Abstract
We recorded extracellular single unit discharges of globus pallidus internal segment (GPi) neurons in monkeys performing a visually-driven forearm rotation movement task in order to quantify how discharge patterns changed in relation to kinematic parameters. Subjects grasped a handle that rotated about its axis while facing a video screen displaying visual targets. Continuous visual feedback of handle rotation position was provided. Monkeys generated forearm rotation movements of ±35° and ±70° amplitude in order to align the cursor and targets. Trial records were aligned to forearm rotation onset in order to compare the discharge patterns that were associated with movements of different amplitudes, velocities, and directions. In addition, we quantified the depth of modulation of neuronal discharge associated with movements generated in two different task phases. Comparisons of discharge patterns were made between the visually-guided, rewarded phase (“cued movements”) and the self-paced, unrewarded phase that returned the monkey to the task start position (“return movements”) by quantifying the goodness of fit between neuronal discharge during cued and return movements.
Our analyses revealed no systematic relationship between the depth of modulation of GPi neurons and forearm rotation amplitude, direction, or velocity. Further, comparisons between the two behavioral contexts revealed a systematic attenuation of modulation that could not be attributed to differences in movement velocity. Collectively, these findings suggest that the GPi neurons that we studied were not significantly involved in mediating movement kinematics, but may have instead been instrumental in the processing of information about the behavioral context during which movements were generated.
Classification Terms: sensorimotor integration, basal ganglia, globus pallidus internal segment, electrophysiology, kinematics, subcortical loops
1. Introduction
Our knowledge of how the various basal ganglia nuclei process information about sensory and motor variables during different task conditions is growing, but still remains rather limited. Pallidal neuron populations having task-selective discharge patterns have been identified by other laboratories in association with sensory-driven eye and limb movement tasks. For instance, context-dependent activity has been observed during tasks in which sensory cues were manipulated during task execution (Jaeger et al., 1993; Mink and Thach, 1991b; Mushiake and Strick, 1995; Turner and Anderson, 2005) and when tasks were structured to allow the prediction of pending movements (Brotchie et al., 1991). However, the characterization of context dependent firing of pallidal neurons in relation to kinematic parameters and reward expectation has only partially been explored.
In other BG regions, including the substantia nigra (pars compacta and pars reticulata) and caudate nucleus, neurons have been shown to be modulated differentially in relation to reward expectation, to cues that predict reward, or to the hedonic value of the reward itself (Hikosaka et al., 1989; Hollerman and Schultz, 1998; Hollerman et al., 1998; Kawagoe et al., 1998; Kawagoe et al., 2004; Lauwereyns et al., 2002; Schultz, 1995; Schultz, 1998; Schultz et al., 1993; Schultz et al., 1992; Takikawa et al., 2004; Wickens et al., 2003). Similar reports suggest that these changes in neuronal activity could also result from differences in cognitive demands or in aspects of the behavioral tasks related to movement sequencing (e.g. moving to a visible versus a remembered target location)(Mushiake and Strick, 1995). Alternatively, some of these differences could arise simply as a consequence of differences in kinematic aspects of forearm rotation in different task settings.
Despite these accounts, our initial observation of context dependence was unexpected, since we had not originally designed our behavioral tasks to elucidate contextual differences (Gdowski et al., 2001). These early findings let us to expand upon those differences in the present study. Most prior studies that have examined movement-related GPi discharge (Jaeger et al., 1993; Mink and Thach, 1991a; Mink and Thach, 1991b; Mushiake and Strick, 1995; Turner and Anderson, 1997) have not emphasized differences in neuronal activity related to the behavioral conditions during which movements were generated. Our behavioral tasks were structured in order to include sensory and motor variables that others have used for the characterization of GPi activity, but also to require animals to make similar movements in during two different behavioral contexts. Accordingly, monkeys learned to generate rotational forearm movements as instructed by visual cues (See Figure 1). Visually cued trials were always initiated with the lighting of a central fixation target followed by an additional instruction target. The animal would acquire each of these targets by rotating a handle to align a cursor with the target. We found that after the instruction target was acquired and the reward was delivered, monkeys would reliably return the handle to the fixation target in anticipation of the next trial. In nearly all trials, this return movement occurred prior to the lighting of the fixation target. While this behavior is likely common to many studies, the neural activity associated with such self-paced movements has rarely been compared with the neural discharge that accompanies the visually cued phase of the task.
Figure 1.

Experimental apparatus and schematic of paradigm design. A) Monkeys sat in a primate chair grasping a handle that rotated about its axis. They faced a computer monitor that displayed a horizontal array of 5 red circular targets (See “face view”). Targets were separated by 35° increments of handle rotation. A green circular cursor on the screen provided continuous visual feedback about hand position. B) Instructional cues and behavioral contingencies are shown in correspondence with handle position (bottom). For simplicity, the target array is shown positioned vertically in the schematic time line instead of in the horizontal orientation as viewed by the monkey. Fixed and variable delay intervals are shown relative to measurable task events.
The goal of the current study was therefore to extend our initial descriptive report of context dependent firing patterns from a single animal by quantitatively comparing discharge during the cued and self-paced task phases in two additional animals. We hypothesized that differences in the kinematics of movements generated in the two conditions would account for the context dependent disparities in modulation. The two animals in the present study were also trained to perform variants of the task to enable assessment of other factors that we reasoned could account for the disparate discharge patterns such as differences in visual cues, reward expectation, or movement velocity. In the present study, we quantify the relationship between movement velocity and discharge of all task-modulated neurons and examine the influence of reward expectation upon a small sample of our neurons. We have also assessed the influence of the presence or absence of visual cues upon the modulation of pallidal neurons, a finding that is briefly addressed herein, but will be examined in greater depth in a separate publication. Lastly, we address whether neurons with similar task-related activity cluster together within the nucleus. Our study confirms the importance of the influence of behavioral context in influencing the modulation pattern of GPi neurons and unveils questions that need to be answered before a definitive role of GPi neurons during voluntary limb movement behaviors can be elucidated. This type of processing is a necessary step in selecting appropriate motor actions in relation to sensory cues and expected outcomes, a presumed role of the collective basal ganglia nuclei. The selective manipulation of variables that influence contextual responses of pallidal neurons is a current focus of our work.
2. Results
A total of 224 neurons were recorded from the internal pallidal segment during 160 electrode penetrations (n=156 Macaca mulatta P7F1; n=258, Macaca fasicularis L9D1; See Table 1). Of the 224 recorded GPi neurons, 82 neurons were classified as task-modulated (n=26 P7F1; n=56 L9D1) and could be definitively assigned to GPi. Task-modulation was determined by the presence of statistically significant changes (p<0.05, Student’s t-test) in discharge rates of either the cued or return limb-movement phases of the task. All task-modulated neuron responses were also evident by visual inspection of the averaged discharge record.
Table 1.
Cumulative Summary of Recorded GPi Neurons
| Monkey ID | P7F1 | L9D1 | Total |
|---|---|---|---|
| Total Neurons Sampled | 73 | 151 | 224 |
| Total Electrode Penetrations | 63 | 97 | 160 |
| Cued Movement Task Modulated Neurons | 25 [34.2] | 55 [36.4] | 80 [35.7] |
In addition to alignment with cued and return movement onsets, the spike trains from all 82 task-modulated GPi neurons were aligned with respect to other significant behavioral events within the trial including the presentation of the fixation target, instruction target, movement cue, and reward. Two of the task-modulated GPi neurons were driven only in association with the return movements and did not exhibit statistically significant changes in discharge rate in association with the cued movements and were thereby excluded from further analysis. Thus, in this paper we focus on the 80 neurons that exhibited changes in discharge during the cued movement phase of the trials in order to compare our data with published studies of GPi and to focus on the neuron type with the prevailing response pattern in our study. Of these 80 neurons, 39 (49%) were modulated by passive movements of the arm, forearm, wrist, hand, or fingers contralateral to the recording chamber. Of the remaining neurons that could not be driven by passive movements of the forelimb, approximately half (20/41) were located within 0.5mm of a passively modulated GPi neuron, a criterion used by others in similar studies for qualifying a neuron as likely to be located within an arm-input receiving area (Turner and Anderson, 2005).
Neurons with Increasing and Decreasing Responses
Representative discharge patterns from isolated GPi neurons P229 and P248 are shown in Figure 2. The two neurons depicted in this figure were significantly modulated in association with cued forearm rotation movements to each of the four targets (p<0.05, small sample test statistic t for difference between two means). Neuron P229 (A) had significant increases in discharge in association with cued movements to all four targets, but not during the return movements. We have observed such differences in GPi discharge previously, which we described as “context dependent” to denote that the neuronal response was contingent upon the behavioral setting during which movements were executed (Gdowski et al., 2001). As indicated in Table 2, 42 neurons (53%) were modulated like neuron P229 shown in Figure 2A, displaying statistically significant increases in discharge in association with the cued movements. On the other hand, only 11 (14%) GPi neurons decreased their discharge in association with cued movements (“decrease neurons”) like the neuron illustrated in Figure 2B. The remaining 27 neurons (34%) exhibited combinations of increases and decreases, either across targets, or during movements to a single target. Both neurons shown clearly illustrate the differences in modulation associated with cued and return movements in this task.
Figure 2.

Examples of context dependent discharge of two representative GPi neurons during visually-cued rotational forearm movements. A) Neuron P229 increased discharge in association with cued movements to all four targets but did not exhibit statistically significant changes in discharge in association with the self-paced movements that returned the handle to the center target (return movements). B) Neuron P248 paused in association with visually-cued movements to all four targets. Slight decreases in discharge rate are noted during the return movements, but these depths of modulation (DOM) were greatly attenuated when compared with those observed during cued movement. Binwidth = 30ms.
Table 2.
Summary of GPi Neuron Response Types
| Monkey ID | P7F1 | L9D1 | Total |
|---|---|---|---|
| Cued Responses | |||
| Increase | 13 | 29 | 42 |
| Decrease | 4 | 7 | 11 |
| Combination | 8 | 19 | 27 |
| Cued Response Total | 25 | 55 | 80 |
| Return Responses | |||
| Increase | 8 | 28 | 36 |
| Decrease | 3 | 1 | 4 |
| Combination | 0 | 0 | 0 |
| Return Response Total | 11 | 29 | 40 |
Analysis of Attenuation of DOM in Return vs. Cued Movements
We used a linear regression method to compare the magnitude of the neuronal responses associated with cued movements to those of return movements (see Methods for details). This analysis produced a gain measure, expressing the ratio between the cued and return modulation. Comparisons were made for each of the four targets, using both the paired cued and return movements for a given target (the paired analysis) and cued movements from one target that had the same amplitude and direction as the return movements from another target (e.g. counterclockwise cued movement from fixation target to −70° target and counterclockwise return movement from +70° to fixation target; the kinematically-similar analysis). The mean gain values (mean of variable a in linear regression calculation for all 4 targets) were calculated for each neuron for both paired and kinematically-similar analyses. Refer to (Gdowski et al., 2001) for further explanation of standard versus kinematically similar analyses.
These gain factors are summarized in Figure 3, which is a scatter plot of the mean gains for the kinematically similar movements (ordinate) plotted against the gains from the paired movements (abscissa). The histograms corresponding to each of these axes indicate that return movement modulation was consistently attenuated by 20 to 80% relative to the cued movement modulation, for both the paired (0.44±0.14, mean gain ± SD) and the kinematically similar (0.48±0.14, mean gain ± SD) analyses. The gains were distributed similarly for the two analyses, and all but 3 points (identified using open circles instead of plus symbols on the scatter plot) were located along the dashed line with unity slope, indicating that the gain or attenuation factor is a characteristic of each neuron, and is independent of the kinematics of the return movement itself. A linear fit of all points on the scatter plot (plus signs and open circles) yielded a slope of 0.76, with ρ = 0.75. Removal of the 3 points demarcated by open circles increased the slope of this line to 1.03 with ρ =0.91 suggesting near perfect correlation between gains in the two types of analyses for the majority of neurons.
Figure 3.

The relative gains obtained from quantitative comparison of cued and return movement DOM calculations obtained during standard (abscissa) and kinematically-similar (“kin sim”, ordinate) analyses reveals that return movement DOMs were attenuated by 20–80% relative to cued movement DOMs. The majority of neurons were modulated similarly during both types of analyses with the exception of the 3 neurons represented by open circles. A linear fit of all points on the scatter plot (plus signs and open circles) yielded a slope of 0.76, with ρ = 0.75 (gray line). Removal of the 3 neurons represented by open circles increased the slope of this line to 1.03 with ρ =0.91 (black line) suggesting near perfect correlation between gains in the two types of analyses for the majority of neurons. Histograms summarizing the distribution of gains and corresponding means ± SD calculated from standard and kinematically similar analysis are shown above and to the right of the scatter plot, respectively.
Evaluation of Amplitude, Direction, and Peak Velocity Upon Neuronal Response
Close inspection of the velocity traces in figure 2 reveals that the return movements were consistently slower than the cued movements. In order to exclude the possibility that the attenuation of depth of modulation during return movements was simply the result of decreased movement velocities, the data were further examined as shown in figures 4 and 5.
Figure 4.
Scatter plot summarizing the influence of peak velocity, amplitude, and direction of forearm movement upon DOM. Velocity is plotted along the abscissa with negative values assigned for counterclockwise rotation (−35°, −70°) movements. Peak velocity versus depth of modulation is shown for the task-modulated GPi neurons that were significantly modulated with exclusively increased (n=53) or decreased (n=11) firing for all four cued movements (±35°, ±70°). Each neuron is represented by 4 points joined by a line. The 4 points represent peak velocity associated with handle rotation movements to each of the 4 targets. Smaller amplitude movements to the near targets (±35°) were always associated with smaller peak velocities. However, no systematic relationship between the cued movement DOM and velocity, direction, or amplitude of targeted forearm rotation was evident.
Figure 5.

Evaluation of the correlation between the ratio of cued versus return movement peak velocity and the fit values obtained from cued and return movement DOM calculations during paired (A) and kinematically-similar (B) analyses. The slope of a line fit to the points (solid lines, slopes = −0.01905 and 0.0097411) suggests little, if any, relationship between peak velocity and DOM. Dashed lines represent unity (x=y) which would indicate a perfect correlation between peak velocity and DOM.
The effect of velocity upon GPi modulation during cued movements is illustrated using a scatter plot in figure 4. For simplicity, only GPi neurons with exclusive increases or decreases in depth of modulation in association with cued movement (n=42+11= 53) are shown. In this scatter plot, each neuron is represented by four points joined by a single line illustrating the depth of modulation and peak velocity that correspond to each of the four cued movements (±35°, ±70°). Negative velocities to correspond to counterclockwise rotations (−35°, −70°) and positive velocities correspond to clockwise rotations. Both monkeys consistently generated the small amplitude movements (±35°) using lower peak velocities than the larger amplitude movements. If the depth of modulation were directly related to the peak velocity, the four points representing each neuron would approach a line having a slope =1. Instead, visual inspection of this plot reveals no consistent relationship between depth of modulation and movement direction or velocity for these neurons. This observation remained true when we plotted the DOM associated with the peak velocity for the 27 neurons that exhibited combinations of increasing and decreasing modulation (data not shown).
These data suggest that the attenuation of the depth of modulation in the return condition relative to the cued phase of the task is not likely a direct consequence of the slower return movements. However, the relation is examined more directly in figure 5, which compares the cue/return depth of modulation attenuation to the velocity of the respective movements. The standard and kinematically-similar analyses are shown in figures 5A and 5B, respectively. Each neuron is again represented using a single point in the scatter plot as in figure 3. These plots reveal no correlation between the velocity of cued and return forearm movements and the corresponding attenuation of modulation during the two movement conditions. Collectively, our data reveal little, if any, evidence for a systematic influence of amplitude, direction, or velocity upon the modulation of neurons in either the cued or return condition.
Analysis of the Timing of the Maximum Depth of Modulation During Cued Movements
The timing of the maximum depth of modulation for each neuron is plotted in Figure 6. Each point represents a single neuron’s maximum change in discharge (either increasing or decreasing) relative to the time of movement onset. This plot reiterates that the typical pattern of modulation of the GPi population from which we recorded was an increase in discharge. The majority of these points fall in quadrant 1: increases in modulation that coincided with the execution phase of movement (50–200ms following movement onset (refer to Fig 2 position and velocity traces for representative raw traces with movement timing information). Peak changes in modulation that preceded movement onset (within the −200–0ms time period) were equally likely to be manifest as increased or decreased discharge (Fig. 6, quadrants II and III, respectively). Decreases in discharge following movement onset (Fig. 6, quadrant IV) were more dispersed in time than any of the other change in modulation, occurring as much as 500ms after movement onset.
Figure 6.

Scatter plot of the time of the occurrence of maximum DOM (abscissa) versus maximum DOM value observed (ordinate). Data were aligned such that time zero (abscissa) represents the onset of cued handle rotation movement, with negative time values preceding movement onset. Negative DOM values were assigned for neurons that decreased firing in association with movement. Maximum DOM was calculated by averaging trials to each of the individual 4 targets and selecting the highest value from 1 of the 4 targeted movements. Consequently, each neuron is represented by a single point on the scatter plot. The timing of the majority of maximum DOM values was concurrent with the actual handle rotation movement, following the onset of handle rotation by 50–200ms (quadrant I). Maximum DOMs that preceded handle movement onset occurred within 200 ms of movement onset (quadrants II and III). Decreases in discharge that accompanied or followed movement occurred over a more widely distributed time range (quadrant IV; 0–500ms post movement onset).
Modulation of GPi Neurons During the Reward Manipulation Task
In order to assess the impact of reward expectation upon GPi discharge, the reward schedule was predictably manipulated as described (See Experimental Procedure “Reward Manipulation Task”). Briefly, in the All Targets Rewarded task, monkeys were rewarded after successful completion of cued movement to each of the 4 targets. In contrast, in the One Target Rewarded task, monkeys were rewarded for successful completion of movements to only one of the 4 possible targets, but movements to the other 3 targets were required as well. Monkeys were familiar with these two task variants and could easily distinguish the reward schedule of the task within a few trials. In total, 7 task-modulated GPi neurons had sufficient numbers of trials to all targets in both the All Targets Rewarded and One Target Rewarded tasks to permit analysis of the impact of reward expectation upon cued movement depth of modulation (4 neurons from monkey P7F1, 3 from monkey L9D1). Of the 7 neurons that we studied, 3 were unchanged by the reward manipulation, meaning that they were preferentially modulated in association with the cued (as opposed to return) movement to any target regardless of whether they would receive a reward for that movement.
The remaining 4 neurons did show some changes in depth of modulation as a consequence of manipulating the reward expectation. However, the changes were not necessarily exclusively related to the rewarded target. For example, 3 GPi neurons exhibited smaller changes in modulation during cued movements during the One Target Rewarded task than they had exhibited when movements to all targets were rewarded. However, the depth of modulation associated with movements to the sole rewarded target did not differ from that associated with the unrewarded targets. This suggests that the task condition was the variable that influenced the different firing patterns during the One Target Rewarded task for these 3 neurons rather than a specific rewarded target. The one remaining neuron exhibited changes in modulation that were more like we expected to see from all neurons tested using this paradigm. This neuron exhibited larger changes in modulation in association with movements to the rewarded target relative to the unrewarded targets in the one target rewarded task. These changes indicate a preference for movements generated to targets for which the animal knew that they would receive a reward. Collectively, these findings suggested that the preferential modulation that we had observed in association with the cued condition was not necessarily exclusively related to the rewarded target, but that the depth of modulation could indeed be influenced by reward expectation or another salient variable (e.g. the task condition itself) that could be associated with cued movement. Figure 7 shows the patterns of modulation associated with one of our best examples of task-modulated neurons (P248, also depicted in Figure 2B) that exhibited strong cued movement modulation, weak return movement modulation, and a lack of preferential modulation in association with the rewarded target during the One Target Rewarded task (+70° target was the only rewarded target). Neuron discharge rate and the corresponding handle position signal for single behavioral trials in different phases during a block of trials in the One Target Rewarded task are shown. Column A shows the decreased firing associated with a single, cued +70° movement. Column B shows the corresponding return movement, which had no movement-related decrease in firing. Spontaneous movements made by the monkey between task trials, such as the +90° spontaneous movement (Column C), also had little or no movement-related firing changes. The prominent decrease in firing for cued movement to the unrewarded −70° target (Column D) was similar to that for the cued, rewarded +70° movement. These data, in combination with the other findings from the reward manipulation task suggest that there is an important variable associated with cued movements that influences the modulation preferentially, but that variable is not necessarily reward expectation. Further study is needed to elucidate the variable or variables that influence modulation during cued and return movements.
Figure 7.
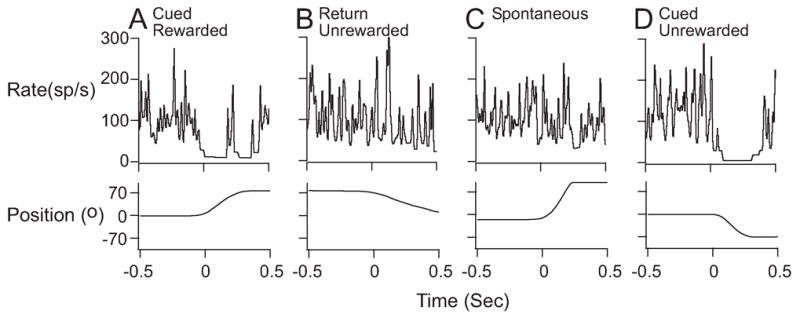
Examples of discharge patterns of GPi neuron P248 during individual behavioral trials that were associated with cued rewarded (A), return unrewarded (B), spontaneous (C), and cued unrewarded (D) movements during the 1TR task. This was one of our best modulated neurons, which exhibited clear task-related modulation within individual trials that remained consistent when averaged across multiple trials (see Fig. 2B for cumulative data from this neuron). Discharge rate and handle position signals are shown with data aligned to movement onset at time zero (BW=30ms). Decreases in firing that were observed during both the cued rewarded and cued unrewarded movements were absent during from return unrewarded and spontaneous movement records.
Assessment of Neurons Associated With Multiple Task Events
We sought to determine whether the differences between cued and return movement modulation might be impacted by the neuron being modulated in association with other task events. Therefore, we tallied the distribution of neurons that were modulated in association with multiple task events during cued and/or return movements (Table 3). All of the 80 neurons included in the table were modulated in association with the onset of movement either during the cued phase of the task (38) or during both cued and return phases (42). However, only 12 of these neurons were modulated in association with an additional behavioral event unrelated to the cued or return phases of limb movement (i.e. instruction target presentation or reward delivery). For details of calculating the modulation related to such behavioral events, refer to “Electrophysiological Recordings and Data Analysis” in Experimental Procedures. Of the 12 neurons, all were modulated in association with both reward delivery and cued forearm movement. Five of the 12 neurons were also modulated in association with return forearm rotation. Only 2 neurons exhibited significant changes in discharge when aligned with the presentation of the visual instruction target, activity that we would expect to be associated with movement preparation. Given these results, it seems unlikely the selectivity of neuronal modulation of the vast majority of our sample was related to the presentation of a visual cue or to reward expectation. However, these variables could clearly have differentially influenced the responses associated with cued and return movements.
Table 3.
Summary of GPi neuron modulation patterns associated with different behavioral events.
| Monkey P7F1 | Monkey L9D1 | Total | |
|---|---|---|---|
| Modulated With Movement Only | |||
| Cued Movement Only | 11 | 22 | 32 |
| Cued and Return Movements | 10 | 25 | 36 |
| Sub-total | 21 | 47 | 68 |
| Modulated With Multiple Events (Cued movement + other event) | |||
| Cued, Reward | 1 | 3 | 5 |
| Cued, Reward, Return | 2 | 4 | 5 |
| IT on, Cued, Reward | 1 | 0 | 1 |
| IT on, Cued, Reward, Return | 0 | 1 | 1 |
| Sub-total | 4 | 8 | 12 |
| Total (movement + multi-event modulation) | 25 | 55 | 80 |
Since some basal ganglia neurons are modulated in conjunction with orofacial movements, neurons were carefully screened as part of the sensory testing process to identify neurons responsive to voluntary or passive stimulation of lips, tongue, or perioral regions. Neurons responding to such stimulation were excluded from the 80 neurons in our data set.
Histological Reconstructions of Recording Sites
The locations of all recording sites and the site of an electrolytic lesion made in the final recording session in monkey P7F1 (left column) and monkey L9D1 (right column) were reconstructed and plotted in coronal section as illustrated in Figure 8. Neurons are plotted relative to the outline of the GPi nucleus at levels corresponding to approximately 15.5, 14.5, 13.5, and 12.5 mm anterior to the interaural line (Paxinos et al., 2000) in rows A through D, respectively. Symbols indicate whether the neuron responded to all four targets with increased (open circle), decreased (filled diamond) or a combined (open triangle) DOM. Although no anatomical clustering of discharge patterns was evident, the modulated neurons did tend to be located in regions of the nucleus that correspond with identified inputs from primary motor cortex, and possibly ventral premotor and supplementary motor areas (Hoover and Strick, 1999; Middleton and Strick, 2000).
Figure 8.

Coronal reconstructions of neurons recorded from GPi of 2 monkeys at approximately 15.5, 14.5, 13.5, and 12.5 mm anterior to the interaural line. Each symbol denotes a different task-modulated neuron. Different symbols denote modulation patterns of increases, decreases, or a combination of increased and decreased firing (see legend). The location of a site in which an electrolytic lesion in monkey P7F1 was placed to facilitate histological reconstruction is also indicated (□).
3. Discussion
This study of GPi neurons yielded the following primary results:
There was a consistent 20–80% reduction in the depth of modulation during the return phase of the task relative to the cued phase.
Although half the neurons were modulated by passive and active limb movement, it is not clear that these neurons play a direct role in specifying the mechanics of limb movement production since differences in kinematics do not appear to be directly related to the observed differences between cued and return depths of modulation.
Task-related neurons were likely to be located in regions receiving inputs from primary motor, ventral premotor, or supplementary motor cortical areas, but there was no clustering of neurons according to discharge pattern.
15% of the movement-related GPi neurons were also significantly modulated with reward delivery, but not passive perioral stimulation. Therefore, the reward component must have reflected cognitive factors like expectation or the encoding of successful task performance.
Minimal Influence of Amplitude, Direction and Peak Velocity Upon Neuronal Responses
Our evaluation of GPi modulation in relation to movement direction, amplitude and velocity yielded few neurons with detectable relationships between these kinematic variables and neuronal discharge. This is the most notable difference between our findings and the directional tuning properties of GPi neurons described earlier (Georgopoulos et al., 1983; Turner and Anderson, 1997). Although there are several potential explanations for this discrepancy, the most plausible is the presence of differences in the motor requirements of the task.
While the cognitive and motivational aspects of our task are comparable, the motor aspects differ, since they are more constrained than in tasks studied by many others. Studies that report directional or amplitude sensitivity typically employ tasks that necessitate multi-joint forearm coordination (i.e. 3-D reaching or button press tasks, or “center-out” target acquisition tasks performed along the surface of a digitizing tablet) (Turner and Anderson, 1997; Turner and Anderson, 2005). In contrast, our task confines the limb movements largely to a single axis of rotation (pronation, supination of the forearm). While we noted occasional shoulder elevation and/or arm abduction during task performance, these movements were not typical, and were excluded from analysis if excessive. Perhaps the simple, single degree of freedom limb movements obviated the need for the coding of signals related to direction or amplitude.
Rather than only kinematics, it is likely that GPi modulation was determined by a composite of movement-related and other types of information which are yet to be determined. The fact that this modulation is more robust in certain behavioral conditions (e.g. cued versus return forearm rotation movements) is consistent with data from other laboratories.
Our findings are also consistent with the idea that distributed processing modules comprised of selective connections between cortical, basal ganglia, and cerebellar neurons serve to embody (i.e. select and or initiate) the voluntary movement commands generated by the loop through the cerebellum (Houk, 2005; Miller et al., 2002). In this model, the basal ganglia have their greatest contributions in relation to movement selection and initiation, while cerebellum is more instrumental in refining the movement selection into a more precise motor command through the programming of movement kinematics such as amplitude, direction, and velocity (Houk et al., 2007). The engagement of certain distributed processing modules in specific behavioral conditions could underlie the discharge of GPi neurons in select behavioral circumstances and could potentially explain the context dependent discharge that we observed in GPi neurons in association with cued and return forearm rotations.
Preferential Modulation of GPi Neurons in Specific Contexts
The recent findings of Turner and Anderson are especially relevant (Turner and Anderson, 2005) to our observation of context-dependent GPi neuron modulation. In their study, Turner and Anderson examined GPi modulation during movements in which sensory feedback was systematically manipulated. The authors observed that larger decreases in firing occurred when sensory cues were available (sensory condition) and that increases in firing were enhanced when the memory demands (precued condition) of the task prevailed. In the sensory condition of Turner and Anderson’s study, targets were visible during forearm rotation and movements were triggered by both auditory and visual cues, very much like in the cued condition of our study. Unlike their results however, we observed both increases and decreases of discharge, but in all 80 neurons that we studied the greater depth of modulation was associated with the cued movement. One possible explanation for this difference between our studies is that the return movements in of our study task tended to consistently have lower velocities than cued movements, which was not true of the data from different conditions in their study. However, the apparent absence of a systematic relationship between velocity and depth of modulation in our data makes this an unlikely explanation. This is further supported by the observation of Turner and Anderson that differences in velocity during sensory and pre-cued conditions did not become manifest as systematic depth of modulation differences. While our data do not directly parallel the Turner and Anderson findings, both studies highlight the potential significance of firing decreases versus increases during externally- versus internally-driven behaviors. Collectively, these findings suggest that further investigation into the underlying associations of increases and decreases in neuronal discharge and the generation of voluntary movements is warranted.
Our observations are more comparable to the context-dependent discharge observed by Brotchie and colleagues (Brotchie et al., 1991), in conjunction with instructed task movements, but not self-paced movements to the target from which all trials began. Similarly, Mink and Thach (Mink and Thach, 1991a) described pallidal neurons that were modulated preferentially during visually guided rather than self-paced wrist movements. In their study, 69% of the neurons that were modulated during the visual task were unmodulated during the self-paced task. Another study to which our observations could be likened was that of pallidal neuron responses, which differed during visually guided or remembered sequential pointing movements (Mushiake and Strick, 1995). However, unlike our results, the majority (65%) of neurons exhibited greater depths of modulation during the remembered task. This apparent discrepancy in results may have been resulted from task-related differences or differences in the regions of GPi that were sampled.
Collectively, these studies reiterate that GPi neurons play a role in both externally- and internally-guided forearm movement tasks, but that these and other behavioral contexts are key determinants of the response patterns that occur in different task conditions. It is reasonable that these neurons could dynamically change their discharge patterns in conjunction with different roles during externally and internally driven behaviors given the diversity of afferent information (e.g. cognitive, limbic, motor, etc.) that they process. The mechanisms through which these diametric functions are mediated remain unclear and represent a current focus of investigation for several laboratories including our own.
Possible Factors That May Influence Context-Dependent Discharge
Current evidence supports a variety of theories purporting that basal ganglia neurons participate in the encoding of information related to reinforcement (Bar-Gad et al., 2003; Kawagoe et al., 1998; Schultz, 1998), expectation of future events(Hollerman et al., 1998), the registration of the presence or absence of salient behavioral stimuli (Hollerman and Schultz, 1998), the mediation of eye and/or limb movements during specific behavioral contexts (Brotchie et al., 1991; Hikosaka and Wurtz, 1983; Jaeger et al., 1993; Kawagoe et al., 2004; Mushiake and Strick, 1995; Turner and Anderson, 2005; van Donkelaar et al., 1999), the integration of sensory cues with motor outputs (Aldridge et al., 1980) and the adaptation of behavior to optimize favorable outcomes (McHaffie et al., 2005; Prescott et al., 2006; Redgrave et al., 1999). Consequently, these types of modulation could underlie our observation of context-dependent responses in the current study.
Neurons could therefore be modulated in conjunction with a specific phase of movement in order to register behavioral salience or likelihood of successful trial completion. Alternatively, task phase-selective modulation could denote the registration of events such as anticipated reward or the pending self-paced limb movement. The reward prediction model of basal ganglia function is thought to be instrumental when a sequence of events occurs as a prelude to reward and enables animals to optimize their decisions in order to ensure successful reward acquisition (Nakahara et al., 2004) by registering outcomes that did or did not match predictions. In support of this theory, a recent study of GPe neurons in a probabilistic visuomotor task indicates that GPe neurons were modulated both by the expected trial outcome (reward or no reward) and by the movement direction, indicating a relationship between limbic and kinematic aspects of movement at the single neuron level (Arkadir et al., 2004). This type of information could easily be conveyed to the context-dependent basal ganglia output neurons that we observed to encode information about the relationship between movement and either successful completion or reward. This finding has been further supported in a recent study of putamen and GPi neurons (Pasquereau et al., 2007). Pasquereau and colleagues demonstrated that the basal ganglia output neurons play a role in both encoding information about the context in which a movement is made, but also appear to execute a computation that can be used to assist the selection of a specific action.
By manipulating reward expectation, we demonstrated that variables in addition to an explicit reward were instrumental in influencing the preferential modulation of GPi neurons during specific behavioral contexts. This seemed to be a difficult task for the animals to perform without frustration. This is likely because they were required to generate a complex series of eye and limb movements that were often unrewarded, since only movements to a single target were rewarded in the One Target Rewarded task. However, even in the latter task, the monkeys generated movements with similar velocities and within the same time allowances that were implemented when all targeted movements were rewarded. However, the animals often grew impatient with these task blocks and would ultimately stop working unless we would resume the All Targets Rewarded condition. These results should therefore be interpreted with some caution, since our manipulations of reward contingency were for a small subset of neurons for which we were able to collect multiple blocks of trial data, with variable results.
It is possible that the modulation during the cued movement phase is stronger because it registers performance accuracy. Context-dependent modulation could also be related to the presence of a lit visual target throughout the cued portion of the trial which is absent during return movements. This possibility would be consistent with the observation that we and others have made that some GPi neurons are preferentially modulated during visually guided tasks, others prefer memory-guided tasks, while still others modulate equivalently in both conditions (Gdowski et al., 2005; Mushiake and Strick, 1993). Lastly, selective modulation could represent a mechanism for maintaining attention to the task or facilitating continued positioning of the handle in the target window until successful trial completion. Our anatomical distribution of task-modulated neurons (see Fig. 8, this paper) overlaps with the GPi neuron population that Francois and colleagues (Francois et al., 2004) labeled using an anterograde tracer injection into a region of external pallidum (see figure 4, Francois and colleagues), that when activated using bicuculline injection elicited behavioral manifestations that mimic attention deficits during natural and simple task behaviors (Grabli et al., 2004). We might predict that such a signal would occur uniformly during all cued movements, and since some neurons in our sample were preferentially modulated during cued movements to fewer than all four targets, it seems likely that these patterns convey something other than attention. However, the discharge pattern that we observed would be consistent with a highly selective and short term signal such as attention that we might expect to be conveyed by striatal medium spiny neurons that are modulated by dopamine. The preferential modulation that we observed during cued movement could also reflect a combination of factors such as cumulative changes in GPi firing pattern, oscillation, or synchronization of neurons within a neural network, all of which could be modulated by dopamine influences (Bergman et al., 1998; Ruskin et al., 2003; Walters et al., 2007). Some of these factors may be directly related to the selection or generation of the movement required by the task, but could perhaps be more indicative of signaling related to the suppression or facilitation of non-task related movements (Mink, 1996).
GPi Neurons Were Rarely Modulated in Association With Multiple Task Events
Many of the dopamine neurons of the substantia nigra pars compacta or the striatal neurons to which the dopamine neurons project are modulated in relation to variables related to reward delivery or preference (Hollerman et al., 1998; Kawagoe et al., 1998; Kawagoe et al., 2004). The breadth of projections from diverse cortical regions into the striatum supports the possible role of BG neurons in encoding information related to motor learning and the modification of behavior in relation to the occurrence of sensory events. A recent report details GPe neuron modulation in association with several behavioral events during visually-guided button pressing (Arkadir et al., 2004). Similarly, another study reported on 26 neurons from both pallidal segments that were modulated in conjunction with the visual cue and movement onset signal in a delayed release Go/No-Go task (Morris et al., 2005). Consequently, we expected to find at least some of our GPi neurons to modulate in association with multiple behavioral events, including reward delivery.
Surprisingly, only a handful of our GPi neurons (n=12; 15% of our sample) were significantly modulated by any event other than the onset of movement. This small proportion is potentially related to our selection criterion (modulation in association with onset of forearm rotation). An alternative criterion may have yielded more multi-modal responses. Nonetheless, the identification of at least some neurons that were modulated in conjunction with multiple behavioral events supports the postulate that GPi neurons may integrate different types of sensory and motor information to guide movements generated in specific behavioral conditions.
Anatomical Localization of Recorded Neurons
The extent to which neurons within pallidal nuclei are functionally segregated according to the cortical origins of their anatomical inputs remains a subject of great debate (Brasted and Wise, 2004; Groenewegen et al., 1990; Haber et al., 2000; Haber and Gdowski, 2004; McFarland and Haber, 2002; Strick et al., 1995a; Strick et al., 1995b; Yelnik et al., 1996). Many studies substantiate the concept of parallel loops or circuits through the basal ganglia that are largely functionally independent (Alexander et al., 1986; Kelly and Strick, 2004; Middleton and Strick, 2000), but whose discharge patterns may be shaped by inputs originating from related cortical and/or striatal regions (Haber, 2003; Percheron and Filion, 1991; Strick et al., 1995b). Moreover, the extent to which pallidal neuron responses are mediated through cerebellar inputs to striatal neurons remains unclear (Hoshi et al., 2005; Houk, 2005). The notion of parallel processing of action selection, valuation of reward, and kinematic parameters of movement through basal ganglia and cerebellar loops is consistent with Houk’s recent motor control theory of distributed processing modules. A distributed processing module is comprised of selective connections between cortical, basal ganglia, and cerebellar neurons which serves to select, initiate, and mediate kinematic aspects of voluntary movements (Houk, 2005). Houk’s theory asserts that the role of the basal ganglia in this process is likely strongest in relation to a ballpark selection and initiation of a movement command, while the cerebellar constituents of the DPM are more instrumental in kinematic aspects of movement control. The engagement of certain DPMs in specific behavioral conditions could underlie the discharge of GPi neurons in conjunction with specific contexts and/or multiple behavioral events during the cued and return forearm rotation movements.
Determining the origin of inputs to individual GPi neurons remains a formidable challenge that, when overcome, will provide great insight into the organization of neural networks within each of the basal ganglia nuclei. Until then, the interpretation of our data is reliant upon existing anatomical studies that delineate territories within the pallidal nuclei largely defined by the regions of cortex that provide their predominant input. Based upon histological reconstruction (e.g. Figure 8) we concluded that the task-modulated neurons from which we recorded resided in regions that were likely to receive fairly selective inputs from primary motor, supplementary motor area, or ventral premotor cortex. However, a study that combines anatomical labeling with physiological characterization of behavioral response properties is necessary in order to determine if neurons with particular functional properties localize within specific regions of the nucleus.
Summary and Interpretations
In summary, the results of this study extend and quantify our prior descriptive analysis of the selective modulation GPi neurons in association with movements generated in specific behavioral contexts (Gdowski et al., 2001). In the present study, nearly 100% (80/82) of the task-modulated GPi neurons had 20–80% reductions of their depths of modulation during the return, unrewarded movements relative to during cued, rewarded movements. There was very little relation between kinematic parameters of movement and the corresponding depth of modulation. Kinematic variables therefore do not likely explain the attenuated return-movement modulation. Other factors influencing the selective modulation that we observed may include the presence or absence of visual cues, the expectation of reward, attentional effects, engagement of specific distributed processing modules and perhaps others that are yet to be recognized. Lastly, we extended our prior findings by comparing the locations of neurons to the nature of their modulation patterns. Task-modulated GPi neurons were distributed within territories likely to receive inputs from primary motor cortex, ventral premotor cortex, or supplementary motor area, but showed little evidence of further clustering in relation to the nature of their task related modulation.
4. Experimental Procedure
Single unit extracellular recordings were acquired from the GPi of 1 adult male (macaca mulatta “monkey P7F1”) and 1 adult female monkey (macaca fascicularis “monkey L9D1”) in accordance with guidelines established by the National Research Council (1996 National Research Council (2003) and the Northwestern University Institutional Animal Care and Use Committee. All surgical and experimental procedures were approved by the Committee for Animal Resources at Northwestern University.
Visually-Guided Forearm Rotation Task
A schematic of the visually-guided forearm rotation task, including the instructional cues and behavioral responses observed in a typical trial is shown in Figure 1. Monkeys faced a 15″ computer monitor with a black background that was positioned 24″ from their eyes (Fig 1A). Five circular targets were drawn in a horizontal array that corresponded to 0°, ±35°, and ±70° of handle rotation (Fig 1B). A representative trial timeline is shown in Figure 1C. For illustrative purposes, the targets have been drawn vertically as opposed to horizontally as the monkey would view them. Targets consisted of a continuously visible gold ring that was filled with red to indicate that the target was being turned “on”. Trials were initiated by turning on the 0° fixation target. After the monkey moved and held the handle within 8° of the center of the fixation target for 250 ms, another target was randomly selected and turned on for a variable instruction period (750–2250ms). Following the instruction period, the fixation target was turned off as a visual cue for the monkey to move to the instruction target. In all trials, primary (water or juice) and secondary reinforcements (100 ms long tone) were delivered after a variable delay (500–1250 ms) following successful completion of the cued movement. Continuous visual feedback of handle position was provided by a cursor that moved linearly along the screen. Cued movements always began from the fixation target (0°) location, while return movements were initiated from any of the ±35° or ±70° targets and returned to the fixation target location. Correct movements to the instruction target always elicited a liquid reinforcement, while return movements to the fixation target were always unrewarded.
Reward Manipulation Task
In order to determine the impact of reward expectation upon the movement-related modulation patterns of GPi neurons, we systematically manipulated the reward schedule for 8 GPi neurons in a manner that would allow subjects to predict whether they would be rewarded for movements to specific targets, analogous to studies that have been performed during targeted eye movement behaviors in other basal ganglia nuclei (Itoh et al., 2003; Kawagoe et al., 1998; Kawagoe et al., 2004). For all tested neurons, the first block of trials consisted of an All Targets Rewarded paradigm, in which subjects were rewarded for correctly performed movements to any of the four possible targets. For the block immediately following the All Targets Rewarded condition block, one target was randomly selected for which movements were rewarded, while correct movements to the other three targets were unrewarded (“One Target Rewarded”). A secondary reinforcement (tone) was delivered on all successful trials for both ATR and 1TR conditions. Data were collected in alternating blocks for as long as neuronal isolation could be maintained. This strategy allowed us to confirm that any changes in modulation were associated with the manipulated reward expectation and were not a consequence of a change in neuronal isolation.
We preferentially selected GPi neurons with robust changes in modulation during cued movements to all four targets for the reward manipulation paradigm, since we expected that only one targeted movement would remain strongly modulated in the One Target Rewarded condition if reward expectation was truly an influential variable. Thus, neurons that were strongly modulated during cued movements to all 4 targets in the All Targets Rewarded condition would provide the best opportunity to observe changes in neuronal discharge that were likely a result of reward manipulation. Unfortunately, this strategy limited the total number of neurons that were recorded since neurons that exhibited minimal or no change in modulation with movement to some of the 4 targets were excluded from further testing using the reward manipulation paradigm.
Electrophysiological Recordings and Data Analysis
Tungsten microelectrodes (0.008″ or 0.010″ diameter, FHC, Bowdoinham, ME) were advanced through a cilux recording chamber and grid (Crist Instrument Co., Inc., Hagerstown, MD) oriented 45° from the sagittal plane. Recording location was selected using coordinates published by others (DeLong et al., 1985; DeLong and Georgopoulos, 1979; Horak and Anderson, 1984) that were adjusted for both monkeys relative to surgically-implanted fiducial markers visualized in MR images acquired prior to chamber implantation. During each electrode penetration, single neurons were discriminated and localized to the internal pallidal segment (GPi) based on standard criteria (electrode depth, firing rate, incidence of pausing (Anderson, 1977; DeLong, 1971; DeLong et al., 1985; DeLong and Georgopoulos, 1981)). Neurons were sampled from the left hemisphere of both monkeys and from the right hemisphere of monkey P7F1 during performance of a visually-guided limb movement using the arm contralateral to the recording chamber.
Data were collected continuously throughout 10–15 minute blocks. For each neuron, data from successful trials were grouped according to the amplitude (35°, 70°) and direction of forearm rotation (clockwise, counterclockwise). Dot rasters and cumulative histograms were generated for all recorded neurons by aligning neuronal discharge to the time of the occurrence of a variety of behavioral events (trial onset, target on, go tone, target off, and reward) and to the onset of both cued and return movements. Movement onset was defined as the point at which the first derivative of the handle position signal (velocity) exceeded three times its standard deviation.
The time of the maximum rate change (burst or pause) within 500 ms of the alignment event was determined for each histogram. Average event-related discharge rate was determined using a 30 ms interval around this maximum. The baseline rate was averaged during a 250 ms interval while the handle was held stationary within the fixation target. DOM was calculated by subtracting the baseline rate from the average event-related discharge rate. Neurons were included in subsequent analyses if the event-related discharge differed significantly from baseline (small-sample test statistic t for the difference between two means, p<0.05). Included neurons typically exhibited visually-detectible peaks or troughs with DOMs >20 spikes/second.
Quantitative Comparisons of Movement Related Activity
In this study, we developed a method to quantitatively compare the modulation patterns during the two task phases (i.e. cued and return movement). Each neuron’s cued movement-related discharge X(n), was fit to the return movement discharge Z(n):
for n= ±17 bins (binwidth = 30 ms) relative to the onset of movement. We performed a least squares regression analysis in order to determine a and b. The coefficient a represented a measurement of gain between cued and return movement responses. The coefficient b was used to compensate for differences in background discharge rate. A value of a<1 indicated that the cued movement modulation was greater than the return modulation. The modulation was compared in this manner for the cued and return movement to individual targets (“paired analysis”) as well as for the return movement to the target that required a movement of the same amplitude and direction as the cued movement, but started in a different position (e.g. cued movement to the −70 target compared with the return movement from the +70 target; “kinematically-similar analysis”).
Reconstruction of Recording Lesions
In order to verify the locations of recordings, histological lesions (50 μA for 20–30 sec) were placed in several locations using stainless steel microelectrodes (FHC, Maine) during the final recording session in each animal. After lesioning, monkeys received a lethal dose of sodium pentobarbital (100 mg/kg) and were perfused with physiological saline followed by 4% paraformaldehyde and 1% potassium ferrocyanide. Brains were removed, blocked, cryoprotected, and sectioned at 50 μm. Sections were stained with thionin for anatomical reconstruction. Alternate sections underwent the Prussian Blue reaction and were counterstained with neutral red to localize the metal deposited from the tips of the stainless steel electrodes (Suzuki and Azuma, 1976). The Prussian blue technique also enabled visualization of the iron concentrated in the globus pallidus facilitating the delineation of pallidal segment boundaries.
Lesions were identified and used to correct for tissue shrinkage during histological processing. The location of each neuron was established in standard stereotaxic coordinates, accounting for the 45° angle of the recording chamber relative to the sagittal plane. Nuclear boundaries were drawn from sections obtained from monkey P7F1 for coronal levels at approximately 12.5, 13.5, 14.5, and 15.5 mm anterior to the interaural line. Notes taken during each recording session recorded the tentative depths at which landmarks such as nuclear boundaries, internal capsule, or the optic tract were encountered. These landmarks were used to confirm the locations of recording sites in relation to the GPi border.
Acknowledgments
The authors thank Ms. Cynthia Meroz for the preparation of histological sections and Ms. Kelly Townsend for assistance with data analysis.
Grants: This work was supported by a National Institute of Mental Health Center Grant (MH-48185-09; JCH, Center Director), a National Institute of Neurological Disorders and Stroke Grant (P01-NS44383, JCH), The Ruggles Fellowship in Movement Disorders, Evanston Hospital Department of Neurology, Evanston, IL, and a grant from the American Parkinson Disease Association (APDA; MJG).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Guide for the Care and Use of Laboratory Animals, vol. National Academy Press; Washington, DC: 1996. [Google Scholar]
- Guidelines for the Care and Use of Mammals in Neuroscience and Behavioral Research, vol. National Academy Press; Washington, DC: 2003. [PubMed] [Google Scholar]
- Aldridge JW, et al. Sensory-motor processing in the caudate nucleus and globus pallidus: a single-unit study in behaving primates. Can J Physiol Pharmacol. 1980;58:1192–201. doi: 10.1139/y80-182. [DOI] [PubMed] [Google Scholar]
- Alexander GE, et al. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Ann Rev Neurosci. 1986;9:357–81. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Anderson ME. Discharge patterns of basal ganglia neurons during active maintenance of postural stability and adjustment to chair tilt. Brain Res. 1977;143:325–338. doi: 10.1016/0006-8993(78)90572-3. [DOI] [PubMed] [Google Scholar]
- Arkadir D, et al. Independent coding of movement direction and reward prediction by single pallidal neurons. J Neurosci. 2004;24:10047–56. doi: 10.1523/JNEUROSCI.2583-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar-Gad I, et al. Information processing, dimensionality reduction and reinforcement learning in the basal ganglia. Prog Neurobiol. 2003;71:439–73. doi: 10.1016/j.pneurobio.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Bergman H, et al. Physiological aspects of information processing in the basal ganglia of normal and parkinsonian primates. Trends Neurosci. 1998;21:32–8. doi: 10.1016/s0166-2236(97)01151-x. [DOI] [PubMed] [Google Scholar]
- Brasted PJ, Wise SP. Comparison of learning-related neuronal activity in the dorsal premotor cortex and striatum. Eur J Neurosci. 2004;19:721–40. doi: 10.1111/j.0953-816x.2003.03181.x. [DOI] [PubMed] [Google Scholar]
- Brotchie P, et al. Motor function of the monkey globus pallidus. 2. Cognitive aspects of movement and phasic neuronal activity. Brain. 1991;114:1685–702. doi: 10.1093/brain/114.4.1685. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Activity of pallidal neurons during movement. J Neurophysiol. 1971;34:414–27. doi: 10.1152/jn.1971.34.3.414. [DOI] [PubMed] [Google Scholar]
- DeLong MR, et al. Primate globus pallidus and subthalamic nucleus: Functional organization. J Neurophys. 1985;53:530–543. doi: 10.1152/jn.1985.53.2.530. [DOI] [PubMed] [Google Scholar]
- DeLong MR, Georgopoulos A. Motor functions of the basal ganglia as revealed by studies of single cell activity in the behaving primate. Adv Neurol. 1979;24:131–140. [Google Scholar]
- DeLong MR, Georgopoulos AP. Motor functions of the basal ganglia. In: Brooks VB, editor. Handbook of Physiology, The Nervous System II, Part 2. Vol. 23. American Physiological Society; Bethesda, MD: 1981. pp. 1017–1061. [Google Scholar]
- Francois C, et al. Behavioural disorders induced by external globus pallidus dysfunction in primates II. Anatomical study. Brain. 2004;127:2055–70. doi: 10.1093/brain/awh239. [DOI] [PubMed] [Google Scholar]
- Gdowski MJ, et al. Context dependency in the globus pallidus internal segment during targeted arm movements. J Neurophysiol. 2001;85:998–1004. doi: 10.1152/jn.2001.85.2.998. [DOI] [PubMed] [Google Scholar]
- Gdowski MJ, et al. Comparison of globus pallidus internal and external segments during visually and memory guided forelimb movements. Society for Neuroscience Abstracts. 2005;30 [Google Scholar]
- Georgopoulos AP, et al. Relations between parameters of step-tracking movements and single cell discharge in the globus pallidus and subthalamic nucleus of the behaving monkey. J Neurosci. 1983;3:1586–98. doi: 10.1523/JNEUROSCI.03-08-01586.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabli D, et al. Behavioural disorders induced by external globus pallidus dysfunction in primates: I. Behavioural study. Brain. 2004;127:2039–54. doi: 10.1093/brain/awh220. [DOI] [PubMed] [Google Scholar]
- Groenewegen HJ, et al. The anatomical relationship of the prefrontal cortex with the striatopallidal system, the thalamus and the amygdala: evidence for a parallel organization. Prog Brain Res. 1990;85:95–116. doi: 10.1016/s0079-6123(08)62677-1. discussion 116–8. [DOI] [PubMed] [Google Scholar]
- Haber SN. The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat. 2003;26:317–30. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Haber SN, et al. Striatonigrostriatal pathways in primates form an ascending spiral from the shell to the dorsolateral striatum. J Neurosci. 2000;20:2369–82. doi: 10.1523/JNEUROSCI.20-06-02369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber SN, Gdowski MJ. The Basal Ganglia. In: Paxinos G, Mai JK, editors. Human Nervous System. Vol. 2. Elsevier Science/Academic Press; San Diego: 2004. pp. 676–738. [Google Scholar]
- Hikosaka O, et al. Functional properties of monkey caudate neurons. III. Activities related to expectation of target and reward. J Neurophysiol. 1989;61:814–32. doi: 10.1152/jn.1989.61.4.814. [DOI] [PubMed] [Google Scholar]
- Hikosaka O, Wurtz RH. Visual and oculomotor functions of monkey substantia nigra pars reticulata. III. Memory-contingent visual and saccade responses. J Neurophysiol. 1983;49:1268–84. doi: 10.1152/jn.1983.49.5.1268. [DOI] [PubMed] [Google Scholar]
- Hollerman JR, Schultz W. Dopamine neurons report an error in the temporal prediction of reward during learning. Nat Neurosci. 1998;1:304–9. doi: 10.1038/1124. [DOI] [PubMed] [Google Scholar]
- Hollerman JR, et al. Influence of reward expectation on behavior-related neuronal activity in primate striatum. J Neurophysiol. 1998;80:947–63. doi: 10.1152/jn.1998.80.2.947. [DOI] [PubMed] [Google Scholar]
- Hoover JE, Strick PL. The organization of cerebellar and basal ganglia outputs to primary motor cortex as revealed by retrograde transneuronal transport of herpes simplex virus type 1. J Neurosci. 1999;19:1446–63. doi: 10.1523/JNEUROSCI.19-04-01446.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horak FB, Anderson ME. Influence of globus pallidus on arm movements in monkeys. I. Effects of kainic acid-induced lesions. J Neurophys. 1984;52:290–304. doi: 10.1152/jn.1984.52.2.290. [DOI] [PubMed] [Google Scholar]
- Hoshi E, et al. The cerebellum communicates with the basal ganglia. Nat Neurosci. 2005;8:1491–3. doi: 10.1038/nn1544. [DOI] [PubMed] [Google Scholar]
- Houk JC. Agents of the mind. Biol Cybern. 2005;92:427–37. doi: 10.1007/s00422-005-0569-8. [DOI] [PubMed] [Google Scholar]
- Houk JC, et al. Action Selection and Refinement in Subcortical Loops Through Basal Ganglia and Cerebellum. Philosophical Transactions of the Royal Society-B. 2007:362. doi: 10.1098/rstb.2007.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh H, et al. Correlation of primate caudate neural activity and saccade parameters in reward-oriented behavior. J Neurophysiol. 2003;89:1774–83. doi: 10.1152/jn.00630.2002. [DOI] [PubMed] [Google Scholar]
- Jaeger D, et al. Primate basal ganglia activity in a precued reaching task: preparation for movement. Exp Brain Res. 1993;95:51–64. doi: 10.1007/BF00229653. [DOI] [PubMed] [Google Scholar]
- Kawagoe R, et al. Expectation of reward modulates cognitive signals in the basal ganglia. Nat Neurosci. 1998;1:411–6. doi: 10.1038/1625. [DOI] [PubMed] [Google Scholar]
- Kawagoe R, et al. Reward-predicting activity of dopamine and caudate neurons--a possible mechanism of motivational control of saccadic eye movement. J Neurophysiol. 2004;91:1013–24. doi: 10.1152/jn.00721.2003. [DOI] [PubMed] [Google Scholar]
- Kelly RM, Strick PL. Macro-architecture of basal ganglia loops with the cerebral cortex: use of rabies virus to reveal multisynaptic circuits. Prog Brain Res. 2004;143:449–59. doi: 10.1016/s0079-6123(03)43042-2. [DOI] [PubMed] [Google Scholar]
- Lauwereyns J, et al. Feature-based anticipation of cues that predict reward in monkey caudate nucleus. Neuron. 2002;33:463–73. doi: 10.1016/s0896-6273(02)00571-8. [DOI] [PubMed] [Google Scholar]
- McFarland NR, Haber SN. Thalamic relay nuclei of the basal ganglia form both reciprocal and nonreciprocal cortical connections, linking multiple frontal cortical areas. J Neurosci. 2002;22:8117–32. doi: 10.1523/JNEUROSCI.22-18-08117.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHaffie JG, et al. Subcortical loops through the basal ganglia. Trends Neurosci. 2005;28:401–7. doi: 10.1016/j.tins.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Middleton FA, Strick PL. Basal ganglia and cerebellar loops: motor and cognitive circuits. Brain Res Brain Res Rev. 2000;31:236–50. doi: 10.1016/s0165-0173(99)00040-5. [DOI] [PubMed] [Google Scholar]
- Miller LE, et al. The role of the cerebellum in modulating voluntary limb movement commands. Arch Ital Biol. 2002;140:175–83. [PubMed] [Google Scholar]
- Mink JW. The basal ganglia: focused selection and inhibition of competing motor programs. Prog Neurobiol. 1996;50:381–425. doi: 10.1016/s0301-0082(96)00042-1. [DOI] [PubMed] [Google Scholar]
- Mink JW, Thach WT. Basal ganglia motor control. I. Nonexclusive relation of pallidal discharge to five movement modes. J Neurophysiol. 1991a;65:273–300. doi: 10.1152/jn.1991.65.2.273. [DOI] [PubMed] [Google Scholar]
- Mink JW, Thach WT. Basal ganglia motor control. II. Late pallidal timing relative to movement onset and inconsistent pallidal coding of movement parameters. J Neurophysiol. 1991b;65:301–29. doi: 10.1152/jn.1991.65.2.301. [DOI] [PubMed] [Google Scholar]
- Morris G, et al. Physiological studies of information processing in the normal and Parkinsonian basal ganglia: pallidal activity in Go/No-Go task and following MPTP treatment. Prog Brain Res. 2005;147:285–93. doi: 10.1016/S0079-6123(04)47021-6. [DOI] [PubMed] [Google Scholar]
- Mushiake H, Strick PL. Preferential activity of dentate neurons during limb movements guided by vision. J Neurophysiol. 1993;70:2660–4. doi: 10.1152/jn.1993.70.6.2660. [DOI] [PubMed] [Google Scholar]
- Mushiake H, Strick PL. Pallidal neuron activity during sequential arm movements. J Neurophysiol. 1995;74:2754–8. doi: 10.1152/jn.1995.74.6.2754. [DOI] [PubMed] [Google Scholar]
- Nakahara H, et al. Dopamine neurons can represent context-dependent prediction error. Neuron. 2004;41:269–80. doi: 10.1016/s0896-6273(03)00869-9. [DOI] [PubMed] [Google Scholar]
- Pasquereau B, et al. Shaping of motor responses by incentive values through the basal ganglia. J Neurosci. 2007;27:1176–83. doi: 10.1523/JNEUROSCI.3745-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, et al. The Rhesus Monkey Brain in Stereotaxic Coordinates, vol. Academic Press; San Diego: 2000. [Google Scholar]
- Percheron G, Filion M. Parallel processing in the basal ganglia: up to a point. Trends Neurosci. 1991;14:55–9. doi: 10.1016/0166-2236(91)90020-u. [DOI] [PubMed] [Google Scholar]
- Prescott TJ, et al. A robot model of the basal ganglia: behavior and intrinsic processing. Neural Netw. 2006;19:31–61. doi: 10.1016/j.neunet.2005.06.049. [DOI] [PubMed] [Google Scholar]
- Redgrave P, et al. The basal ganglia: a vertebrate solution to the selection problem? Neuroscience. 1999;89:1009–23. doi: 10.1016/s0306-4522(98)00319-4. [DOI] [PubMed] [Google Scholar]
- Ruskin DN, et al. Correlated multisecond oscillations in firing rate in the basal ganglia: modulation by dopamine and the subthalamic nucleus. Neuroscience. 2003;117:427–38. doi: 10.1016/s0306-4522(02)00921-1. [DOI] [PubMed] [Google Scholar]
- Schultz W. The Primate Basal Ganglia Between the Intention and Outcome ofAction. In: Kimura M, Graybiel AM, editors. Functions of the Cortico-Basal Ganglia Loop vol. Springer-Verlag; New York: 1995. pp. 31–48. [Google Scholar]
- Schultz W. Predictive reward signal of dopamine neurons. J Neurophysiol. 1998;80:1–27. doi: 10.1152/jn.1998.80.1.1. [DOI] [PubMed] [Google Scholar]
- Schultz W, et al. Responses of monkey dopamine neurons to reward and conditioned stimuli during successive steps of learning a delayed response task. J Neurosci. 1993;13:900–13. doi: 10.1523/JNEUROSCI.13-03-00900.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W, et al. Neuronal activity in monkey ventral striatum related to the expectation of reward. J Neurosci. 1992;12:4595–610. doi: 10.1523/JNEUROSCI.12-12-04595.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strick PL, et al. Basal Ganglia “Loops” with the Cerebral Cortex. In: Kimura M, Graybiel AM, editors. Functions of the Cortico-Basal Ganglia Loop vol. Springer-Verlag; New York: 1995a. pp. 106–124. [Google Scholar]
- Strick PL, et al. Motor Functions and Working Memories vol. 1995b. Macro-organization of the Circuits Connecting the Basal Ganglia with the Cortical Motor Areas; pp. 117–130. [Google Scholar]
- Suzuki H, Azuma M. A glass-insulated “Elgiloy” microelectrode for recording unit activity in chronic monkey experiments. Electroencephalogr Clin Neurophysiol. 1976;41:93–5. doi: 10.1016/0013-4694(76)90218-2. [DOI] [PubMed] [Google Scholar]
- Takikawa Y, et al. A possible role of midbrain dopamine neurons in short- and long-term adaptation of saccades to position-reward mapping. J Neurophysiol. 2004;92:2520–9. doi: 10.1152/jn.00238.2004. [DOI] [PubMed] [Google Scholar]
- Turner RS, Anderson ME. Pallidal discharge related to the kinematics of reaching movements in two dimensions. J Neurophysiol. 1997;77:1051–1074. doi: 10.1152/jn.1997.77.3.1051. [DOI] [PubMed] [Google Scholar]
- Turner RS, Anderson ME. Context-dependent modulation of movement-related discharge in the primate globus pallidus. J Neurosci. 2005;25:2965–76. doi: 10.1523/JNEUROSCI.4036-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Donkelaar P, et al. Neuronal activity in the primate motor thalamus during visually triggered and internally generated limb movements. J Neurophysiol. 1999;82:934–45. doi: 10.1152/jn.1999.82.2.934. [DOI] [PubMed] [Google Scholar]
- Walters JR, et al. Phase relationships support a role for coordinated activity in the indirect pathway in organizing slow oscillations in basal ganglia output after loss of dopamine. Neuroscience. 2007;144:762–76. doi: 10.1016/j.neuroscience.2006.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickens JR, et al. Neural mechanisms of reward-related motor learning. Curr Opin Neurobiol. 2003;13:685–90. doi: 10.1016/j.conb.2003.10.013. [DOI] [PubMed] [Google Scholar]
- Yelnik J, et al. A spatial and quantitative study of the striatopallidal connection in the monkey. Neuroreport. 1996;7:985–8. doi: 10.1097/00001756-199604100-00006. [DOI] [PubMed] [Google Scholar]



