Abstract
Previously, we have demonstrated that STAT-5B plays a role in thrombin-induced vascular smooth muscle cell (VSMC) growth and motility. To learn more about the role of STAT-5B in vessel wall remodeling, we examined its involvement in platelet-derived growth factor-BB (PDGF-BB)-stimulated VSMC growth and motility and balloon injury-induced neointima formation. PDGF-BB activated STAT-5B as measured by its tyrosine phosphorylation, DNA binding, and reporter gene activity. PDGF-BB induced cyclin D1 expression, CDK4 activity, and Rb protein phosphorylation, leading to VSMC growth and motility, and these responses were suppressed by the blockade of STAT-5B. Increased cyclin D1 levels, CDK4 activity, and Rb protein phosphorylation were observed in 1-week balloon-injured arteries compared with uninjured arteries, and these responses were also suppressed by adenovirus-mediated expression of dnSTAT-5B. In addition, adenovirus-mediated expression of dnSTAT-5B attenuated balloon injury-induced smooth muscle cell migration from media to intima and their proliferation in intima, resulting in reduced neointima formation. These observations indicate that STAT-5B plays an important role in PDGF-BB-induced VSMC growth and motility in vitro and balloon injury-induced neointima formation in vivo.
Increased vascular smooth muscle cell (VSMC) mass is a major contributing factor in atherosclerosis and neointima formation after percutaneous transluminal angioplasty.1,2,3 A broad spectrum of molecules including cytokines, growth factors, hormones, and oxidants that are produced by a variety of cell types at the sites of vascular injury have been reported to influence the growth of VSMCs in vitro.4,5,6,7,8,9,10 Because a large number of molecules are apparently involved in these vessel wall diseases, understanding the biochemical mechanisms that are common to the mitogenic effects of many of these substances may lead to the development of better therapeutic agents against the progression of these vascular lesions. Indeed, approaches that target the inhibition of VSMC proliferation have been found to be more promising in arresting the development of neointima.11,12,13,14 Toward elucidation of signal transduction mechanisms that are ubiquitously involved in the mitogenic actions of various VSMC agonists, we have shown previously that both receptor tyrosine kinase (RTK) and G protein-coupled receptor (GPCR) agonists such as platelet-derived growth factor-BB (PDGF-BB) and thrombin, respectively, activate nuclear factor of activated T cells (NFATs) and signal transducer and activator of transcription-3 (STAT-3) in stimulating VSMC growth and/or motility.9,15,16,17,18
Early studies from various laboratories have shown that STATs play an important role in the regulation of cytokine-induced gene expression.19,20,21 However, subsequent studies have revealed their involvement in a variety of cellular responses including cell differentiation, proliferation, and apoptosis.22,23,24,25,26,27,28 In addition, selectivity among various STATs in mediating these cellular effects has been observed.22,23,24,25,26,27,28 Specifically, whereas STAT-1 has been found to be involved in the modulation of apoptosis,27,28 STAT-3 and STAT-5 were reported to play a role in the regulation of cell growth and differentiation.22,23,24 Recently, we have observed a role for STAT-5B in GPCR agonist thrombin-induced VSMC growth and motility.29 PDGF-BB is the most potent VSMC mitogen and chemoattractant.1,3,16,17 Therefore, to understand the role of STAT-5B in vascular wall remodeling further, in the present investigation we have studied its involvement in VSMC growth and motility in response to PDGF-BB in vitro and balloon injury in vivo. Here, we report for the first time that PDGF-BB stimulates STAT-5B activation leading to induction of cyclin D1 expression, CDK4 activity, and Rb protein phosphorylation in VSMCs and thereby the growth and motility of these cells. In addition, increases in cyclin D1 levels, CDK4 activity, Rb protein phosphorylation, smooth muscle cell (SMC) migration from media to intima, and their proliferation in intima were observed in arteries after balloon injury as compared with uninjured arteries, and these responses were suppressed by adenovirus-mediated expression of dnSTAT-5B resulting in reduced neointima formation. Together, these results point to a potential role for STAT-5B in vessel wall remodeling in response to injury.
Materials and Methods
Reagents
Aprotinin, phenylmethyl sulfonyl fluoride, sodium orthovanadate, leupeptin, HEPES, β-glycerophosphate, and sodium fluoride were purchased from Sigma Chemical Company (St. Louis, MO). Recombinant human PDGF-BB (220-BB) was from R&D Systems Inc. (Minneapolis, MN). Anti-STAT-3 antibodies (SC-482), anti-STAT-5 antibodies (SC-835, SC-836), anti-STAT-5B antibodies (SC-1656), anti-CDK4 antibodies (SC-260), and STAT-5 consensus-binding oligonucleotides (5′-AGATTTCTAGGAATTCAATCC-3′) (SC-2565) were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). Anti-phospho STAT-3 antibodies (9145), anti-phospho STAT-5 antibodies (9351), anti-PY20 antibodies (9411), and anti-phospho pRb antibodies (9307) were supplied by Cell Signaling Technology (Beverly, MA). Anti-cyclin D1 antibodies (RB-010-P), anti-STAT-5B antibodies (RB-096-P1ABX), and anti-proliferating cell nuclear antigen (PCNA) antibodies (MS-106-P) were from NeoMarkers (Fremont, CA). Anti-STAT-5B antibodies (06-969) were also obtained from Upstate (Lake Placid, NY). Monoclonal anti-histone antibodies (MAB 1276) were from Chemicon (Temecula, CA). The in situ cell death detection kit, TMR Red, was purchased from Roche Diagnostics (Indianapolis, IN). Truncated Rb protein (3109) was obtained from QED Bioscience, San Diego, CA. Rat cyclin D1 short interfering RNA (siRNA) duplexes (siRNA3: sense, 5′-GCGCGUACCCUGACACCAAUU-3′; antisense, 5′-UUGGUGUCAGGGUACGCGCUU-3′; siRNA4: sense, 5′-CCGAGAAGUUGUGCAUCUAUU-3′; antisense, 5′-UAGAUGCACAACUUCUCGGUU-3′); scrambled control siRNA (5′-UAGCGACUAAACACAUCAA-3′) were made by Dharmacon (Lafayette, CO). T4 polynucleotide kinase was obtained from Promega (Madison, WI). [γ32P]ATP (3000 Ci/mmol), [14C]chloramphenicol (59 mCi/mmol), and protein-A-Sepharose (CL-4B) were from Amersham Biosciences (Piscataway, NJ). Thymidine [methyl-3H] (20 Ci/mmol) was obtained from Perkin-Elmer (Boston, MA). All of the primers and the putative STAT-binding oligonucleotides from rat cyclin D1 promoter were made by IDT (Coralville, IA).
Cell Culture
Rat VSMCs were isolated and subcultured as described previously.29 VSMCs were used between 4 and 12 passages. Human aortic smooth muscle cells (HASMCs) were obtained from Cascade Biologics, Inc. (Portland, OR) and subcultured in Medium 231 containing smooth muscle growth supplements, 10 μg/ml gentamicin, and 0.25 μg/ml amphotericin B. Cultures were maintained at 37°C in a humidified 95% air and 5% CO2 atmosphere. HASMCs were growth-arrested by incubating in Medium 231 without smooth muscle growth supplements for 48 hours and used to perform the experiments unless otherwise stated. HASMCs were used between 6 and 10 passages.
DNA Synthesis
VSMC DNA synthesis was measured by pulse-labeling cells with 1 μCi/ml [3H]thymidine for the last 12 hours of the 24-hour incubation period as described previously.29
Cell Number
VSMCs at 72 hours of appropriate treatments were trypsinized, rinsed with and suspended in 1 ml of phosphate-buffered saline (PBS), and counted using hemocytometer.
Cell Migration
VSMC motility was measured using a wounding assay as described previously.29
In Vivo SMC Migration Assay
Although migration is difficult to quantify in vivo, the accumulation of cells in the intima early (∼4 days) after injury is considered to be mostly attributable to the migration of SMCs from the injured media.30 Therefore, the in vivo SMC migration was determined as described by Bendeck and colleagues.31 In brief, 4 days after balloon injury, the carotid arteries were fixed in vivo with 10% buffered formalin at physiological pressure. The middle 1 cm of the denuded (injured) and uninjured common carotid arteries were cut and fixed in ice-cold acetone for 10 minutes. The arteries were then opened longitudinally and pinned down onto an agar plate with the luminal surface facing upward. The arteries were rinsed in PBS and then placed in 3% H2O2 to block endogenous peroxidase activity. Nonspecific protein binding was blocked by incubating the arteries in 5% normal goat serum in PBS for 30 minutes. The arteries were incubated with anti-histone monoclonal antibody diluted 1:100 in PBS for 1 hour, followed by incubation in biotinylated goat anti-mouse IgG for 30 minutes. Peroxidase labeling was performed by using the ABC kit (Vector Laboratories, Burlingame, CA), and the signals were visualized by using the DAB kit (Vector Laboratories). After each step, the slides were rinsed three times for 5 minutes each in PBS. Finally, the opened arteries were placed intimal side up on glass slides with coverslips. As a negative control, samples of the same specimens without the primary antibody were used. The intimal surface of the vessel was examined under a light microscope at ×200, and the total number of intimal cell nuclei per square millimeter of surface area were counted.
Electrophoretic Mobility Shift Assay
After appropriate treatments, VSMC nuclear extracts were prepared and analyzed for STAT-5 DNA binding activity as described previously.29
CAT Assay
VSMCs were transfected with 1× pSp1GLECAT plasmid in serum- and antibiotic-free Dulbecco’s modified Eagle’s medium using FuGENE 6 reagent (Invitrogen, Carlsbad, CA). The 1× pSp1GLECAT contains STAT-5-responsive sequence of the human Sp2.1 promoter and thereby drives the CAT reporter gene expression.32 Cells were then quiesced and treated with and without PDGF-BB (20 ng/ml) for the indicated times, and cell extracts were prepared. In the case of testing the role of STAT-5B, cells were transduced first with the control Ad-GFP or Ad-dnSTAT-5B virus followed by transfection with pSp1GLECAT plasmid DNA. Cell extracts were normalized for protein and assayed for CAT activity using [14C]chloramphenicol and acetyl coenzyme A as substrates as described previously.18
Chromatin Immunoprecipitation (ChIP) Assay
ChIP assay was performed on VSMCs by using the ChIP assay kit following the supplier’s protocol (Upstate Biotechnology). STAT-5B-DNA complexes were immunoprecipitated using anti-STAT-5B antibodies (catalog no. SC-1656 from Santa Cruz Biotechnology or catalog no. 06-969 from Upstate Biotechnology). Preimmune serum (SC-2338) was used as a negative control. Precipitated DNA was extracted using phenol-chloroform, and the DNA fragments from the aqueous phase were purified using QIAquick columns (catalog no. 28104; Qiagen, Valencia, CA). The purified DNA was used as template for polymerase chain reaction (PCR) amplification using primers (forward, 5′-CAACGAAGCCAATCGGGAAGCTTC-3′; reverse, 5′-CACCCTATACTTAAGCGGAGAGAA-3′) flanking the putative STAT-binding site located at −978 in rat cyclin D1 promoter region.33 The PCR products were resolved on 2% agarose gel and stained with ethidium bromide.
CDK4 Assay
After appropriate treatments, protein extracts from cells or tissues were prepared using lysis buffer (20 mmol/L HEPES, pH 7.4, 150 mmol/L NaCl, 1% Nonidet P-40, 10 μg/ml aprotinin, 10 μg/ml leupeptin, 50 mmol/L glycerophosphate, 10 mmol/L NaF, and 1 mmol/L sodium orthovanadate). An equal quantity of total protein (200 μg) from each sample was immunoprecipitated with anti-CDK4 antibodies (2 μg) overnight at 4°C followed by incubation with protein-A-Sepharose beads for 1 hour at room temperature. CDK4 activity in the immunocomplexes was assayed at 30°C in a kinase reaction mix that contained 25 mmol/L HEPES, pH 7.4, 10 mmol/L MgCl2, 1 mmol/L EGTA, 200 μg/ml truncated Rb protein, 1 μCi of [γ32P]ATP, and 50 μmol/L ATP. After 30 minutes of incubation, the kinase reaction was stopped by the addition of sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer and subsequent boiling for 5 minutes. After heat denaturation, the reaction products were separated by electrophoresis on 0.1% sodium dodecyl sulfate and 10% polyacrylamide gels. The 32P-labeled Rb protein was visualized by autoradiography, and the band intensities were quantitated using NIH Image J (National Institutes of Health, Bethesda, MD).
Construction of GFP, dnSTAT-5A, and dnSTAT-5B Adenoviral Vectors
DnSTAT-5A and dnSTAT-5B expression plasmids were constructed by deletion of their C-terminal regions starting from amino acids 713 to 793 in the case of the former one and from amino acids 718 to 793 in the case of the latter one.34 Construction of Ad-GFP, Ad-dnSTAT-5A, and Ad-dnSTAT-5B were described previously.15,29
Delivery of Adenoviruses into Injured Arteries
After balloon injury, solutions (100 μl) of Ad-GFP (1010 pfu/ml) or Ad-dnSTAT-5B (1010 pfu/ml) were infused into the ligated segment of the common carotid artery for 30 minutes as described previously.15 Viral transductions were evaluated at 1 week after balloon injury by isolation of arteries followed either by cryostat cross-sectioning and examining for green fluorescence protein expression or by preparing tissue extracts and Western blot analysis of GFP levels using its specific antibodies.
Carotid Artery Balloon Injury
All of the animal protocols were performed in accordance with the relevant guidelines and regulations approved by the Internal Animal Care and Use Committee of the University of Tennessee Health Science Center. Balloon injury was performed essentially as described previously by us.15 In brief, rats weighing 250 to 300 g were anesthetized by injecting (intraperitoneally) ketamine (60 mg/kg) and xylazine (5 mg/kg). Under a stereomicroscope, the right common, external and internal, carotid arteries were exposed by a longitudinal midline cervical incision, and blood flow was temporarily interrupted by ligation of the common and internal carotid arteries using vessel clips. External carotid artery was ligated permanently. A 2F Fogarty arterial embolectomy catheter was introduced through an arteriotomy in the external carotid artery just below the ligature and advanced to the common carotid artery. To produce carotid artery injury, the balloon was inflated with saline and passed six times with rotation from just under the proximal edge of the omohyoid muscle to the carotid bifurcation. After this, the balloon was deflated, and the catheter was withdrawn. The external carotid artery was ligated with a 6-0 silk suture, and the blood flow was restored by removing the clips at the common and internal carotid arteries. After inspection to ascertain adequate pulsation of the common carotid artery, the surgical incision was closed, and the rats were allowed to recover from anesthesia in a humidified and warmed chamber for 2 to 4 hours. At 1 or 2 weeks after balloon injury, the animals were sacrificed with an overdose of pentobarbital (200 mg/kg), and the carotid arteries were collected for protein isolation, PCNA staining, and morphometric analysis.
Histochemistry
After dissecting out, arteries were fixed in 10% formalin and embedded in paraffin. Sections were cut with 5-μm thickness and immunostained for PCNA using specific antibodies or stained with hematoxylin and eosin (H&E). SMC proliferation was measured by counting PCNA-positive cells. The intimal (I) and medial (M) areas in H&E-stained sections were measured using NIH Image J program, and the I:M ratios and lumen sizes were calculated.
Terminal dUTP Nick-End Labeling (TUNEL) Assay
VSMCs after appropriate treatments were fixed with paraformaldehyde and examined for cell death using in situ cell death detection kit essentially as per the instructions of the manufacturer. Similarly, cryostat cross sections of 2-week balloon-injured and Ad-GFP- or Ad-STAT-5B-transduced arteries were used to evaluate cell death using the above-mentioned kit.
Western Blot Analysis
Equal amounts of protein from VSMCs or tissue extracts were analyzed by Western blotting for the protein of interest using its specific antibodies as described previously.29
Statistics
All of the experiments were repeated several times with similar results. Data are presented as mean ± SD. The treatment effects were analyzed by Student’s t-test. P values <0.05 were considered to be statistically significant. In the case of CAT activity, electrophoretic mobility shift assay, and Western blotting, one representative set of data is shown.
Results
PDGF-BB Activates STAT-5B in VSMCs
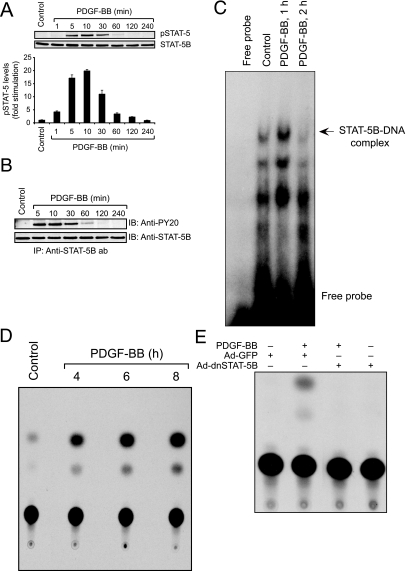
To understand the role of STAT-5 in vessel wall remodeling, we studied its involvement in PDGF-BB-induced VSMC growth and motility. Quiescent VSMCs were treated with and without PDGF-BB (20 ng/ml) for various times, cell extracts were prepared, and an equal amount of protein from control and each treatment was analyzed by Western blotting for STAT-5 phosphorylation using its phosphospecific antibodies. PDGF-BB stimulated the tyrosine phosphorylation of STAT-5 (Tyr694) in a time-dependent manner with a maximum increase at 10 minutes and declining thereafter, reaching basal levels by 2 hours (Figure 1A). Anti-phosphospecific STAT-5 antibodies recognize both the tyrosine-phosphorylated STAT-5A and -5B. Therefore, to ascertain that PDGF-BB stimulates tyrosine phosphorylation of STAT-5B, an equal amount of protein from control and various times of PDGF-BB (20 ng/ml)-treated VSMCs was immunoprecipitated with monoclonal anti-STAT-5B antibodies (SC-1656), and the immunocomplexes were subjected to immunoblotting analysis using anti-PY20 antibodies. Immunoblotting analysis of anti-STAT-5B immunocomplexes with anti-PY20 antibodies revealed a time-dependent increase in tyrosine phosphorylation of STAT-5B in response to PDGF-BB (Figure 1B). Together, these results indicate that PDGF-BB stimulates STAT-5B in VSMCs. To find whether the increased tyrosine phosphorylation of STAT-5B leads to its transcriptional transactivation capacity, nuclear extracts were prepared from various times of PDGF-BB (20 ng/ml)-treated and untreated VSMCs, and an equal amount of nuclear protein from control and each treatment was analyzed for STAT-5 DNA-binding activity using its 32P-labeled consensus double-stranded oligonucleotides as a probe. PDGF-BB induced STAT-5-DNA binding activity compared with control (Figure 1C). Maximum increase in its DNA binding activity occurred at 1 hour after PDGF-BB treatment. To gain additional support for these findings, VSMCs were transfected with a reporter gene, pSp1GLECAT, quiesced, and treated with and without PDGF-BB (20 ng/ml) for various times, and cell extracts were prepared and assayed for CAT activity. Consistent with its effect on STAT-5-DNA binding activity, PDGF-BB induced Sp1GLE-mediated CAT activity (Figure 1D). Because both STAT-5A and -5B are able to bind to STAT-5 consensus-binding oligonucleotides and drive Sp1GLE-mediated reporter gene activity, we next used a dominant-negative mutant approach to test the role of STAT-5B in these responses. Adenovirus-mediated expression of dnSTAT-5B suppressed PDGF-BB-induced Sp1GLE-mediated CAT activity (Figure 1E). Together, these results suggest that PDGF-BB stimulates STAT-5B-dependent transcriptional transactivation in VSMCs.
Figure 1.
PDGF-BB activates STAT-5B in VSMCs. Quiescent VSMCs were treated with and without PDGF-BB (20 ng/ml) for the indicated times, and either cell or nuclear extracts were prepared. A: Cell extracts containing an equal amount of protein from control and each treatment were analyzed by Western blotting for pSTAT-5 using its phosphospecific antibodies. The blot was reprobed with anti-STAT-5B antibodies for normalization. The bar graph at the bottom represents the quantitative analysis of three independent experiments. B: An equal amount of protein (500 μg) from control and PDGF-BB-treated VSMCs was immunoprecipitated with 3 μg of anti-STAT-5B antibodies, and the immunocomplexes were subjected to immunoblotting analysis using anti-PY20 antibodies. C: The nuclear extracts containing an equal amount of protein from control and each treatment were assayed for STAT-5 DNA-binding activity using its 32P-labeled consensus double-stranded oligonucleotides as a probe. D: VSMCs were transfected with 1× pSp1GLECAT plasmid DNA, quiesced, and treated with and without PDGF-BB (20 ng/ml) for the indicated times, and cell extracts were prepared and assayed for CAT activity using [14C]chloramphenicol and acetyl coenzyme A as substrates. E: VSMCs were transduced first with Ad-GFP (control) or Ad-dnSTAT-5B at a MOI of 80, and 24 hours later they were transfected with 1× pSp1GLECAT plasmid DNA and quiesced. Cells were treated with and without PDGF-BB (20 ng/ml) for 8 hours, and cell extracts were prepared and assayed for CAT activity as described in D.
STAT-5B Mediates PDGF-BB-Induced VSMC Growth and Motility
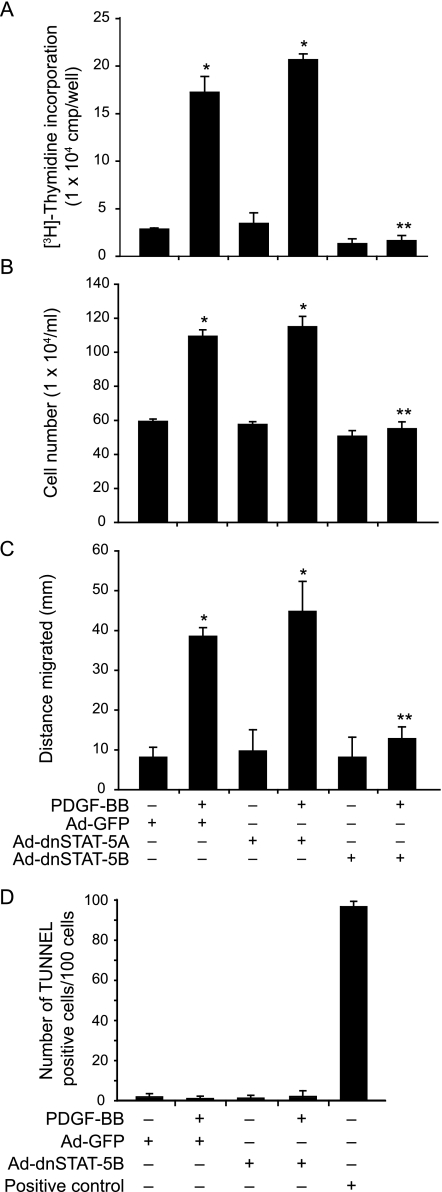
To learn the role of STAT-5B in PDGF-BB-induced VSMC growth and motility, we studied the effects of dnSTAT-5A and dnSTAT-5B. Quiescent VSMCs that were transduced with adenovirus [80 multiplicity of infection (MOI)] harboring either GFP, dnSTAT-5A, or dnSTAT-5B were treated with and without PDGF-BB (20 ng/ml) for 24 hours, and DNA synthesis was measured by pulse-labeling cells with 1 μCi/ml [3H]thymidine for the last 12 hours of the 24-hour incubation period. PDGF-BB induced VSMC DNA synthesis by several fold as compared with Ad-GFP-transduced control cells, and adenovirus-mediated expression of dnSTAT-5B but not dnSTAT-5A suppressed this effect (Figure 2A). To substantiate the role of STAT-5B in PDGF-BB-induced VSMC growth, cells that were transduced with Ad-GFP (80 MOI), Ad-dnSTAT-5A (80 MOI), or Ad-dnSTAT-5B (80 MOI) were treated with and without PDGF-BB (20 ng/ml) for 72 hours, and cell numbers were measured. Consistent with their effects on DNA synthesis, adenovirus-mediated expression of dnSTAT-5B but not dnSTAT-5A inhibited PDGF-BB-induced VSMC numbers (Figure 2B). To test the role of STAT-5B in PDGF-BB-induced VSMC motility, cells were transduced with Ad-GFP (80 MOI), Ad-dnSTAT-5A (80 MOI), or Ad-dnSTAT-5B (80 MOI), quiesced, and subjected to PDGF-BB-induced motility using wounding assay. PDGF-BB (20 ng/ml) induced VSMC motility by fourfold compared with control, and this effect was suppressed by dnSTAT-5B but not dnSTAT-5A (Figure 2C). To exclude that the decreased cell numbers and motility are not attributable to apoptosis induced by blockade of STAT-5B, we tested the effect of dnSTAT-5B on VSMC death using TUNEL assay. Very few TUNEL-stained positive cells were observed in Ad-GFP-transduced cells in response to PDGF-BB treatment, and blockade of STAT-5B signaling had no additional effect on the induction of apoptosis (Figure 2D). These results suggest that the decreased cell numbers and their migration by blockade of STAT-5B signaling are not attributable to an increase in apoptosis but attributable to a role of STAT-5B in PDGF-BB-induced VSMC growth and motility.
Figure 2.
PDGF-BB-induced VSMC growth and motility require activation of STAT-5B. VSMCs that were transduced with Ad-GFP (control), Ad-dnSTAT-5A, or Ad-dnSTAT-5B with a MOI of 80 were quiesced and subjected to PDGF-BB-induced DNA synthesis and cell numbers and motility. A: DNA synthesis was measured by [3H]thymidine incorporation. B: Cell numbers were counted by hemocytometer. C: Cell motility was determined by wounding assay. D: Apoptosis was measured by TUNEL assay. DNase I treatment was used as a positive control. *P < 0.01 versus control; **P < 0.01 versus PDGF-BB treatment alone.
STAT-5B Mediates PDGF-BB-Induced Cyclin D1 Expression, CDK4 Activity, and Rb Protein Phosphorylation in VSMCs
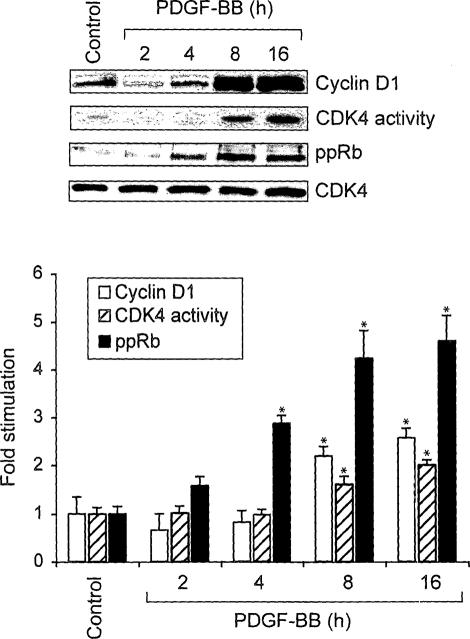
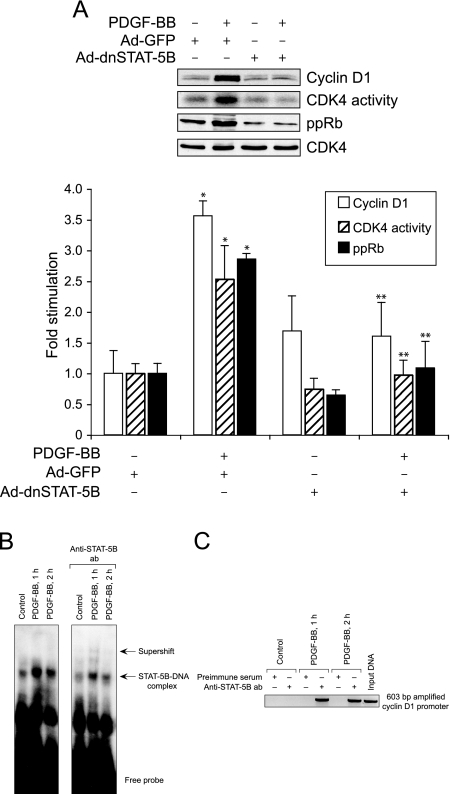
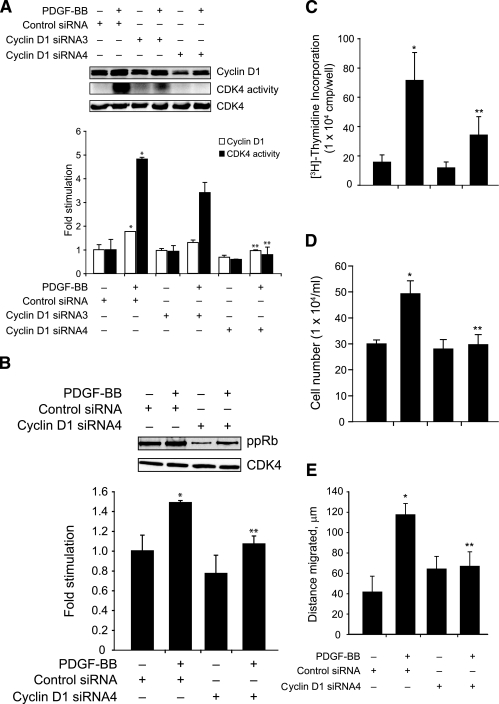
Earlier it was reported that STAT-5 plays a role in the regulation of cytokine-induced cyclin D1 expression in hematopoietic cells.35 Therefore, to understand the molecular mechanisms by which STAT-5B is involved in PDGF-BB-induced VSMC growth, we first tested the effect of PDGF-BB on cyclin D1 expression. PDGF-BB induced the expression of cyclin D1 in a time-dependent manner with a maximum increase of approximately threefold at 8 hours of treatment as compared with control and these levels were sustained at least for 16 hours (Figure 3). Cyclin D1 forms a complex with and exerts a positive effect on CDK4 activity.36 To test whether PDGF-BB-induced increases in cyclin D1 levels led to up-regulation of CDK4 activity, quiescent VSMCs were treated with and without PDGF-BB (20 ng/ml) for various times, and cell extracts were prepared. An equal amount of protein from control and each treatment was immunoprecipitated with anti-CDK4 antibodies, and the kinase activity in the immunocomplexes was measured using truncated recombinant retinoblastoma protein and [γ32P]ATP as substrates. As expected, PDGF-BB induced CDK4 activity in a time-dependent manner with a maximum effect of twofold increase at 8 hours (Figure 3). CDK4 phosphorylates and inactivates Rb protein.37 The phosphorylated Rb protein, in turn, dissociates from E2Fs and thereby facilitates the activation of the latter transcriptional factors, which in their turn, influence the transcription of genes required for DNA synthesis.38,39 Therefore, to test whether PDGF-BB-induced CDK4 activity correlates with increased phosphorylation of its substrate(s), we measured Rb protein phosphorylation. Quiescent VSMCs were treated with and without PDGF-BB (20 ng/ml) for various times, cell extracts were prepared, and an equal amount of protein from each condition was analyzed by Western blotting for phosphorylated Rb protein levels using its phosphospecific antibodies. PDGF-BB stimulated Rb protein phosphorylation (Ser780) in a time-dependent manner with a maximum effect of fivefold increase at 8 hours (Figure 3). Thus, the time course study showed a correlation between PDGF-BB-induced cyclin D1 expression, CDK4 activity, and Rb protein phosphorylation in VSMCs. To test the role of STAT-5B in PDGF-BB-induced cyclin D1 expression, CDK4 activity, and Rb protein phosphorylation, we next tested the effect of dominant-negative STAT-5B on these responses. PDGF-BB induced cyclin D1 expression, CDK4 activity, and Rb protein phosphorylation in Ad-GFP-transduced control VSMCs, and these responses were completely suppressed in cells that were transduced with Ad-dnSTAT-5B (Figure 4A). TRANSFAC analysis of the cloned rat cyclin D1 promoter33 revealed the presence of one putative STAT-binding site spanning from −978 to −986 (5′-TTCCTGGAA-3′). To find whether STAT-5B binds to cyclin D1 promoter, we have performed both electrophoretic mobility shift and ChIP assays. A threefold increase in STAT-5B DNA-binding activity was observed with rat cyclin D1 promoter sequence, 5′-TCTGGTTCCTGGAAGGGCAA-3′, encompassing the putative STAT-binding site as a 32P-labeled probe in response to PDGF-BB (Figure 4B). In addition, anti-STAT-5B antibodies caused a supershift of this protein-DNA complex, suggesting the presence of STAT-5B. ChIP of control and various time periods of PDGF-BB-treated VSMCs with anti-STAT-5B antibodies followed by PCR amplification using primers spanning −1013 to −411 region of cyclin D1 promoter revealed the binding of STAT-5B to this region in response to PDGF-BB treatment only (Figure 4C). These results clearly indicate that STAT-5B is indeed involved in PDGF-BB-induced cyclin D1 expression in VSMCs. To find whether cyclin D1 expression is required for PDGF-BB-induced VSMC growth and motility, we used an siRNA approach. VSMCs were transfected with scrambled control or cyclin D1 siRNA (100 nmol/L), quiesced, treated with and without PDGF-BB (20 ng/ml) for 16 hours, cell extracts were prepared and analyzed for cyclin D1 levels, CDK4 activity, and Rb protein phosphorylation as described above. Compared with the effects of scrambled control siRNA, cyclin D1 siRNA inhibited PDGF-BB-induced increases in cyclin D1 expression, CDK4 activity, and Rb protein phosphorylation (Figure 5, A and B). Similarly, cyclin D1 siRNA also inhibited PDGF-BB-induced VSMC DNA synthesis, their numbers, and motility (Figure 5, C–E).
Figure 3.
PDGF-BB induces cyclin D1 expression, CDK4 activity, and Rb protein phosphorylation in VSMCs. Quiescent VSMCs were treated with and without PDGF-BB (20 ng/ml) for the indicated times, and cell extracts were prepared. An equal amount of protein from control and each treatment was analyzed by Western blotting for cyclin D1 expression, Rb protein phosphorylation, and CDK4 levels using their specific antibodies. For CDK4 assay, an equal amount of protein from control and each treatment was incubated with anti-CDK4 antibodies, and the kinase activity in the immunocomplexes was measured using recombinant retinoblastoma protein and [γ32P]ATP as substrates. The bar graph at the bottom represents the quantitative analysis of three independent experiments. *P < 0.01 versus control.
Figure 4.
STAT-5B mediates PDGF-BB-induced cyclin D1 expression, CDK4 activity, and Rb protein phosphorylation in VSMCs. A: The conditions were the same as in Figure 3 except that cells were transduced with either Ad-GFP (control) or Ad-dnSTAT-5B with a MOI of 80 and quiesced. Cells were then treated with and without PDGF-BB (20 ng/ml) for 16 hours and analyzed for cyclin D1 expression, CDK4 activity, Rb protein phosphorylation, and CDK4 levels as described above in Figure 3. The bar graph at the bottom represents the quantitative analysis of three independent experiments. B: Nuclear extracts were prepared from quiescent control and various times of PDGF-BB (20 ng/ml)-treated VSMCs, and an equal amount of protein from each condition was analyzed for DNA binding activity using a 32P-labeled putative STAT-binding sequence from rat cyclin D1 promoter as a probe. To detect the presence of STAT-5B in the protein-DNA complexes, anti-STAT-5B antibodies (2 μg) were added to the reaction mix and incubated for an additional 3 hours on ice. C: ChIP was performed with control and various time periods of PDGF-BB-treated VSMCs using anti-STAT-5B antibodies, and the resulting DNA fragments were subjected to PCR amplification using primers spanning −1013 to −411 region of rat cyclin D1 promoter. *P < 0.01 versus control; **P < 0.01 versus PDGF-BB treatment alone.
Figure 5.
Cyclin D1 siRNA attenuates PDGF-BB-induced cyclin D1 expression, CDK4 activity, and Rb protein phosphorylation in VSMCs and their growth and motility. VSMCs were transfected with scrambled control or cyclin D1 siRNA, quiesced, and treated with and without PDGF-BB (20 ng/ml) for 16 hours to measure cyclin D1 expression, CDK4 activity, Rb protein phosphorylation, and CDK4 levels: 24 hours to measure DNA synthesis, 72 hours to count cell numbers, and 24 hours to measure cell migration. A and B: Cyclin D1 expression (A), CDK4 activity (A), Rb protein phosphorylation (B), and CDK4 levels (A and B) were measured as described above in Figure 3. C and D: DNA synthesis (C) and cell numbers (D) were measured as described in Figure 2. E: Cell motility was determined by wounding assay. The bar graphs represent the quantitative analysis of three independent experiments. *P < 0.01 versus control; **P < 0.01 versus PDGF-BB treatment alone.
STAT-5B Mediates Balloon Injury-Induced Cyclin D1 Expression, CDK4 Activity, Rb Protein Phosphorylation, SMC Migration from Media to Intima, and Their Proliferation in Intima in Rat Carotid Artery
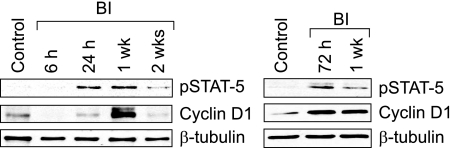
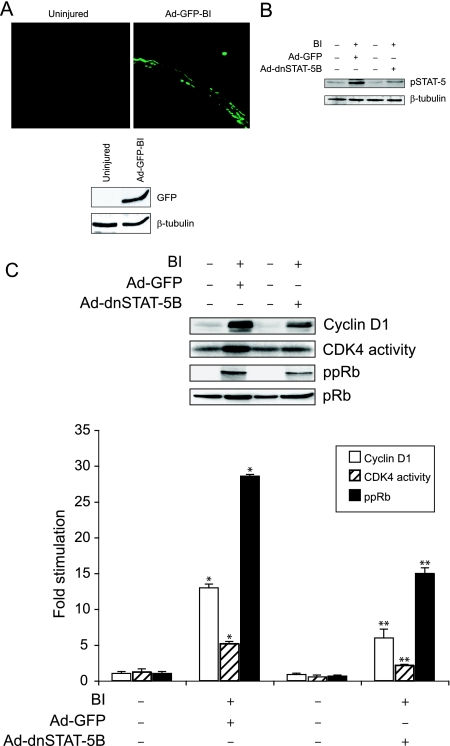
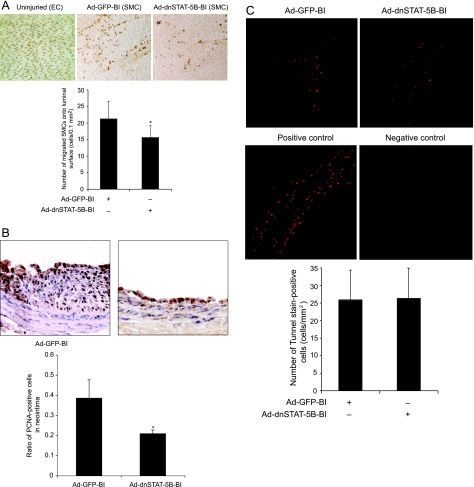
SMC migration from media to intima and their proliferation in intima play an important role in neointima formation.3,4,5,13,14,30,31,40 Maximum aortic SMC proliferation occurs between 3 and 7 days after balloon injury with peak DNA synthesis in the media and intima after the 2nd and 4th days, respectively.13,14,40,41,42,43 Because blockade of STAT-5B suppressed both PDGF-BB- and thrombin-induced VSMC growth and motility (present study),29 we next examined its role in SMC migration and proliferation in response to injury in vivo. Because cyclin D1 expression appears to be linked to VSMC growth and motility, we first studied a time course effect of balloon injury on cyclin D1 levels. At various times after balloon injury, the left uninjured and the right injured common carotid arteries were isolated, and an equal amount of protein from these tissues was analyzed by Western blotting for STAT-5 phosphorylation level and cyclin D1 expression using their specific antibodies. Balloon injury induced tyrosine phosphorylation of STAT-5 in arteries with threefold to fourfold increases at 24 hours and 1 week after injury (Figure 6). At 2 weeks after balloon injury, the STAT-5 tyrosine phosphorylation levels returned almost to basal levels in injured arteries. A fivefold increase in cyclin D1 expression was observed at 1 week after balloon injury, and by 2 weeks these levels returned to basal levels (Figure 6). In another time course experiment that includes 3-day and 1-week time points, a twofold increase in cyclin D1 expression was observed at 3 days after balloon injury as compared with its levels in control uninjured arteries. These results reveal that balloon injury-induced STAT-5 tyrosine phosphorylation precedes cyclin D1 expression. Based on these findings, we have chosen 1-week time point to test the role of STAT-5B on balloon-injured cyclin D1 levels. To ensure that adenovirus-mediated gene transfer leads to its sustained expression, balloon-injured arteries were transduced with Ad-GFP (1010 pfu/ml) and isolated 1 week after injury, and then either cryostat cross sections were made and examined for green fluorescence protein expression or extracts were prepared and analyzed by Western blotting for GFP using its specific antibodies. As shown in Figure 7A, transduction of Ad-GFP into balloon-injured arteries led to substantial expression of GFP even after 1 week of injury. Based on this observation, we next studied the effect of Ad-dnSTAT5B on balloon injury-induced vascular wall remodeling. One week after balloon injury, the left uninjured and the right injured Ad-GFP- or Ad-dnSTAT-5B-transduced common carotid arteries were isolated, extracts were prepared, and an equal amount of protein from each condition was analyzed for tyrosine-phosphorylated STAT-5 levels, cyclin D1 expression, CDK4 activity, and Rb protein phosphorylation as described above in VSMCs. Tyrosine phosphorylation of STAT-5 was found to be increased in balloon-injured and Ad-GFP-transduced arteries compared with uninjured arteries, and adenovirus-mediated expression of dnSTAT-5B blunted this effect (Figure 7B). Similarly, increases in cyclin D1 expression, CDK4 activity, and Rb protein phosphorylation were observed in injured Ad-GFP-transduced arteries as compared with uninjured arteries, and adenovirus-mediated expression of dnSTAT-5B significantly suppressed these effects (Figure 7C). To test the role of STAT-5B in injury-induced SMC migration from media to intima and their proliferation in intima, 4 days and 2 weeks after balloon injury, the right injured and Ad-GFP- or Ad-dnSTAT-5B-transduced arteries and the left uninjured common carotid arteries were isolated and fixed. To measure SMC migration, arteries were opened longitudinally, stained with anti-histone antibodies, and the histone-positive cells were counted. For determination of SMC proliferation, cross sections were made, stained with anti-PCNA antibodies followed by counter staining with hematoxylin, and the ratio of the PCNA-positive cells was determined. Increased SMC migration from media to intimal region occurred in response to balloon injury, and this effect was inhibited by dnSTAT-5B to ∼30% (Figure 8A). In regard to SMC growth, ∼35% of cells were found to be PCNA-positive in the neointimal region of balloon-injured and Ad-GFP-transduced arteries (Figure 8B). In contrast, only ∼15 to 18% of cells were found to be PCNA-positive in neointimal region of injured and Ad-dnSTAT-5B-transduced arteries, suggesting a 50% decrease in SMC growth (Figure 8B). To determine whether the decrease in the PCNA-positive cells in neointimal region is attributable to increased cell death by the suppression of the STAT-5B signaling, we next measured apoptosis in the cryostat cross sections of balloon-injured and Ad-GFP- or Ad-dnSTAT-5B-transduced arteries using a TUNEL assay. TUNEL-positive cells were present in balloon-injured, Ad-GFP- and Ad-dnSTAT-5B-transduced arteries, but the difference in the number of positive cells between Ad-GFP- and Ad-dnSTAT-5B-transduced arteries was statistically insignificant (Figure 8C). This finding clearly infers that suppression of STAT-5B signaling by adenovirus-mediated expression of its dominant-negative mutant did not cause an additional effect on the observed cell death while causing significant blockade of cell proliferation and motility. Together, these results demonstrate that blockade of STAT-5B signaling inhibits SMC migration from media to intima and their neointimal proliferation by ∼30% and 50%, respectively.
Figure 6.
Balloon injury induces STAT-5 tyrosine phosphorylation and cyclin D1 expression in a time-dependent manner in rat carotid artery. At various times after balloon injury, rats were sacrificed, the injured right common carotid arteries and uninjured left common carotid arteries were dissected out, and tissue extracts were prepared. An equal amount of protein from each sample was analyzed for STAT-5 tyrosine phosphorylation and cyclin D1 levels using their specific antibodies. The blots were reprobed with anti-β-tubulin antibodies for normalization. For time points, six rats were used, and arteries from two rats were pooled.
Figure 7.
Blockade of STAT-5B signaling suppresses balloon injury-induced STAT-5B phosphorylation, cyclin D1 expression, CDK4 activity, and Rb protein phosphorylation in the arteries. Soon after balloon injury, the rats received adenovirus expressing either GFP or dnSTAT-5B by infusion into the injured arteries. One week after balloon injury, rats were sacrificed, and the injured right common carotid arteries and uninjured left common carotid arteries were dissected out; either cryostat cross sections were made, or tissue extracts were prepared. A: Cryostat cross sections were fixed and examined for green fluorescence protein expression (top), or an equal amount of protein from the same group of arteries was analyzed by Western blotting for GFP using its specific antibodies. B: An equal amount of protein from uninjured and balloon-injured Ad-GFP- or Ad-dnSTAT-5B-transduced arteries was analyzed for STAT-5 phosphorylation using its phosphospecific antibodies. The blot was reprobed with anti-tubulin antibodies for normalization. C: An equal amount of protein from the samples described in B was analyzed for cyclin D1 expression, CDK4 activity, and Rb protein phosphorylation as described in Figure 3. The bar graph in C represents the quantitative analysis of three independent experiments. *P < 0.01 versus control; **P < 0.01 versus PDGF-BB treatment alone (n = 8).
Figure 8.
Blockade of STAT-5B signaling suppresses balloon injury-induced SMC migration from media to intima and their neointimal proliferation without affecting cell death. All treatment conditions were the same as in Figure 6 except that 4 days and 1 week after balloon injury rats were sacrificed, arteries were isolated and fixed, and SMC migration from media to intima, SMC neointimal proliferation, and apoptosis were measured as described in Materials and Methods. A: For the measurement of SMC migration, arteries were opened longitudinally, stained for nucleus using anti-histone antibodies (top), and the histone-positive cells in the luminal region were counted (bottom). B: Top panel shows the representative pictures of balloon-injured Ad-GFP- or Ad-dnSTAT-5B-transduced carotid artery sections that were stained for PCNA. The bar graph at the bottom shows the quantitative analysis of the PCNA-positive cells counted in 10 randomly selected fields of the immunostained sections of the balloon-injured Ad-GFP- or Ad-dnSTAT-5B-transduced arteries. C: Top panel shows the representative pictures of balloon-injured Ad-GFP- or Ad-dnSTAT-5B-transduced artery cross sections that were stained with TUNEL kit for detection of apoptosis. The bar graph at the bottom shows the quantitative analysis of the TUNEL-positive cells counted in 12 randomly selected fields of the balloon-injured Ad-GFP- or Ad-dnSTAT-5B-transduced arteries. *P < 0.05 versus Ad-GFP (n = 6).
STAT-5B Mediates Injury-Induced Neointima Formation
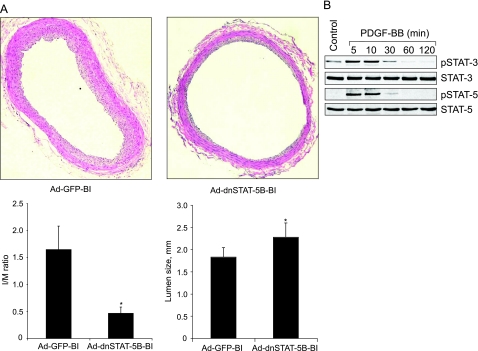
We next studied the effect of dnSTAT-5B on balloon injury-induced neointima formation. Right common carotid arteries that were balloon-injured and transduced with Ad-GFP or Ad-dnSTAT-5B (1010 pfu/ml) were dissected out 2 weeks after injury, fixed, sectioned, and stained with H&E, and the intimal (I) to medial (M) area ratios and the lumen size were determined by morphometric analysis. As shown in Figure 9A, substantial neointima formation occurred in injured and Ad-GFP-transduced arteries. Infusion of adenovirus harboring dnSTAT-5B into injured arteries inhibited neointima formation by ∼50% as measured by I:M ratio and increased lumen size by 24% (Figure 9A). These results suggest that STAT-5B is involved in vascular wall remodeling in response to injury and plays an important role in neointima formation. To extrapolate the relevance of these findings to vascular wall remodeling in humans, we have also studied the effect of PDGF-BB on activation of various STATs in HASMCs. Western blot analysis of an equal amount of protein from control and various time periods of PDGF-BB (20 ng/ml)-treated HASMCs using phosphotyrosine-specific antibodies of STAT-3 and STAT-5 showed a time-dependent increase in their phosphorylation (Figure 9B). This result clearly suggests the existence of a similar mechanism of activation of STAT-5 in HASMCs as well.
Figure 9.
A: Blockade of STAT-5B signaling inhibits balloon injury-induced neointima formation. All treatment conditions were the same as in Figure 6 except that 2 weeks after balloon injury, rats were sacrificed, arteries were isolated and fixed, and cross-sections were made and stained with H&E. After morphometry analysis, I:M ratios and lumen sizes were calculated. Top shows the representative pictures of balloon-injured Ad-GFP- or Ad-dnSTAT-5B-transduced carotid artery cross sections that were stained with H&E. The bar graphs at the bottom show the quantitative analysis of the I/M ratios and lumen sizes of the balloon-injured Ad-GFP- or Ad-dnSTAT-5B-transduced rat carotid arteries. *P < 0.05 versus Ad-GFP (n = 6). B: PDGF-BB stimulates tyrosine phosphorylation of both STAT-3 and STAT-5 in HASMCs. Quiescent HASMCs were treated with and without PDGF-BB (20 ng/ml) for the indicated time periods, and cell extracts were prepared. Cell extracts containing an equal amount of protein from control and each time period of PDGF-BB treatment were analyzed by Western blotting for tyrosine phosphorylation of STAT-3 and STAT-5 using their phosphospecific antibodies.
Discussion
The major findings of the present study are as follows. i) PDGF-BB activated STAT-5 in VSMCs as measured by its tyrosine phosphorylation, DNA binding, and reporter gene activity. ii) PDGF-BB induced cyclin D1 expression, CDK4 activity, and Rb protein phosphorylation in VSMCs as well as the growth and motility of these cells. iii) Blockade of STAT-5B but not STAT-5A by adenovirus-mediated expression of their dominant-negative mutants suppressed PDGF-BB-induced cyclin D1 expression, CDK4 activity, and Rb protein phosphorylation in VSMCs as well as the growth and motility of these cells. iv) siRNA-targeted depletion of cyclin D1 expression also inhibited PDGF-BB-induced CDK4 activity and Rb protein phosphorylation, growth, and motility of VSMCs. v) Increased cyclin D1 expression, CDK4 activity, and Rb protein phosphorylation occurred in balloon-injured arteries compared with uninjured control arteries. vi) Similarly, increased SMC migration from media to intima, and their proliferation in intima resulting in neointima formation, was observed after balloon injury. vii) Transduction of adenovirus harboring dnSTAT-5B into arteries immediately after balloon injury attenuated injury-induced increases in cyclin D1 expression, CDK4 activity, Rb protein phosphorylation, and SMC migration from media to intima and their proliferation in intima resulting in reduced neointima formation.
STAT-5 has been reported to be involved in the regulation of epithelial cell survival and proliferation.23 It has been reported that, by mediating cyclin D1 expression, STAT-5 plays a role in cytokine-induced hematopoietic cell growth.35 Cyclin D1 association is required for CDK4 activity,37,38 and CDK4, in turn, phosphorylates Rb protein.39 The phosphorylated Rb protein dissociates from and thereby facilitates activation of transcriptional factors, E2Fs, which in their turn influence the expression of genes required for cell-cycle progression.38,39 The finding that blockade of STAT-5B completely suppresses the most potent vascular mitogen, PDGF-BB-induced cyclin D1 expression, CDK4 activity, and Rb protein phosphorylation, indicates that STAT-5B plays an important role in the regulation of VSMC growth. A large number of molecules account for increased SMC growth in the neointimal region.1,2,3,13,14 Because suppression of STAT-5B signaling abolishes injury-induced cyclin D1 expression, CDK4 activity, and Rb protein phosphorylation in the arteries, it is likely that STAT-5B is also an important regulator of SMC growth in vivo.
The other notable finding is that blockade of STAT-5B signaling inhibits PDGF-BB-induced VSMC motility. Similarly, down-regulation of cyclin D1 levels attenuated PDGF-BB-induced VSMC migration. A role for cyclin D1 in the regulation of cell migration has also been demonstrated.44,45 In addition, we recently reported that cyclin D1 mediates SMC migration induced by interleukin-6 and balloon injury.46 These findings suggest that cyclin D1, in addition to its role in cell-cycle regulation, is involved in the modulation of cell motility. Although cyclin D1 seems to be one of the molecules required for cell-cycle progression, and possibly cell migration, other molecules whose actions are required for cell proliferation and migration may also be targets of STAT-5B in VSMCs. Recent work from our laboratory showed that STAT-5B mediates thrombin-induced Hsp27 and FGF-2 expression leading to VSMC growth and motility.29 In fact, PDGF-BB also increased FGF-2 expression in a STAT-5B-dependent manner in VSMCs (V.K.-S. and G.N.R., unpublished observations). These observations may lead to the speculation that STAT-5B influences the transcription of several genes whose products may be involved in the signaling events underlying cell proliferation and/or migration. Some recent studies have reported that cyclin D1 interacts with transcriptional factors and influences gene regulation.47,48 Therefore, the other scenario by which cyclin D1 could be involved in the regulation of SMC motility is via its possible role in the transcriptional induction of FGF-2 expression in response to PDGF-BB in vitro and injury in vivo. However, further studies are required to address these assumptions.
Earlier studies from other laboratories have shown that STAT-3 plays a role in balloon injury-induced neointima formation.49 Previously, we have demonstrated that STAT-3, via targeting the expression of cPLA2, mediates PDGF-BB-induced VSMC growth and motility.16,17 Our work also revealed a role for STAT-3-cPLA2 axis in thrombin-induced VSMC motility.18 In addition, the recent studies from our laboratory showed that thrombin-induced VSMC growth and motility require the activation of STAT-5B and its association with STAT-3.29 Based on these observations, it is also possible that STAT-5B in association with STAT-3 targets the induction of expression of a number of genes such as cPLA2, cyclin D1, and FGF-2 and thereby influences the growth and motility of SMCs in response to PDGF-BB in vitro and balloon injury in vivo. Because PDGF-BB activated STAT-5 and STAT-3 in HASMCs, a similar role for these transcriptional factors can also be extrapolated to human vascular wall remodeling. Therefore, therapeutics targeting both STAT-5B and STAT-3 may be more effective in the treatment of proliferative vascular lesions such as restenosis.
Acknowledgments
We thank Dr. J.C. Lacal from the Instituto de Investigaciones Biomedicas, Consejo Superior de Investigaciones Cientificas, Madrid, Spain, for providing us with 1× Sp1GLECAT plasmid.
Footnotes
Address reprint requests to Gadiparthi N. Rao, Ph.D., Department of Physiology, The University of Tennessee Health Science Center, 894 Union Ave., Memphis, TN 38163. E-mail: grao@physio1.utmem.edu.
Supported by the National Institutes of Health (grant HL64165 to G.N.R.).
V.K.-S. and D.W. contributed equally to this work.
References
- Ross R. Atherosclerosis—an inflammatory disease. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- Hansson GK, Libby P, Schonbeck U, Yan ZQ. Innate and adaptive immunity in the pathogenesis of atherosclerosis. Circ Res. 2002;91:281–291. doi: 10.1161/01.res.0000029784.15893.10. [DOI] [PubMed] [Google Scholar]
- Schwartz SM, deBlois D, O’Brien ER. The intima. Soil for atherosclerosis and restenosis. Circ Res. 1995;77:445–465. doi: 10.1161/01.res.77.3.445. [DOI] [PubMed] [Google Scholar]
- Itoh H, Mukoyama M, Pratt RE, Gibbons GH, Dzau VJ. Multiple autocrine growth factors modulate vascular smooth muscle cell growth response to angiotensin II. J Clin Invest. 1993;91:2268–2274. doi: 10.1172/JCI116454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent MA, Karnovsky MJ, Edelman ER. Vascular cell-derived heparan sulfate shows coupled inhibition of basic fibroblast growth factor binding and mitogenesis in vascular smooth muscle cells. Circ Res. 1993;73:1051–1060. doi: 10.1161/01.res.73.6.1051. [DOI] [PubMed] [Google Scholar]
- Rauch BH, Millette E, Kenagy RD, Daum G, Fischer JW, Clowes AW. Syndecan-4 is required for thrombin-induced migration and proliferation in human vascular smooth muscle cells. J Biol Chem. 2005;280:17507–17511. doi: 10.1074/jbc.M410848200. [DOI] [PubMed] [Google Scholar]
- Abid MR, Yano K, Guo S, Patel VI, Shrikhande G, Spokes KC, Ferran C, Aird WC. Forkhead transcription factors inhibit vascular smooth muscle cell proliferation and neointimal hyperplasia. J Biol Chem. 2005;280:29864–29873. doi: 10.1074/jbc.M502149200. [DOI] [PubMed] [Google Scholar]
- Spinetti G, Wang M, Monticone R, Zhang J, Zhao D, Lakatta EG. Rat aortic MCP-1 and its receptor CCR2 increase with age and alter vascular smooth muscle cell function. Arterioscler Thromb Vasc Biol. 2004;24:1397–1402. doi: 10.1161/01.ATV.0000134529.65173.08. [DOI] [PubMed] [Google Scholar]
- Liu Z, Dronadula N, Rao GN. A novel role for nuclear factor of activated T cells in receptor tyrosine kinase and G protein-coupled receptor agonist-induced vascular smooth muscle cell motility. J Biol Chem. 2004;279:41218–41226. doi: 10.1074/jbc.M406917200. [DOI] [PubMed] [Google Scholar]
- Rao GN, Berk BC. Active oxygen species stimulate vascular smooth muscle cell growth and proto-oncogene expression. Circ Res. 1992;70:593–599. doi: 10.1161/01.res.70.3.593. [DOI] [PubMed] [Google Scholar]
- Ahn JD, Morishita R, Kaneda Y, Lee SJ, Kwon KY, Choi SY, Lee KU, Park JY, Moon IJ, Park JG, Yoshizumi M, Ouchi Y, Lee IK. Inhibitory effects of novel AP-1 decoy oligonucleotides on vascular smooth muscle cell proliferation in vitro and neointimal formation in vivo. Circ Res. 2002;90:1325–1332. doi: 10.1161/01.res.0000023200.19316.d5. [DOI] [PubMed] [Google Scholar]
- Johnson TW, Wu YX, Herdeg C, Baumbach A, Newby AC, Karsch KR, Oberhoff M. Stent-based delivery of tissue inhibitor of metalloproteinase-3 adenovirus inhibits neointimal formation in porcine coronary arteries. Arterioscler Thromb Vasc Biol. 2005;25:754–759. doi: 10.1161/01.ATV.0000157582.33180.a9. [DOI] [PubMed] [Google Scholar]
- Morishita R, Gibbons GH, Horiuchi M, Ellison KE, Nakama M, Zhang L, Kaneda Y, Ogihara T, Dzau VJ. A gene therapy strategy using a transcription factor decoy of the E2F binding site inhibits smooth muscle proliferation in vivo. Proc Natl Acad Sci USA. 1995;92:5855–5859. doi: 10.1073/pnas.92.13.5855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner FC, Boehm M, Akyurek LM, San H, Yang ZY, Tashiro J, Nabel GJ, Nabel EG. Differential effects of the cyclin-dependent kinase inhibitors p27kip1, p21cip1 and p16ink4 on vascular smooth muscle cell proliferation. Circulation. 2000;101:2022–2025. doi: 10.1161/01.cir.101.17.2022. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhang C, Dronadula N, Li Q, Rao GN. Blockade of nuclear factor of activated T cells activation signaling suppresses balloon injury-induced neointima formation in a rat carotid artery model. J Biol Chem. 2005;280:14700–14708. doi: 10.1074/jbc.M500322200. [DOI] [PubMed] [Google Scholar]
- Yellaturu CR, Rao GN. Cytosolic phospholipase A2 is an effector of janus-activated kinase/signal transducers and activators of transcription signaling and is involved in platelet-derived growth factor BB-induced growth in vascular smooth muscle cells. J Biol Chem. 2003;278:9986–9992. doi: 10.1074/jbc.M211276200. [DOI] [PubMed] [Google Scholar]
- Neeli I, Liu Z, Dronadula N, Ma ZA, Rao GN. An essential role of Jak-2/STAT-3/cPLA2 axis in platelet-derived growth factor BB-induced vascular smooth muscle cell motility. J Biol Chem. 2004;279:46122–46128. doi: 10.1074/jbc.M406922200. [DOI] [PubMed] [Google Scholar]
- Dronadula N, Liu Z, Wang C, Cao H, Rao GN. STAT-3-dependent expression of cPLA2 is required for thrombin-induced vascular smooth muscle cell motility. J Biol Chem. 2005;280:3112–3120. doi: 10.1074/jbc.M409739200. [DOI] [PubMed] [Google Scholar]
- Darnell JE, Jr, Kerr IM, Stark GR. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994;264:1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Horvath CM, Wen Z, Darnell JE., Jr A STAT protein domain that determines DNA sequence recognition suggests a novel DNA-binding domain. Genes Dev. 1995;9:984–994. doi: 10.1101/gad.9.8.984. [DOI] [PubMed] [Google Scholar]
- Teglund S, McKay C, Schuetz E, vanDeursen JM, Stravopodis D, Wang D, Brown M, Bodner S, Grosveld G, Ihle JN. Stat5a and Stat5b proteins have essential and nonessential, or redundant, roles in cytokine responses. Cell. 1998;93:841–850. doi: 10.1016/s0092-8674(00)81444-0. [DOI] [PubMed] [Google Scholar]
- Wu YY, Bradshaw RA. Activation of the Stat3 signaling pathway is required for differentiation by interleukin-6 in PC12–E2 cells. J Biol Chem. 2000;275:2147–2156. doi: 10.1074/jbc.275.3.2147. [DOI] [PubMed] [Google Scholar]
- Iavnilovitch E, Groner B, Barash I. Overexpression and forced activation of stat5 in mammary gland of transgenic mice promotes cellular proliferation, enhances differentiation, and delays postlactational apoptosis. Mol Cancer Res. 2002;1:32–47. [PubMed] [Google Scholar]
- Bromberg JF, Wrzeszczynska MH, Devgan G, Zhao Y, Pestell RG, Albanese C, Darnell JE., Jr Stat3 as an oncogene. Cell. 1999;98:295–303. doi: 10.1016/s0092-8674(00)81959-5. [DOI] [PubMed] [Google Scholar]
- Cui Y, Riedlinger G, Miyoshi K, Tang W, Li C, Deng CX, Robinson GW, Hennighausen L. Inactivation of Stat5 in mouse mammary epithelium during pregnancy reveals distinct functions in cell proliferation, survival, and differentiation. Mol Cell Biol. 2004;24:8037–8047. doi: 10.1128/MCB.24.18.8037-8047.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udy GB, Towers RP, Snell RG, Wilkins RJ, Park SH, Ram PA, Waxman DJ, Davey HW. Requirement of STAT5b for sexual dimorphism of body growth rates and liver gene expression. Proc Natl Acad Sci USA. 1997;94:7239–7244. doi: 10.1073/pnas.94.14.7239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephanou A, Brar BK, Scarabelli TM, Jonassen AK, Yellon DM, Marber MS, Knight RA, Latchman DS. Ischemia-induced STAT-1 expression and activation play a critical role in cardiomyocyte apoptosis. J Biol Chem. 2000;275:10002–10008. doi: 10.1074/jbc.275.14.10002. [DOI] [PubMed] [Google Scholar]
- Huang YQ, Li JJ, Karpatkin S. Thrombin inhibits tumor cell growth in association with upregulation of p21waf/cip1 and caspases via a p53-independent STAT-1-dependent pathway. J Biol Chem. 2000;275:6462–6468. doi: 10.1074/jbc.275.9.6462. [DOI] [PubMed] [Google Scholar]
- Cao H, Dronadula N, Rizvi F, Li Q, Gerthoffer WT, Rao GN. A novel role for STAT-5B in the regulation of Hsp27-FGF-2 axis facilitating thrombin-induced vascular smooth muscle cell DNA synthesis and motility. Circ Res. 2006;98:913–922. doi: 10.1161/01.RES.0000216954.55724.a2. [DOI] [PubMed] [Google Scholar]
- Jackson CL, Raines EW, Ross R, Reidy MA. Role of endogenous platelet-derived growth factor in arterial smooth muscle cell migration after balloon catheter injury. Arterioscler Thromb. 1993;13:1218–1226. doi: 10.1161/01.atv.13.8.1218. [DOI] [PubMed] [Google Scholar]
- Bendeck MP, Zempo N, Clowes AW, Galardy RE, Reidy MA. Smooth muscle cell migration and matrix metalloproteinase expression after arterial injury in the rat. Circ Res. 1994;75:539–545. doi: 10.1161/01.res.75.3.539. [DOI] [PubMed] [Google Scholar]
- Benitah SA, Valeron PF, Rui H, Lacal JC. STAT5a activation mediates the epithelial to mesenchymal transition induced by oncogenic RhoA. Mol Biol Cell. 2003;14:40–53. doi: 10.1091/mbc.E02-08-0454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitazawa S, Kitazawa R, Maeda S. Transcriptional regulation of rat cyclin D1 gene by CpG methylation status in promoter region. J Biol Chem. 1999;274:28787–28793. doi: 10.1074/jbc.274.40.28787. [DOI] [PubMed] [Google Scholar]
- Wang D, Stravopodis D, Teglund S, Kitazawa J, Ihle JN. Naturally occurring dominant negative variants of Stat5. Mol Cell Biol. 1996;16:6141–6148. doi: 10.1128/mcb.16.11.6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura I, Kitamura T, Wakao H, Tanaka H, Hashimoto K, Albanese C, Downward J, Pestell RG, Kanakura Y. Transcriptional regulation of the cyclin D1 promoter by STAT5: its involvement in cytokine-dependent growth of hematopoietic cells. EMBO J. 1999;18:1367–1377. doi: 10.1093/emboj/18.5.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao GH, Gallagher R, Shetler J, Skele K, Altomare DA, Pestell RG, Jhanwar S, Testa JR. The NF2 tumor suppressor gene product, merlin, inhibits cell proliferation and cell cycle progression by repressing cyclin D1 expression. Mol Cell Biol. 2005;25:2384–2394. doi: 10.1128/MCB.25.6.2384-2394.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace M, Ball KL. Docking-dependent regulation of the Rb tumor suppressor protein by CDK4. Mol Cell Biol. 2004;24:5606–5619. doi: 10.1128/MCB.24.12.5606-5619.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ, Robersts JM. Living with or without cyclins and cyclin-dependent kinases. Genes Dev. 2004;18:2699–2711. doi: 10.1101/gad.1256504. [DOI] [PubMed] [Google Scholar]
- Weinberg RA. The retinoblastoma protein and cell cycle control. Cell. 1995;81:323–330. doi: 10.1016/0092-8674(95)90385-2. [DOI] [PubMed] [Google Scholar]
- Boehm M, Nabel EG. Cell cycle and cell migration. New pieces to the puzzle. Circulation. 2001;103:2879–2881. doi: 10.1161/01.cir.103.24.2879. [DOI] [PubMed] [Google Scholar]
- Miano JM, Kelly LA, Artacho CA, Nuckolls TA, Piantedosi R, Blaner WS. All-trans-retinoic acid reduces neointimal formation and promotes favorable geometric remodeling of the rat carotid artery after balloon withdrawal injury. Circulation. 1998;98:1219–1227. doi: 10.1161/01.cir.98.12.1219. [DOI] [PubMed] [Google Scholar]
- Vlasic N, Ramos L, Mittelman A, Stemerman MB. Inhibition of smooth muscle cell proliferation and DNA synthesis by Vasoprin—a biologic response modifier. Atherosclerosis. 1993;104:79–85. doi: 10.1016/0021-9150(93)90178-w. [DOI] [PubMed] [Google Scholar]
- Clowes AW, Reidy MA, Clowes MM. Kinetics of cellular proliferation after arterial injury. I. Smooth muscle growth in the absence of endothelium. Lab Invest. 1983;49:327–333. [PubMed] [Google Scholar]
- Neumeister P, Pixley FJ, Xiong Y, Xie H, Wu K, Ashton A, Cammer M, Chan A, Symons M, Stanley ER, Pestell RG. Cyclin D1 governs adhesion and motility of macrophages. Mol Cell Biol. 2003;14:2005–2015. doi: 10.1091/mbc.02-07-0102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Wang C, Jiao X, Lu Y, Fu M, Quong AA, Dye C, Yang J, Dai M, Ju X, Zhang X, Li A, Burbelo P, Stanley ER, Pestell RG. Cyclin D1 regulates cellular migration through the inhibition of thrombospondin 1 and ROCK signaling. Mol Cell Biol. 2006;26:4240–4256. doi: 10.1128/MCB.02124-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D, Liu Z, Li Q, Karpurapu M, Kundumani-Sridharan V, Cao H, Dronadula N, Rizvi F, Bajpai AK, Zhang C, Muller-Newen G, Harris KW, Rao GN. An essential role for gp130 in neointima formation following arterial injury. Circ Res. 2007;100:807–816. doi: 10.1161/01.RES.0000261350.61711.9e. [DOI] [PubMed] [Google Scholar]
- Bienvenu F, Barre B, Giraud S, Avril S, Coqueret O. Transcriptional regulation by a DNA-associated form of cyclin D1. Mol Biol Cell. 2005;16:1850–1858. doi: 10.1091/mbc.E04-08-0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M, Wang C, Rao M, Wu X, Bouras T, Zhang X, Li Z, Jiao X, Yang J, Li A, Perkins ND, Thimmapaya B, Kung AL, Munoz A, Giordano A, Lisanti MP, Pestell RG. Cyclin D1 represses p300 transactivation through a cyclin-dependent kinase-independent mechanism. J Biol Chem. 2005;280:29728–29742. doi: 10.1074/jbc.M503188200. [DOI] [PubMed] [Google Scholar]
- Shibata R, Kai H, Seki Y, Kato S, Wada Y, Hanakawa Y, Hashimoto K, Yoshimura A, Imaizumi T. Inhibition of STAT3 prevents neointima formation by inhibiting proliferation and promoting apoptosis of neointimal smooth muscle cells. Hum Gene Ther. 2003;14:601–610. doi: 10.1089/104303403321618128. [DOI] [PubMed] [Google Scholar]