Figure 2C). Although the differences between young and old mice were statistically significant, those between old and atherosclerotic animals (old/ApoE+/+ versus old/ApoE−/−) were not. This suggests that the process of host aging as such may impact oxygenation and growth of the emerging tumors.
Impact of Host Aging and Atherosclerosis on Tumor Microvasculature
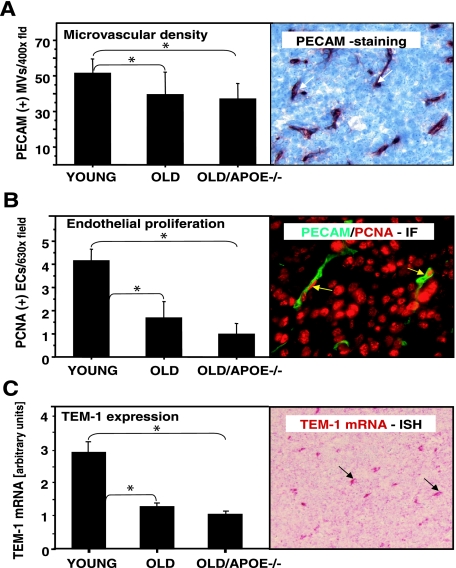
We reasoned that the aforementioned changes in tumor hypoxia could originate as a consequence of perturbations within the tumor microvasculature. Indeed, microvascular changes were detected at the structural, functional, and molecular level. We first assessed the MVD of LLC tumors in young, old, and atherosclerotic mice by staining the respective tissue sections for the panendothelial marker CD31/PECAM, followed by MVD quantification in vascular hot spots. Interestingly, MVD counts were markedly higher in LLC tumors growing in young mice than those of tumors in old, or atherosclerotic (old/ApoE−/−) recipients (Figure 3A). Moreover, dual-immunofluorescent staining of LLC tumor sections for CD31 and PCNA revealed that tumor-associated endothelial cells are more mitotically active (ie, contain a higher fraction of CD31/PCNA double-positive cells) in young tumor recipients than corresponding cell populations in old and/or atherosclerotic mice (Figure 3B and Supplemental Figure 3 at http://ajp.amjpathol.org). Finally, we used in situ hybridization to assess the expression of mRNA for tumor endothelial marker 1 (TEM-1), a molecular indicator of active angiogenesis.23 TEM-1 expression levels in tumor-associated endothelial cells were significantly greater in tumor microvasculature of young mice compared with old and atherosclerotic (old/ApoE−/−) hosts (Figure 3C, and Supplemental Figure 4 at http://ajp.amjpathol.org). As with hypoxia, in all these instances the changes were somewhat more pronounced in atherosclerotic (old/ApoE−/−) mice than in their age-matched but atherosclerosis-free old/ApoE+/+ counterparts. However, these differences were not statistically significant (Figure 3, A–C). Collectively, these results suggest that the sluggish growth of tumors in old and atherosclerotic mice could be linked, at least in part, with the age-dependent angiogenic dysfunction of the endothelium in tumor blood vessels.
Figure 3.
Impact of aging and atherosclerosis on changes within the vascular compartment of LLC tumors. A: Staining for endothelial markers (right) reveals robust PECAM1/PECAM staining (red) of LLC tumors in young recipients. Reduced MVD was observed in tumors growing in old and/or atherosclerotic (old/ApoE−/−) mice. B: LLC tumors in old and old/ApoE−/− mice exhibit reduced endothelial cell proliferation as measured by the number of PCNA/PECAM-double-positive cells. Right: Example of immunofluorescent (IF) double staining for PECAM (green) and PCNA (red, LLC in a young mouse). C: Differential expression of the TEM-1 mRNA in young, old/ApoE+/+ (old), and atherosclerotic (old/ApoE−/−) mice (left). The in situ hybridization signal (right, example of TEM-1 staining) was quantified morphometrically (*, statistically significant; details in Results).
The Impact of Age and Atherosclerosis on Endothelial Cells
To understand the nature of host- and age-dependent microvascular perturbations in LLC tumors, we chose to examine more directly the properties of resident and circulating endothelial cells in young, old, and atherosclerotic mice. First, the aortic segments from the respective tumor-free animals were used to assess spontaneous ingrowth of endothelial capillary sprouts into the surrounding matrix (Figure 4A).21 In this setting, aortic explants from young mice gave rise to robust capillary growth, which reached a plateau within only 4 to 7 days. In contrast, cultures derived from old and atherosclerotic mice exhibited a marked delay (by at least 24 to 48 hours) in deployment of capillary sprouts, and some aortic rings failed to form them altogether (Figure 4B). Moreover, even after 7 days in culture, the numbers of sprouts per aortic ring (sprouting indices) were still significantly lower in the case of aortas of old and atherosclerotic mice than when explants originated from their young and healthy counterparts (Figure 4C). Although in this assay sprouting capacity of endothelial cells from old/ApoE−/− (atherosclerotic) mice was somewhat more impaired than that of cells derived from old/ApoE+/+ (nonatherosclerotic) donors, the difference was not statistically significant (Figure 4, B and C). Thus, once again, aging leads to permanent and intrinsic changes in properties of resident endothelial cells, which impairs their ability to efficiently form angiogenic sprouts.
Figure 4.
The impact of age and atherosclerosis on the angiogenic potential of endothelial cells. A–C: Age- and atherosclerosis-dependent diminution of microvascular sprouting ex vivo from isolated aortic rings. A: Aortas were isolated from young, old, and atherosclerotic (old/ApoE−/−) mice, their fragments embedded in Matrigel, and monitored for formation of endothelial sprouts. In comparison to their young counterparts, isolates from old and atherosclerotic mice exhibited a significantly (*) reduced efficiency in time-dependent formation of endothelial outgrowths (B, number of aortas with sprouts) and a markedly lower number of sprouts per aorta (C, mean ± SD). Aortas of atherosclerotic mice displayed a consistent but statistically insignificant reduction of sprouting capacity as compared with vessels of old mice (see Results).
In addition to stimulating local vascular growth, tumor angiogenesis often entails mobilization of endothelial-like (progenitor) cells into the circulation.22,24 Therefore, we tested the presence of such CD45−/VEGFR-2+ circulating cells (CCs) in blood of mice harboring LLC tumors (Figure 5) and found them readily detectable in both young and old nonatherosclerotic (ApoE+/+), tumor-bearing mice (4 to 8 cells/μl). In contrast, numbers of these cells were significantly diminished (by up to eightfold) in blood of atherosclerotic (old/ApoE−/−) tumor recipients. It is noteworthy that levels of CCs were also extremely low in tumor-free atherosclerotic mice. The lack of an appreciable increase of CCs in this particular context is in contrast to old mice (without atherosclerosis), in which despite some variability, we noted an increase in the numbers of these cells in the presence of LLC tumors.
Figure 5.
Diminished number of circulating endothelial-like cells (CD45−/VEGFR-2+) in tumor-bearing atherosclerotic mice versus nonatherosclerotic (old and young) counterparts (*, statistically significant; details in Results). Baseline levels of these CCs have been examined in at least three tumor-free mice in each age category and are indicated as dotted/dashed lines.
Overall our results suggest that, at least in the experimental settings explored thus far, aging per se diminishes mainly the function of resident endothelial cells, whereas atherosclerosis (in old/ApoE−/−) leads to an additional depletion of circulating endothelial-like cells in both tumor-free and tumor-bearing mice (Figure 5). The striking parallel between these changes and the aforementioned pattern of tumor growth retardation (Figure 1) may suggest a convergence of multiple mechanisms, by which vascular disease and aging could alter tumor neovascularization, progression, and possibly (by extension) outcomes of therapy.
Aging and Atherosclerosis as Modulators of Tumor Responsiveness to Metronomic Chemotherapy
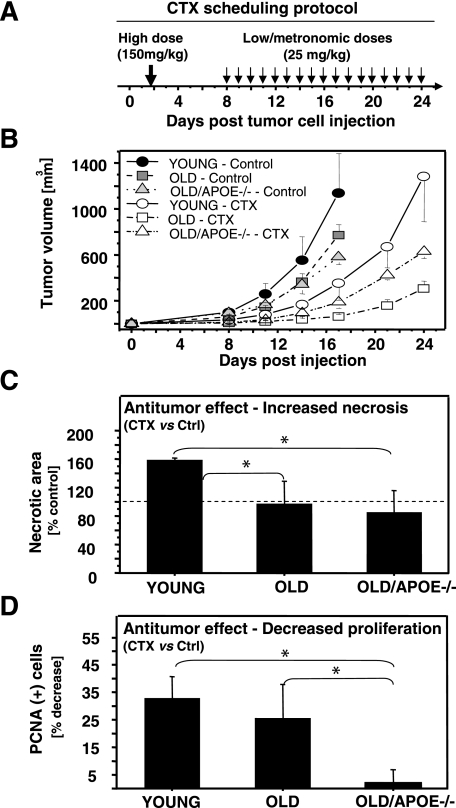
It is of considerable practical interest to determine whether atherosclerosis and vascular aging affect the course of anticancer therapy, especially with antiangiogenic agents. To explore this question we chose to use the optimized protocol of metronomic chemotherapy with CTX, the antiangiogenic effects of which were recently described.18,25 In this setting CTX is initially given in a single high dose (150 mg/kg, day 2), possibly causing both direct and indirect antitumor effects (Figure 6, A and B). Subsequently, the drug is given in daily low doses (25 mg/kg), and its cytotoxic action is thought to be redirected primarily toward angiogenic endothelial cells instead of cancer cells themselves.18 In agreement with these reports, we observed that this treatment was generally well tolerated and blocked gross volume increases of LLC tumors by up to 70 to 90% in young, old, and atherosclerotic mice, with tumors in old mice being somewhat more affected (Figure 6B). We also observed a similar pattern of growth inhibition in the case of B16F1 tumors (data not shown). This global profile of responsiveness is understandable because the treatment includes a directly cytotoxic, early, high dose of CTX, something that would unlikely be affected by vascular aging or atherosclerosis.
Figure 6.
Age- and atherosclerosis-dependent changes in tumor responses to metronomic protocol of chemotherapy with CTX. A: Schema of the CTX therapy as described by Bocci and colleagues.18 The protocol includes an initial higher dose (150 mg/kg) followed by a rest period and a sequence of daily, low metronomic doses of the drug (25 mg/kg i.p.). B: Early treatment-related inhibition of global tumor growth. C: Treatment-related increase in the extent of necrosis within established LLC tumors extracted from young mice, while on metronomic dosing of CTX. Interestingly, this effect is not observed in tumors of old and old/ApoE−/− mice. The values represent the percentage of necrotic area measured morphometrically in H&E-stained sections of tumors exposed to CTX relative to necrosis in vehicle-treated corresponding control tumors (100%, dashed line). D: Host-dependent changes in mitogenic activity of cancer cells in mice subjected to metronomic CTX or vehicle (percent decrease in PCNA staining). Of note, metronomic therapy translates into reduced tumor cell proliferation (PCNA positivity) in nonatherosclerotic mice (young and old) but does not change mitotic indices of LLC cells growing as tumors in old/ApoE−/− recipients (*, statistically significant; details in Results).
However, more detailed analysis of tumors collected during the later metronomic phase of the CTX treatment (Figure 6A) revealed several subtle, but potentially important, host-dependent differences. For instance, CTX treatment increased significantly the extent of tumor necrosis (necrotic area) in young mice, whereas such changes were not observed in old or atherosclerotic tumor recipients (Figure 6C). Moreover, staining for PCNA revealed that metronomic treatment resulted in a decrease in proliferation of cancer cells (LLC) in young and old (old/ApoE+/+) animals by ∼30% and 25%, respectively (versus corresponding vehicle-treated controls). In contrast, this treatment evoked no such changes in proliferation of LLC tumors growing in atherosclerotic (old/ApoE−/−) recipients (Figure 6D). We have also observed a trend toward an increase in expression of certain angiogenesis-related transcripts in tumors subjected to metronomic CTX, including ubiquitous up-regulation of tissue factor and preferential up-regulation of thrombospondin-1 in atherosclerotic mice. These latter changes were modest in magnitude, variable, and ultimately statistically insignificant (data not shown). Although much remains to be understood with regard to the scope and antiangiogenic nature of metronomic chemotherapy,18 our results suggest that vascular aging and related co-morbidities may interfere with its biological effects in cancer.
Discussion
The results of our study raise the possibility that age-related vascular diseases may influence the course of tumor progression and treatment. In this regard, we observed that the chronic atherogenic process in ApoE−/− mice not only leads to the well-known damage to the macrovasculature2 but also affects microvascular events relevant to tumorigenesis. It is noteworthy that unlike in humans, atherosclerosis in mice is not a common component of the organismal aging and can only be induced by genetically engineered predisposition.7 In this regard, by using age-matched ApoE+/+ and ApoE−/− mice, we were able to document (and separate) the contribution of aging and atherosclerosis to alterations in tumor progression and angiogenesis.
We observed a striking growth retardation of two unrelated tumors (B16F1 and LLC) in both old/ApoE+/+ and old/ApoE−/− mice. This was coupled with a diminished MVD, lowered microvascular proliferation index, and a decrease in the expression of TEM-1 by tumor endothelial cells. TEM-1, also known as endosialin, was recently identified as one of the unique markers of tumor-associated vasculature, the expression of which is indicative of the pathological hyperstimulation of endothelial cells.23 We have also observed a decrease in expression of mRNA encoding the type 2 receptor for vascular endothelial growth factor (VEGFR-2) by tumor-associated endothelial cells in both old and atherosclerotic (old/ApoE−/−) mice, albeit the pattern of these changes differed somewhat from that of TEM-1 expression (Supplemental Figure 4, see http://ajp.amjpathol.org). Moreover, endothelial cells derived from these respective mice (even in the absence of tumors) were significantly impaired in their angiogenic capacity in vitro, as determined using the aortic ring-sprouting assay. Collectively, these results support and extend previous observations26,27,28,29 indicating that aging has a profound detrimental effect on endothelial cell properties, vascular integrity,30,31 and angiogenic proficiency, including in cancer.26
Although the apparent dysfunction of resident endothelial cells was consistently more severe in old/ApoE−/− mice than in their old/ApoE+/+ counterparts, the respective differences did not reach statistical significance when applied to individual assays. This may reflect insufficient sensitivity of these assays, or suggest a predominant influence of age-related antiangiogenic effects rather than atherosclerosis. Interestingly, the key role of the latter condition in modulating vascular homeostasis was revealed by our novel observation that in tumor-bearing (and tumor-free) old/ApoE−/− mice the levels of circulating CD45−/VEGFR-2+ cells were primarily depleted relative to nonatherosclerotic animals, both young and old. This parallels and potentially explains the more severe tumor growth suppression observed in old/ApoE−/− animals compared with old/ApoE+/+ nonatherosclerotic tumor recipients. We refer to CD45−/VEGFR-2+ cells as endothelial-like or circulating cells (CCs), rather than using more common terms such as circulating endothelial cells, or circulating endothelial progenitors/endothelial progenitor cells.24 This is because of the still unsettled debate as to the exact nature and function of circulating endothelial cells and circulating endothelial progenitors/endothelial progenitor cells32 and also because we were unable to conclusively and consistently determine the presence or absence of progenitor markers (eg, CD117) on the surface of the cells analyzed in our experiments. Still, these observations suggest that both aging and atherosclerosis may play important and distinctive (cumulative) roles in the modulation of tumor-vascular interactions.
Our results shed new light on the emerging linkage between aging, atherosclerosis, and tumor angiogenesis. Thus, recent reports indicate that certain proatherogenic stimuli may induce signs of cellular aging in human endothelial cells in vitro33 and that experimental angiogenesis may be impaired in atherosclerotic animals.9 In patients at risk for atherosclerosis, aging affects the levels of endothelial progenitor cells and this in turn may impact the severity of the atherosclerotic process.6 It is thought, that endothelial progenitor cells could participate in repair of atherosclerotic lesions, and therefore a decrease in their levels could accelerate progression of the vascular disease.6 In one study transfer of bone marrow-derived endothelial progenitor cells from young mice to their older atherosclerotic counterparts resulted in attenuation of vascular damage.34 Moreover, changes in levels of endothelial progenitor cells parallel ongoing angiogenesis and antiangiogenesis in the context of cancer.22,24 Here we provide experimental evidence that both resident and circulating endothelial cells are in different ways affected by age and atherosclerosis, and these changes correspond to the dynamics of tumor angiogenesis and growth.
We cannot formally exclude the possibility that tumor growth retardation in old and particularly in atherosclerotic mice may also be affected by inflammatory, metabolic, or physiological changes. However, we observed that tumor growth rates in severely dyslipidemic young/ApoE−/− and normolipidemic young/ApoE+/+ mice (both nonatherosclerotic; Figure 1) were, indeed, very comparable,8 an observation that suggests that high cholesterol levels are unlikely to be responsible for impaired tumor growth in old/ApoE−/− mice. Interestingly, body weight and fatty tissue accumulation was markedly reduced in the latter animals in comparison with their age-matched old/ApoE+/+ counterparts (data not shown). Because accumulation of body fat is thought to depend on angiogenesis (Ref. 10 and references therein) it remains to be established whether atherosclerosis also affects cancer-unrelated forms of vascular growth.
Importantly, our study suggests that aging and atherosclerosis may alter at least some of the consequences of anticancer therapy. In this regard, administration of low daily doses of CTX is thought to act selectively (but not exclusively) on the endothelial cell compartment (including both resident and circulating cells). The resulting angiogenesis inhibition and tumor hypoxia are thought to interfere with the mitogenesis and survival of cancer cells.24,25 Interestingly, our experiments revealed that at least some of these host-mediated responses (ie, a reduction in mitotic indices and an increase in tumor necrosis) were significantly alleviated in old and atherosclerotic mice. It should be noted, however, that the protocol we used, although originally described as metronomic,18 may also exert nonantiangiogenic effects on tumor growth. This is attributable to both complexities of CTX action itself and the inclusion of one high (cytotoxic) dose of the drug at the beginning of the treatment (compare Figure 6A).
We propose that aging and atherosclerosis may alter the state, availability, and/or sensitivity of antiangiogenic target cells in tumor-bearing animals. In this regard an interesting study published recently by Izumi and colleagues35 examined therapeutic responses of autochthonous tumors (mainly sarcoma or adenocarcinoma) occurring spontaneously in 21- to 25-month-old C3Hf/Sed mice. This study revealed that despite the increasing aggressiveness of such old-age tumors on their reinoculation into secondary recipients, they still responded to potent antiangiogenic inhibitors of VEGFR-2 (DC101).35 It would be of considerable interest to assess whether this class of agents would have a similar impact on tumors growing in atherosclerotic hosts. It should be mentioned that the antiangiogenic (and other) activities of agents, such as VEGF inhibitors, or metronomic CTX could also be modulated indirectly, namely by aging/atherosclerosis-dependent changes in drug metabolism, biodistribution, and pharmacokinetics. Collectively, the impact of atherosclerosis on tumor responses may entail multiple mechanisms and be relevant to several types of anticancer treatment, including targeted agents and conventional therapeutics, all of which depend on tumor microcirculation for drug delivery and/or efficacy.14
It should be mentioned that the murine model systems used in our study are not fully optimized to accurately reflect mitogenic, tumorigenic, and angiogenic properties of human cancers (analysis of additional, slow-growing, spontaneous, and human tumors is warranted). Still, our results are reminiscent of certain intriguing clinical observations. For instance, reduced disease aggressiveness is sometimes (but not always) observed in elderly patients with breast, colorectal, and lung cancers,36,37,38 although it is presently unclear whether these changes are of a vascular nature. In some instances the impact of age on the effects of antiangiogenic agents has been noticed in experimental39 and clinical malignancies.40
Out of necessity our study left a number of question unanswered. Of particular interest is the possibility that thrombosis, vascular damage, inflammation, altered neovascularization, and other constituents of the atherosclerotic process could affect (exacerbate) tumor cell dissemination, secondary metastatic growth, and therapeutic responsiveness.2,41 Studies in this direction are currently underway.
In conclusion, we propose that age and vascular status should perhaps be considered more fully when designing therapies for pediatric versus adult cancer patients (Figure 7) or extrapolating the respective treatment efficacies from standard experimental models involving young mice. In this regard we suggest that old and atherosclerotic mice used in the present study could offer a useful model for testing anticancer agents destined for use in adult patients.
Figure 7.
The possible impact of vascular co-morbidities on angiogenesis and anti-angiogenesis in cancer. In standard experimental mice (usually 4 to 8 weeks old), the responses of the microvasculature to angiogenic and anti-angiogenic influences are generally unperturbed and relatively robust. The impact of several metabolic, inflammatory, and degenerative changes associated with aging and vascular diseases may alter these responses in old and atherosclerotic mice and in human cancer patients, the majority of whom are individuals in later stages of life (see Results). We postulate that young mice and pediatric cancer patients may therefore respond differently to anti-angiogenic agents than adult patients.
Supplementary Material
Acknowledgments
We thank our colleagues Drs. Gavin Thurston and Wojciech Kalas for their advice and help, our families and colleagues for their support and inspiration, and Anna and Danuta Rak for their inexhaustible patience.
Footnotes
Address reprint requests to Janusz Rak, Montreal Children’s Hospital Research Institute, McGill University, 4060 Ste Catherine West, Montreal QC, H3Z 2Z3 Canada. E-mail: janusz.rak@mcgill.ca.
Supported by the Canadian Cancer Society, the Terry Fox Foundation, and the National Cancer Institute of Canada (grants NCIC 015267 and 015290 to J.R.).
J.R. is a recipient of the scientist award from the National Cancer Institute of Canada and the Jack Cole Chair in Pediatric Oncology; H.K. is a recipient of a doctoral fellowship funded by the Henderson Research Centre in Hamilton to support international exchange of students; and J.L.Y. is a recipient of a postdoctoral award from the Canadian Institutes of Health Research.
Supplemental material for this article can be found on http://ajp. amjpathol.org.
References
- Gloeckler Ries LA, Reichman ME, Lewis DR, Hankey BF, Edwards BK. Cancer survival and incidence from the Surveillance, Epidemiology, and End Results (SEER) program. Oncologist. 2003;8:541–552. doi: 10.1634/theoncologist.8-6-541. [DOI] [PubMed] [Google Scholar]
- Lusis AJ. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demierre MF, Higgins PD, Gruber SB, Hawk E, Lippman SM. Statins and cancer prevention. Nat Rev Cancer. 2005;5:930–942. doi: 10.1038/nrc1751. [DOI] [PubMed] [Google Scholar]
- Isner JM. Cancer and atherosclerosis: the broad mandate of angiogenesis. Circulation. 1999;99:1653–1655. doi: 10.1161/01.cir.99.13.1653. [DOI] [PubMed] [Google Scholar]
- Stokes KY, Granger DN. The microcirculation: a motor for the systemic inflammatory response and large vessel disease induced by hypercholesterolaemia? J Physiol. 2005;562:647–653. doi: 10.1113/jphysiol.2004.079640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q. The impact of progenitor cells in atherosclerosis. Nat Clin Pract Cardiovasc Med. 2006;3:94–101. doi: 10.1038/ncpcardio0396. [DOI] [PubMed] [Google Scholar]
- Fazio S, Linton MF. Mouse models of hyperlipidemia and atherosclerosis. Front Biosci. 2001;6:D515–D525. doi: 10.2741/fazio. [DOI] [PubMed] [Google Scholar]
- Meir KS, Leitersdorf E. Atherosclerosis in the apolipoprotein-E-deficient mouse: a decade of progress. Arterioscler Thromb Vasc Biol. 2004;24:1006–1014. doi: 10.1161/01.ATV.0000128849.12617.f4. [DOI] [PubMed] [Google Scholar]
- Jang JJ, Ho HK, Kwan HH, Fajardo LF, Cooke JP. Angiogenesis is impaired by hypercholesterolemia: role of asymmetric dimethylarginine. Circulation. 2000;102:1414–1419. doi: 10.1161/01.cir.102.12.1414. [DOI] [PubMed] [Google Scholar]
- Folkman J, Kalluri R. Tumor angiogenesis. Kufe DW, Pollock RE, Weichselbaum RR, Bast RC Jr, Gansler TS, Holland JF, Frei E III, editors. Hamilton: BC Decker Inc.,; Cancer Medicine. 2003:pp 161–194. [Google Scholar]
- Carmeliet P. Angiogenesis in life, disease and medicine. Nature. 2005;438:932–936. doi: 10.1038/nature04478. [DOI] [PubMed] [Google Scholar]
- Rak J, Milsom C, May L, Klement P, Yu J. Tissue factor in cancer and angiogenesis: the molecular link between genetic tumor progression, tumor neovascularization, and cancer coagulopathy. Semin Thromb Hemost. 2006;32:54–70. doi: 10.1055/s-2006-933341. [DOI] [PubMed] [Google Scholar]
- Yu JL, Rak JW. Host microenvironment in breast cancer development: inflammatory and immune cells in tumour angiogenesis and arteriogenesis. Breast Cancer Res. 2003;5:83–88. doi: 10.1186/bcr573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat Clin Pract Oncol. 2006;3:24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature. 2005;438:967–974. doi: 10.1038/nature04483. [DOI] [PubMed] [Google Scholar]
- Kerbel RS. Human tumor xenografts as predictive preclinical models for anticancer drug activity in humans: better than commonly perceived—but they can be improved. Cancer Biol Ther. 2003;2:S134–S139. [PubMed] [Google Scholar]
- Rak J, Mitsuhashi Y, Bayko L, Filmus J, Sasazuki T, Kerbel RS. Mutant ras oncogenes upregulate VEGF/VPF expression: implications for induction and inhibition of tumor angiogenesis. Cancer Res. 1995;55:4575–4580. [PubMed] [Google Scholar]
- Bocci G, Francia G, Man S, Lawler J, Kerbel RS. Thrombospondin 1, a mediator of the antiangiogenic effects of low-dose metronomic chemotherapy. Proc Natl Acad Sci USA. 2003;100:12917–12922. doi: 10.1073/pnas.2135406100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Rak JW, Klement G, Kerbel RS. VEGF isoform expression as a determinant of blood vessel patterning in human melanoma xenografts. Cancer Res. 2002;62:1838–1846. [PubMed] [Google Scholar]
- Carson-Walter EB, Watkins DN, Nanda A, Vogelstein B, Kinzler KW, St Croix B. Cell surface tumor endothelial markers are conserved in mice and humans. Cancer Res. 2001;61:6649–6655. [PubMed] [Google Scholar]
- Zhu WH, Iurlaro M, MacIntyre A, Fogel E, Nicosia RF. The mouse aorta model: influence of genetic background and aging on bFGF- and VEGF-induced angiogenic sprouting. Angiogenesis. 2003;6:193–199. doi: 10.1023/B:AGEN.0000021397.18713.9c. [DOI] [PubMed] [Google Scholar]
- Monestiroli S, Mancuso P, Burlini A, Pruneri G, Dell’Agnola C, Gobbi A, Martinelli G, Bertolini F. Kinetics and viability of circulating endothelial cells as surrogate angiogenesis marker in an animal model of human lymphoma. Cancer Res. 2001;61:4341–4344. [PubMed] [Google Scholar]
- Nanda A, St Croix B. Tumor endothelial markers: new targets for cancer therapy. Curr Opin Oncol. 2004;16:44–49. doi: 10.1097/00001622-200401000-00009. [DOI] [PubMed] [Google Scholar]
- Shaked Y, Bertolini F, Man S, Rogers MS, Cervi D, Foutz T, Rawn K, Voskas D, Dumont DJ, Ben David Y, Lawler J, Henkin J, Huber J, Hicklin DJ, D’Amato RJ, Kerbel RS. Genetic heterogeneity of the vasculogenic phenotype parallels angiogenesis; implications for cellular surrogate marker analysis of antiangiogenesis. Cancer Cell. 2005;7:101–111. doi: 10.1016/j.ccr.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Browder T, Butterfield CE, Kraling BM, Shi B, Marshall B, O’Reilly MS, Folkman J. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer. Cancer Res. 2000;60:1878–1886. [PubMed] [Google Scholar]
- Pili R, Guo Y, Chang J, Nakanishi H, Martin GR, Passaniti A. Altered angiogenesis underlying age-dependent changes in tumor growth. J Natl Cancer Inst. 1994;86:1303–1314. doi: 10.1093/jnci/86.17.1303. [DOI] [PubMed] [Google Scholar]
- Rivard A, Fabre JE, Silver M, Chen D, Murohara T, Kearney M, Magner M, Asahara T, Isner JM. Age-dependent impairment of angiogenesis. Circulation. 1999;99:111–120. doi: 10.1161/01.cir.99.1.111. [DOI] [PubMed] [Google Scholar]
- Franco S, Segura I, Riese HH, Blasco MA. Decreased B16F10 melanoma growth and impaired vascularization in telomerase-deficient mice with critically short telomeres. Cancer Res. 2002;62:552–559. [PubMed] [Google Scholar]
- Shimada T, Takeshita Y, Murohara T, Sasaki K, Egami K, Shintani S, Katsuda Y, Ikeda H, Nabeshima Y, Imaizumi T. Angiogenesis and vasculogenesis are impaired in the precocious-aging Klotho mouse. Circulation. 2004;110:1148–1155. doi: 10.1161/01.CIR.0000139854.74847.99. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Shimokawa T, Yi H, Isobe K, Kojima T, Loskutoff DJ, Saito H. Aging and obesity augment the stress-induced expression of tissue factor gene in the mouse. Blood. 2002;100:4011–4018. doi: 10.1182/blood-2002-03-0945. [DOI] [PubMed] [Google Scholar]
- Sagripanti A, Carpi A. Natural anticoagulants, aging, and thromboembolism. Exp Gerontol. 1998;33:891–896. doi: 10.1016/s0531-5565(98)00047-3. [DOI] [PubMed] [Google Scholar]
- Peters BA, Diaz LA, Polyak K, Meszler L, Romans K, Guinan EC, Antin JH, Myerson D, Hamilton SR, Vogelstein B, Kinzler KW, Lengauer C. Contribution of bone marrow-derived endothelial cells to human tumor vasculature. Nat Med. 2005;11:261–262. doi: 10.1038/nm1200. [DOI] [PubMed] [Google Scholar]
- Breitschopf K, Zeiher AM, Dimmeler S. Pro-atherogenic factors induce telomerase inactivation in endothelial cells through an Akt-dependent mechanism. FEBS Lett. 2001;493:21–25. doi: 10.1016/s0014-5793(01)02272-4. [DOI] [PubMed] [Google Scholar]
- Rauscher FM, Goldschmidt-Clermont PJ, Davis BH, Wang T, Gregg D, Ramaswami P, Pippen AM, Annex BH, Dong C, Taylor DA. Aging, progenitor cell exhaustion, and atherosclerosis. Circulation. 2003;108:457–463. doi: 10.1161/01.CIR.0000082924.75945.48. [DOI] [PubMed] [Google Scholar]
- Izumi Y, di Tomaso E, Hooper A, Huang P, Huber J, Hicklin DJ, Fukumura D, Jain RK, Suit HD. Responses to antiangiogenesis treatment of spontaneous autochthonous tumors and their isografts. Cancer Res. 2003;63:747–751. [PubMed] [Google Scholar]
- Balducci L, Ershler WB. Cancer and ageing: a nexus at several levels. Nat Rev Cancer. 2005;5:655–662. doi: 10.1038/nrc1675. [DOI] [PubMed] [Google Scholar]
- Jayasinghe UW, Taylor R, Boyages J. Is age at diagnosis an independent prognostic factor for survival following breast cancer? ANZ J Surg. 2005;75:762–767. doi: 10.1111/j.1445-2197.2005.03515.x. [DOI] [PubMed] [Google Scholar]
- Lin JT, Wang WS, Yen CC, Liu JH, Yang MH, Chao TC, Chen PM, Chiou TJ. Outcome of colorectal carcinoma in patients under 40 years of age. J Gastroenterol Hepatol. 2005;20:900–905. doi: 10.1111/j.1440-1746.2005.03893.x. [DOI] [PubMed] [Google Scholar]
- Kaptzan T, Skutelsky E, Itzhaki O, Sinai J, Huszar M, Siegal A, Ben-Zvi R, Jossiphov J, Michowitz M, Schiby G, Leibovici J. Efficacy of anti-angiogenic treatment of tumors in old versus young mice. Mech Ageing Dev. 2006;127:398–409. doi: 10.1016/j.mad.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M, Negrier S, Chevreau C, Solska E, Desai AA, Rolland F, Demkow T, Hutson TE, Gore M, Freeman S, Schwartz B, Shan M, Simantov R, Bukowski RM. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med. 2007;356:125–134. doi: 10.1056/NEJMoa060655. [DOI] [PubMed] [Google Scholar]
- Petralia GA, Lemoine NR, Kakkar AK. Mechanisms of disease: the impact of antithrombotic therapy in cancer patients. Nat Clin Pract Oncol. 2005;2:356–363. doi: 10.1038/ncponc0225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.