Abstract
We investigated capillary density in 12 subjects with leukoaraiosis (LA), in 9 age-matched normal subjects, in 7 cases of Alzheimer’s disease (AD), and 4 after whole-brain irradiation for brain tumors. In the LA study (which as been published), autopsy brains were evaluated by MRI. The presence of LA was indicated by confluent or patchy areas of hyperintensity in the deep white matter. We employed a stereology method using computerized image processing and analysis to determine microvascular density. Afferent vessels (arterioles and capillaries, but not veins or venules) were stained for alkaline phosphatase in 100 μm thick celloidin sections. Microvascular density in LA lesions in the deep white matter (2.56%) was significantly lower than in the corresponding deep white matter of normal subjects (3.20%, p = 0.0180). LA subjects demonstrated decreased vascular density at early ages (55 – 65 years) when compared to normal subjects. Our findings indicate that LA affects the brain globally, with capillary loss, although the parenchymal damage is found primarily in the deep white matter. In ongoing studies of the deep white matter in AD brains, we found a pattern of decreased vascular density compared to normal, as well as an age-related decline. In the four irradiated brains, we found very low vessel densities, similar to those found in LA, without an additional age-related decline.
Keywords: Leukoaraiosis, Capillary loss, Vascular dementia, Aging changes, Alkaline phosphatase staining, Stereology, Vessel density
1. Introduction
Leukoaraiosis (LA) is an age-related degeneration of white matter (WM) in the centrum semiovale characterized on MRI by hyperintensity on T2-weighted imaging [1]. Histopathologic findings in LA include demyelination, loss of glial cells (especially oligodendrocytes), axon damage, and spongiosis [2–4]. LA is thought to be due to insufficient blood supply to the cerebral deep white matter (DWM) due to vascular pathology [5–8]. The arterioles supplying the cerebral DWM may be affected by tortuosity, hyalinization, or atherosclerosis [9, 10]. In addition, collagenous thickening and occlusion of venules has been shown in LA lesions [11, 12]. The DWM is particularly susceptible to injury from hypoperfusion because this area is supplied exclusively by vessels arising from the leptomeningeal border zone, an area with a precarious blood supply, and these arterioles travel for a considerable distance to reach the deep white matter [13]. The tortuosity of some of these arterioles leads to losses in hemodynamic kinetic energy [14]. Studies with MRI [15] and PET [16, 17] have found decreased blood flow in the white matter of subjects with LA, and the blood flow was even reduced in normal-appearing white matter [15]. Thus, the vascular pathology and blood flow changes suggest that LA results from chronic ischemia [5, 8, 16–18].
In this article we present an updated interpretation of our previously published data [19] on microvascular damage in LA, with a particular focus on capillary loss, not only in LA lesions, but in the normal-appearing white matter, and even the cortex of LA subjects. We also present our findings of capillary loss in the brain white matter in cases of Alzheimer’s disease and after whole-brain irradiation for brain tumors.
2. Materials and methods
Details on our method of determining vascular density in the brain in the LA study have been previously published [19]. Twelve male and 9 female subjects comprised the study group with an average age of 72.5 years. Brains were cut with an oblique-coronal slice through the frontal lobes, parallel to the penetrating vessels. MRI of the brain slice was performed and T2-weighted images were acquired parallel to the brain slice. Brain slices were fixed, dehydrated, embedded in celloidin, sectioned at 100 μm, and stained for alkaline phosphatase (AP). The hyperintense LA area was mapped onto the AP-stained slides. In the 12 LA subjects, control areas of normal-appearing white matter were also outlined. In the 9 normal subjects, areas were outlined in the periventricular DWM and areas of white matter apart from the periventricular DWM.
In new studies, we investigated vascular density in the white matter of 7 Alzheimer’s disease cases, 4 patients who had undergone whole-brain irradiation for brain tumors (in the contralateral hemisphere from the site of the tumor), and in a normal 25 year-old.
Images of the areas to be measured were captured with a SPOT RT digital camera. Color images were 1600 × 1200 pixels and encompassed a 1.2 × 0.9 mm area. The data included only afferent vessels less than 200 μm, i.e., small arteries, arterioles, and capillaries. The area fraction is calculated as the vessel area in pixels/total image area in pixels and is expressed as a percentage. Image processing included the following steps: pixel intensity data were filtered from color images, hue and saturation information were discarded, and the image was converted to grayscale. Background staining was then removed by background subtraction. The image was then converted to a black/white image in binary mode for calculation of percent vessel density. Statistical analysis in the LA study employed a series of repeated measures linear mixed models. The data for AD and brain irradiation have not been tested for statistical significance as we are still accumulating cases.
3. Results
Histologic study of the sections from the 12 LA subjects confirmed classic changes in the LA lesions (spongiosis, loss of glial cells, and demyelination). White matter that appeared normal by MRI also appeared normal on histologic examination. The cortex in LA subjects also appeared normal. Both the LA lesion and the DWM in normal subjects contained vessels ranging in size from arteries and arterioles to capillaries (Figure 1). Figure 2 shows the data points from the 21 subjects in our published study of vessel density in LA [19]. However, the lines through those data points are not the original computer-generated regression lines. They have been redrawn to show our updated interpretation of the data, as explained in the figure legend.
Figure 1.
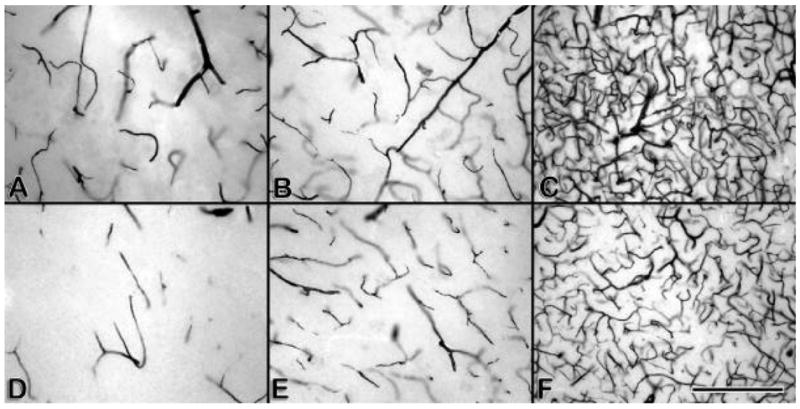
(a–f) AP stained, 100 μm celloidin sections from normal subjects (a–c) and LA subjects (d–f). Only afferent vessels are stained. (a) DWM of normal, (b) LA lesion, (c) normal white matter away from ventricle, (d) normal-appearing white matter in LA subject, (e) cortex of normal subject, (f) cortex of LA subject. Note the decrease in vessels in the LA subjects.
Figure 2.
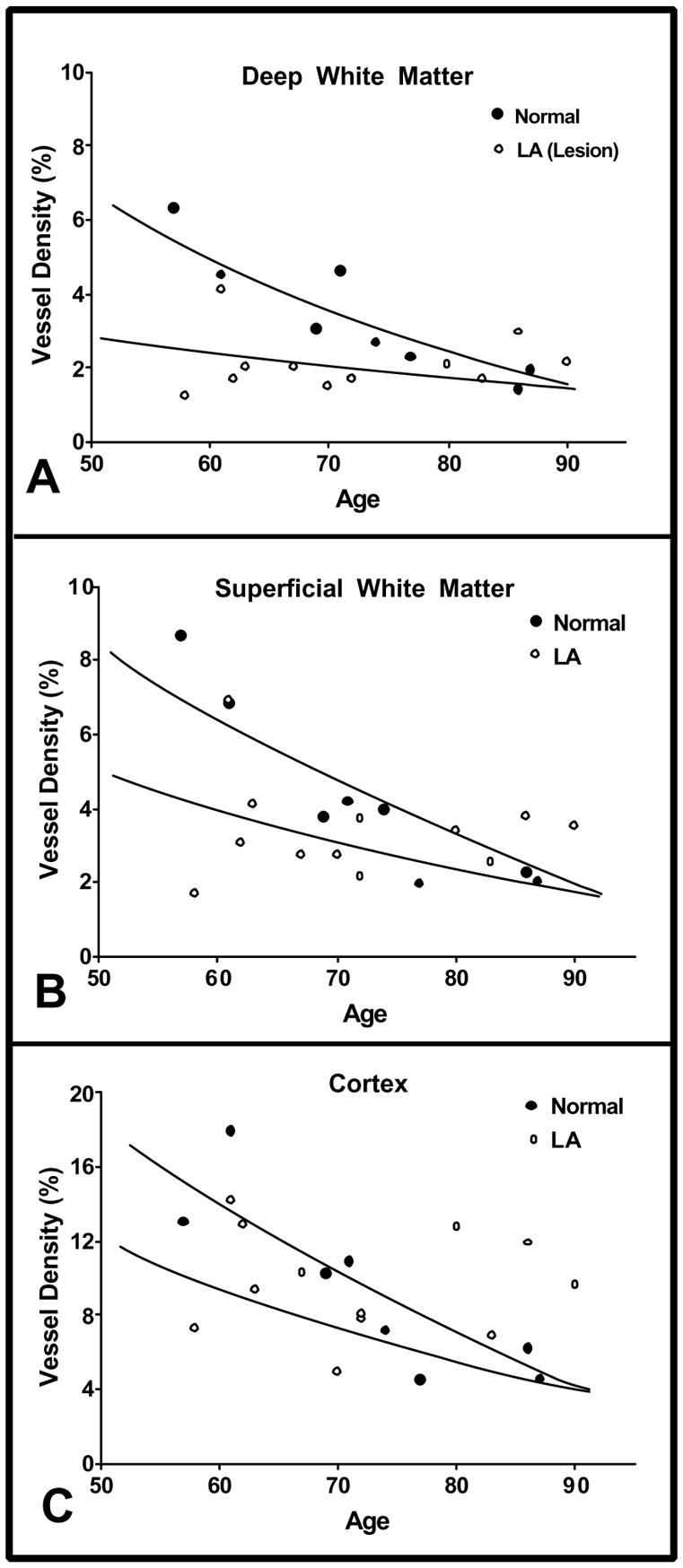
Graphs of vascular density (% area) in deep white matter, white matter, and cortex of normal and leukoaraosis subjects. The vessel density in normal subjects (closed circles, solid lines) and LA subjects (open circles, dashed lines) are shown as a function of age. The statistical analysis was based on computer-generated regression lines from the data points shown. However, the lines drawn here are our interpretation of the data. They are based on the regression lines, but the scatter of the data points offers wide latitude for interpretation. A great many more data points would be needed to clearly establish the true shape of these curves. Note that the normal and LA lines meet at about 90 years of age. The computer-generated regression lines always crossed at about 75 years of age, giving the impression that vessel density in the normal subjects was lower than in the LA subjects after 75 years of age. That is biologically implausible. (a) Vessel density in the deep white matter of normal subjects was significantly greater than that in LA (p=0.0180). In normal subjects, vessel density decreased with age from approximately 6% at 55–60 years to 2% at 85–90 years. Vessel density in the LA lesion in the deep white matter was 2% to 3% at 55–60 years, less than half the level observed in normal subjects, and it did not show much tendency to decline further with increasing age. (b) The average vessel density in superficial white matter in normal subjects was not significantly different than that in normal-appearing, superficial white matter of LA subjects. In the normal subjects, vessel density decreased from approximately 8% at 55–60 years to 2% at 85–90 years. For the LA subjects, vessel density in normal-appearing white matter was 3% to 4% at 55–60 years, almost half of that seen in similar areas in normal subjects, and it did not show much decline with increasing age. (c) The density of vessels in the cortex of normal subjects was not significantly different from that in the cortex of LA subjects. Vessel density in normal cortex declined from approximately 16% at 55–60 years to approximately 6% by 90 years. Vessel density in cortex of LA subjects appeared somewhat reduced at 55–60 years, and it did not show much decline with increasing age.
Vessel density was significantly decreased in LA DWM compared to normal DWM (p = 0.0180). There was a significant age-related decline in vessel density (p = 0.0005) between years 57 to 90 in the DWM of normal subjects, whereas vessel density in the lesions of LA subjects did not decrease significantly with age (p = 0.9940). The slopes of the two regression lines were significantly different (p = 0.0091). There was a 54% lower vessel density in those LA subjects who died early, but because of the age-related loss of vessels in normal subjects, vessel densities after 80 years appeared equivalent in the DWM of LA and non-LA subjects.
Vascular densities were also measured in more superficial, normal-appearing areas of the centrum semiovale. When plotted in relation to age, there was a significant difference (p = 0.0058) in the slopes of the lines for vascular density in LA versus normal subjects. Vessel density in the superficial white matter decreased significantly with age in normal subjects (p = 0.0447), but not in those with LA. The vessel density in the normal-appearing, superficial white matter of subjects with LA was decreased by 47% in those subjects who died before 60 years of age, but it did not decrease thereafter. In summary, in LA subjects, both the lesion and non-lesion areas of white matter showed decreased vascular density, particularly in the youngest cases.
The cortex in LA and non-LA subjects appeared normal by MRI and microscopic examination. While there was no significant difference in the average vessel density in normal versus LA cortex, there was a nearly significant difference in the slopes of the lines (p = 0.0512). In subjects 60 years of age or less, vessel density in the LA cortex was decreased by 38% compared to normal cortex. Vessel density in cortex of normal subjects decreased with age, while that of LA subjects appeared not to decline further from an initially decreased level.
In the seven subjects with AD, we found vessel densities in the DWM that tended to decline with age (Fig. 3). The graph lines suggest that vessel density in the youngest AD subjects may be lower than in normal subjects (Fig. 3).
Figure 3.
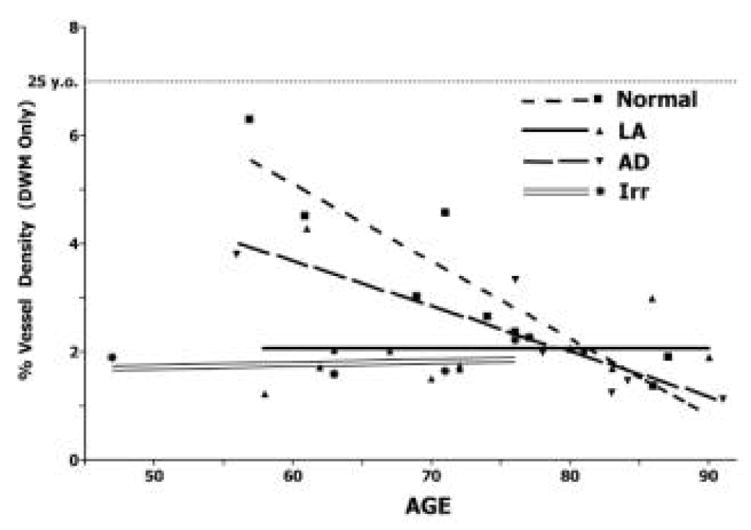
Graph of vascular density in normal, LA, AD, and brain irradiation.
In four patients who received whole-brain irradiation for brain tumors, we found leukoaraiosis-like lesions in the centrum semiovale, in the contralateral hemisphere from the site of the tumor. The DWM in these cases showed low vessel densities (Fig. 3), similar to those found in LA subjects. Also like LA, there did not appear to be a further decline in vessel density with age. Vascular density in the white matter of a normal 25 year-old is also presented for comparison with aging and the three pathologic brain conditions (LA, AD, and irradiation).
4. Discussion
As we previously reported [19], LA lesions were associated with a significantly decreased vascular density and that this decrease was especially apparent at the earliest ages examined, i.e., subjects who died between 55 and 60 yrs. The LA lesions had an average 20% decrease in vessel density compared to the DWM in normal subjects. It is not surprising that vascular density was decreased in the LA lesions in view of known LA-associated pathologies, such as loss of cells and spongiosis. Although it could be suggested that blood vessel loss may be a response to reduced metabolic requirements, our study provides evidence of an alternative hypothesis, that vascular loss precedes parenchymal cell loss.
In a comparison of normal and LA subjects, we found decreased vascular density in the LA lesion, in the normal-appearing white matter, and in the cortex. Despite an apparent decrease of vessels in the cortex, there is no MRI or histologically detectable alteration to the neuropil of the cortex. Similarly, a significant loss of vessels in normal-appearing white matter in LA subjects was not associated with histologically detectable abnormality. Thus, our findings indicate that the vessel loss appears to precede any visible damage to the parenchyma, and the areas most severely affected in the white matter are those most susceptible to ischemia.
Our measurements of decreased vascular density indicate that a 55 year-old dying with deep white matter hyperintensities has the brain arteriolar/capillary bed volume typical of an 80 year-old. The diagnosis of LA has been viewed as an indicator of localized deep white matter degeneration in the aged. Our findings of decreased vascular density beyond the LA lesion in the white matter, and even in the cortex, suggest that LA affects the brain globally, with capillary loss, although the parenchymal damage is found primarily in the centrum semiovale.
The finding of a substantial age-related decline in vascular density in the brains of normal subjects is in itself an important finding. Furthermore, it is important to note that the use of age-matched controls is not always sufficient to identify changes that may vary with increasing age. Subjects with LA who die before the eighth decade show substantially decreased vascular density, whereas those dying around 80 years of age show no difference compared to age-matched controls. Thus, heavy sampling in subjects over 70 years of age may show no significant changes. Graphs showing changes over time provide a better view. An additional observation derived from the LA data is the potentially significant finding of an apparent “floor” on vascular density in the LA lesions. We now suggest that vascular densities below a certain level may be incompatible with viability. Perhaps lower vessels densities than those found would lead to frank infarction. However, in our experience and observations it seems that LA does not commonly progress to a condition of infarction in the deep white matter. If this is the case, then it may be that despite considerable loss of capillaries, there usually remain enough arteriolar and capillary channels to provide enough blood flow to avoid infarction. In contrast, in patients who receive brain irradiation for brain tumors, it is not uncommon to find leukoaraiosis-like lesions in the centrum semiovale that do progress to frank necrosis. That these two brain pathologies might show different progressions is not surprising, since they have different precipitating mechanisms. (We do not know the mechanism causing age-related leukoaraiosis, but it is not radiation).
We found leukoaraiosis to affect the brain globally, with capillary loss most prominent in the deep white matter and spreading outward into the cortex. The initial stages can be observed histologically before MRI abnormalities are apparent. The presence of DWM hyperintensities on MRI may be used as a clinical sign alerting physicians to the existence of influences acting to the detriment of the entire brain microvasculature. A precise knowledge of the disease process could facilitate the development of promising interventional therapy that could be applied before the disease inflicts neurological disability.
Acknowledgments
We thank Patricia Wood and Carolyn Cox for preparing the histological specimens. This work was supported by NIH grants NS20618 to D. M. Moody and CA113321 to W. R. Brown.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hachinski VC, Potter P, Merskey H. Leuko-araiosis. Arch Neurol. 1987;44:21–3. doi: 10.1001/archneur.1987.00520130013009. [DOI] [PubMed] [Google Scholar]
- 2.Munoz DG, Hastak SM, Harper B, Lee D, Hachinski VC. Pathologic correlates of increased signals of the centrum ovale on magnetic resonance imaging. Arch Neurol. 1993;50:492–7. doi: 10.1001/archneur.1993.00540050044013. [DOI] [PubMed] [Google Scholar]
- 3.Brown WR, Moody DM, Thore CR, Challa VR. Apoptosis in leukoaraiosis. AJNR: Am J Neuroradiol. 2000;21:79–82. [PMC free article] [PubMed] [Google Scholar]
- 4.Pantoni L, Garcia JH. The significance of cerebral white matter abnormalities 100 years after Binswanger’s report. A review Stroke. 1995;26:1293–301. doi: 10.1161/01.str.26.7.1293. [DOI] [PubMed] [Google Scholar]
- 5.Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke. 1997;28:652–9. doi: 10.1161/01.str.28.3.652. [DOI] [PubMed] [Google Scholar]
- 6.Moody DM, Brown WR, Challa VR, Ghazi-Birry HS, Reboussin DM. Cerebral microvascular alterations in aging, leukoaraiosis, and Alzheimer’s disease. Ann N Y Acad Sci. 1997;826:103–16. doi: 10.1111/j.1749-6632.1997.tb48464.x. [DOI] [PubMed] [Google Scholar]
- 7.Brown WR, Moody DM, Thore CR, Challa VR. Cerebrovascular pathology in Alzheimer’s disease and leukoaraiosis. Ann N Y Acad Sci. 2000;903:39–45. doi: 10.1111/j.1749-6632.2000.tb06348.x. [DOI] [PubMed] [Google Scholar]
- 8.Pantoni L. Pathophysiology of age-related cerebral white matter changes. Cerebrovascular Diseases. 2002;13 (Suppl 2):7–10. doi: 10.1159/000049143. [DOI] [PubMed] [Google Scholar]
- 9.Spangler KM, Challa VR, Moody DM, Bell MA. Arteriolar tortuosity of the white matter in aging and hypertension. A microradiographic study. J Neuropathol Exp Neurol. 1994;53:22–6. doi: 10.1097/00005072-199401000-00003. [DOI] [PubMed] [Google Scholar]
- 10.Yamauchi H, Fukuyama H, Nagahama Y, et al. Brain arteriolosclerosis and hemodynamic disturbance may induce leukoaraiosis. Neurology. 1999;53:1833–8. doi: 10.1212/wnl.53.8.1833. [DOI] [PubMed] [Google Scholar]
- 11.Brown WR, Moody DM, Challa VR, Thore CR, Anstrom JA. Venous collagenosis and arteriolar tortuosity in leukoaraiosis. J Neurol Sci. 2002;203–204:159–63. doi: 10.1016/s0022-510x(02)00283-6. [DOI] [PubMed] [Google Scholar]
- 12.Moody DM, Brown WR, Challa VR, Anderson RL. Periventricular venous collagenosis: association with leukoaraiosis. Radiology. 1995;194:469–76. doi: 10.1148/radiology.194.2.7824728. [DOI] [PubMed] [Google Scholar]
- 13.Moody DM, Bell MA, Challa VR. Features of the cerebral vascular pattern that predict vulnerability to perfusion or oxygenation deficiency: an anatomic study. AJNR: Am J Neuroradiol. 1990;11:431–9. [PMC free article] [PubMed] [Google Scholar]
- 14.Moody DM, Santamore WP, Bell MA. Does tortuosity in cerebral arterioles impair down-autoregulation in hypertensives and elderly normotensives? A hypothesis and computer model Clin Neurosurg. 1991;37:372–87. [PubMed] [Google Scholar]
- 15.O’Sullivan M, Lythgoe DJ, Pereira AC, et al. Patterns of cerebral blood flow reduction in patients with ischemic leukoaraiosis. Neurology. 2002;59:321–6. doi: 10.1212/wnl.59.3.321. [DOI] [PubMed] [Google Scholar]
- 16.Kobari M, Meyer JS, Ichijo M, Oravez WT. Leukoaraiosis: correlation of MR and CT findings with blood flow, atrophy, and cognition. AJNR: Am J Neuroradiol. 1990;11:273–81. [PMC free article] [PubMed] [Google Scholar]
- 17.Meguro K, Hatazawa J, Yamaguchi T, et al. Cerebral circulation and oxygen metabolism associated with subclinical periventricular hyperintensity as shown by magnetic resonance imaging. Ann Neurol. 1990;28:378–83. doi: 10.1002/ana.410280313. [DOI] [PubMed] [Google Scholar]
- 18.Yamauchi H, Fukuyama H, Yamaguchi S, Miyoshi T, Kimura J, Konishi J. High-intensity area in the deep white matter indicating hemodynamic compromise in internal carotid artery occlusive disorders. Arch Neurol. 1991;48:1067–71. doi: 10.1001/archneur.1991.00530220089024. [DOI] [PubMed] [Google Scholar]
- 19.Moody DM, Thore CR, Anstrom JA, Challa VR, Langefeld CD, Brown WR. Quantification of afferent vessels shows reduced brain vascular density in subjects with leukoaraiosis. Radiology. 2004;223:883–90. doi: 10.1148/radiol.2333020981. [DOI] [PubMed] [Google Scholar]


