Abstract
Endothelin 1 (EDN1) plays a primary role in the pathophysiology of hypoxia-induced fetal growth restriction in the rat. In this study we evaluated the effects of chronic maternal hypoxia on the expression of endothelin and its receptors and on receptor binding activity in the uterus and placenta of the rat, in order to elucidate their roles in hypoxia-induced fetal growth restriction. Timed-pregnant Sprague-Dawley rats were maintained in either a normoxic or a normobaric hypoxic (12% O2) atmosphere from Gestational Days 18–21. Uterine and placental tissues collected on Gestational Day 21 were assayed for Edn1, Ednra, and Ednrb (endothelin receptors) mRNA expression by real-time quantitative RT-PCR, for localization of EDN1 and its receptors by immunohistochemistry, for EDNRA and EDNRB protein expression by Western blot, and for receptor binding activity by homologous competitive binding assays. EDN1 mRNA expression was significantly increased in the hypoxic placenta, but not in the uterus, compared with normoxic controls. Immunohistochemistry revealed increased EDN1 specifically in the labyrinth of the placenta. Receptor mRNA levels were not significantly affected by hypoxia, but EDNRA protein expression was significantly decreased specifically in the uterine placental beds. Receptor binding decreased significantly in response to hypoxia in all tissues investigated, compared with controls. These results suggest that chronic maternal hypoxia results in increased expression of EDN1 in the placenta but not in the uterus, and that reduced binding activity, rather than regulation of receptor expression, is a mechanism by which these tissues regulate the local hemodynamic response to increased endogenous placental EDN1 in the setting of hypoxia.
Keywords: environment, placenta, pregnancy, uterus
INTRODUCTION
The endothelins are a family of peptides that includes three isoforms, EDN1, EDN2, and EDN3. Each consists of 21 amino acids and is produced mainly by endothelial or epithelial cells. The endothelins act primarily as paracrine mediators. Their most prominent effect is vasoconstriction, though they also affect cell proliferation, differentiation, and migration as well as chemokinesis and apoptosis [1, 2]. Endothelin 1 (EDN1), the most physiologically relevant isoform in humans, is one of the most potent vasoconstrictors known.
Endothelins mediate their effects via two types of receptors in mammalian species. The EDNRA receptor, which has a high affinity for EDN1, is located on smooth muscle cells and is responsible principally for the vasoconstrictive and proliferative effects of EDN1. The EDNRB receptor, which has an equal affinity for all three isoforms, is located on endothelial cells and is responsible for a vasodilatory effect that is mediated primarily by nitric oxide [3]. EDNRB also functions as a clearance receptor to remove excess endothelins from the vascular system [4].
Chronic late-term hypoxia in the pregnant rat is a well-established model for the study of fetal growth restriction. We have previously demonstrated that EDN1 (acting via EDNRA) plays a major role in the pathophysiology of hypoxia-induced fetal growth restriction [5]. The purpose of this study was to evaluate the expression of EDN1 and its receptors in the uterus and placenta in normoxic and hypoxic pregnant rats.
MATERIALS AND METHODS
Materials
[125I]EDN1 and [125I]EDN3 (each 2200 Ci/mmol) were obtained from Amersham (Arlington Heights, IL). EDN1 and EDN3 were purchased from American Peptide Company (Sunnyvale, CA). Antibodies to the following proteins were purchased from the sources indicated: EDN1 (Phoenix Pharmaceuticals, Burlingame, CA), EDNRA and EDNRB (Alomone Laboratories, Jerusalem, Israel), and Na+/K+ transporting ATPase alpha 1 polypeptide (ATP1A1; Abcam, Cambridge, MA). Other reagents were purchased from Sigma (St. Louis, MO) unless indicated otherwise.
Animals
Female Sprague-Dawley rats were purchased from Harlan Sprague Dawley (Madison, WI), housed in the Evanston Northwestern Healthcare Research Institute Center for Comparative Medicine, and bred with male rats from the same source. All rats received a standard laboratory rodent diet (PMI Feeds, St. Louis, MO), water ad libitum, and were kept on a 12L:12D cycle. Animal care and the conduct of all experiments were in accord with guidelines approved by the Institutional Animal Care and Use Committee of Evanston Northwestern Healthcare Research Institute.
Experimental Hypoxia
Half the rats (n = 6) were housed in a normoxic environment in the laboratory while the other half (n = 6) were housed in a hypoxic environment for Gestational Days 18–21 (term = 22 days). This maternal hypoxia model is an established model for intrauterine growth restriction in the rat [5]. The hypoxic environment was maintained in a 120-L plexiglass glove box by titrating compressed air and compressed nitrogen to produce the desired O2 concentration of 12%, which was monitored by an O2 analyzer (Sensorlabs, Lexington, MA). A gas-flow rate of 3.0 L/min was adequate to control humidity (50%–70%), and barium hydroxide pellets (Baralyme; Chemtron, St. Louis, MO) were used as a CO2 absorbent. The hypoxic environment housed two rats at a time, and both groups were run concurrently. This environment produced average maternal pO2 values that were 37% lower than normoxic controls, as reported previously [5].
Tissue Collection
On Gestational Day 21, rats were killed and a laparotomy was performed. The uterine horns were opened to expose the fetuses and placentas. Placentas were collected and frozen in liquid nitrogen for storage. The uterine placental beds (placental attachment sites) were dissected free of the remaining uterine wall. The uterine placental bed is the full thickness of the uterine wall at the sites where each placenta was attached. Uterine free wall is the remaining uterine wall where no placental attachment sites were present. We had previously observed differences in perfusion in uterine placental bed compared to free wall. Therefore, we chose to evaluate these regions independently. Both the uterine placental beds and the uterine free wall were individually frozen and stored at −80°C for further use.
Real-Time Quantitative RT-PCR
Tissue samples stored at −80°C were homogenized in RNA STAT-60 (a phenol/guanidinium thiocyanate reagent; TEL-TEST, Friendswood, TX) on ice for extraction of total RNA. Genomic DNA, a possible impurity in the RNA extract, was removed by adding RNase-free DNase (1 unit/μg DNA) and incubating at 37°C for 60 min. The concentration of RNA was determined by absorbance at 260 nm and the purity was checked by the 260:280 nm ratio (greater than 1.8). RNA integrity was verified by electrophoresis in a 1% agarose gel. For each sample, 3 μg total RNA was reverse transcribed at 37°C for 1 h in a total of 20 μl reaction mixture: 50 mM Tris-HCl, 75 mM KCl, 2.0 mM MgCl2, 10 mM dithiothreitol, 1.25 mM of each deoxynucleotide-triphosphate, 7.5 pM random hexamer, 1 U/μl RNaseOUT (RNase inhibitor; Invitrogen, Carlsbad, CA), and 10 U/μl Moloney-murine leukemia virus reverse transcriptase (Gibco-BRL, Grand Island, NY). Amplification of cDNA by PCR with 0.1 U/μl AmpliTaq DNA polymerase was done on an Applied Biosystems (Foster City, CA) GeneAmp 5700 real-time quantitative PCR instrument in a total volume of 50 μl consisting of 1.0–3.0 μl RT product, 10 mM Tris-HCl, 50 mM KCl, 2 mM MgCl2, 0.1 mM of each dNTP, and 0.2–1.0 μM of each primer, sense and antisense, respectively, and 0.2–1.0 μM TaqMan-MGB fluorescent detection probe (Applied Biosystems). For each PCR reaction a probe and primer titration was performed to determine optimal concentrations for each. The reaction mixtures were heated at 95°C for 30 sec and then immediately carried through 60 cycles of PCR using the following schedule: 30 sec denaturation at 94°C and 2 min annealing plus extension at 60°C. Primers and probes specific for rat Edn1, Ednra, Ednrb, and cytoplasmic beta actin (Actb, internal control gene) were designed by Primer Express (Applied Biosystems). In preliminary experiments Actb was shown to be expressed similarly in both normoxic and hypoxic rat placenta and uterus (data not shown). No-template controls (also carried through 60 PCR cycles) were used to exclude the possibility of primer dimer formation. The following represent the primer sense and antisense and the TaqMan probe sequences, respectively:
Rat Edn1: 5′-GACCAGCGTCCTTGTTCCAA-3′, 5′-TTGCTACCAGCGGATG-CAA-3′, 5′-(6FAM)-TCCAAGAGAGGTTGAGGTGT-(MGBNFQ)-3′
Rat Ednra: 5′-CTCAACGCCACGACCAAGTT-3′, 5′-GCAAGCTCCCATTCCTTCTG-3′, 5′-(6FAM)-ATGGAGTTTTACCAAGACGT-(MGBNFQ)-3′
Rat Ednrb: 5′-TGGCCATTTGGAGCTGAGAT-3 ′, 5′-TCCAAGAAGCAACAGCTCGAT-3′, 5′-(6FAM)-TGCCCTTCATACAGAAGG-(MGBNFQ)-3′
Rat Actb: 5′-AGGCCAACCGTGAAAAGATG-3′, 5′-ACCAGAGGCATACAGGGACAA-3′, 5′-(6FAM)-CCCAGATCATGTTTGAGAC-(MGBNFQ)-3′
Membrane Preparations
Frozen tissues were homogenized on ice in 10 volumes (w/v) of 10 mM Hepes (pH 7.4) containing 0.25 M sucrose, 3 mM EDTA, and protease inhibitors (0.1 mM PMSF and 5 μg/ml Pepstatin A) using a stainless steel Polytron tissue homogenizer (Brinkmann, Westbury, NY) at 13 500 rpm. The homogenate was centrifuged at 1000 × g for 15 min at 4°C. The supernatant was collected and the pellet was resuspended in the same buffer, rehomogenized, and recentrifuged. The combined supernatant was centrifuged at 60 000 × g for 1 h at 4°C. The precipitate was resuspended in 50 mM Tris (pH 7.4) containing 50 mM EDTA and the previously listed protease inhibitors and then centrifuged at 30 000 × g for 30 min at 4°C. The final pellet was resuspended in the Tris buffer and stored at −80°C. In addition to the placental and uterine tissues, membranes were prepared from an untreated adult rat brain (contains abundant EDNRA and EDNRB receptors) for use as control membranes in the Western blot analyses. Protein content was determined by the Bradford assay (Bio-Rad, Hercules, CA).
Western Immunoblots
From each tissue membrane preparation, 20 μg of protein was separated by 12% SDS-PAGE and then transferred to PVDF blotting membranes. Immunoblots were performed using rabbit polyclonal primary antibodies specific for EDNRA or EDNRB, or ATP1A1 (a plasma membrane marker protein used as a loading control to assure equal amounts of protein in all lanes). After blocking nonspecific binding with 5% normal goat serum (NGS) in 1 M Tris-buffered saline (TBS; pH 7.5) containing 0.1% Tween 20 (TBST), primary antibodies were incubated on the membranes at 1:250 or 1:200 (anti-EDNRA and -EDNRB, respectively; dilutions determined in preliminary antibody titration experiments) overnight at 4°C in TBS containing 5% NGS, 0.5% Tween 20, 10% glycerol, and 18% glucose. Following six 10-min washes in TBST and 1 h of blocking, the membranes were incubated for 2 h at 25°C with secondary goat anti-rabbit IgG antibodies labeled with horseradish peroxidase (1:5000 dilution; Santa Cruz Biotechnology, Santa Cruz, CA). Membranes were washed again as before. Immunoreactive bands were identified using the ECL+Plus Chemifluorescent Detection System (Amersham, Piscataway, NJ) as directed by the manufacturer and then quantified using a Storm Imager (Molecular Dynamics, Sunnyvale, CA). Quantitative comparisons were made between bands run together on the same gel. Band intensity for uterine and placental samples was normalized to the ET receptor-rich control membrane preparation used throughout all Western analyses. Transfer of proteins to the membranes was ascertained by the inclusion of Rainbow molecular weight markers (Amersham, Arlington Heights, IL) on each gel, and specific bands for EDNRA (48 Mr × 10−3), EDNRB (49 Mr × 10−3), and ATP1A1 (112 Mr × 10−3) were identified by Cruz molecular weight standards (Santa Cruz Biotechnology).
Immunohistochemistry
Placental and uterine wall tissues were fixed for 2 h in 10% neutral buffered formalin and either embedded in paraffin (for EDN1) or infiltrated with 10% sucrose in PBS and frozen in OCT compound (Miles Labs, Naperville, IL) for cryosectioning (for EDNRA and EDNRB). Sections were deparaffinized (when appropriate) and hydrated in PBS. The Envision system (Dako, Carpinteria, CA) was used for all immunohistochemistry according to the manufacturer’s instructions, beginning with an endogenous horseradish peroxidase block. Sections were then incubated with primary antibody for 2 h at room temperature (all diluted 1:500 in PBS) followed by three 5-min rinses in PBS. The remaining immunohistochemistry steps were conducted as directed in the staining kit. A light methyl green counterstain was applied (1% for 5 min). Sections were dehydrated through a graded series of ethyl alcohols and xylene and then mounted in Permount for bright-field microscopy. Controls included elimination of the primary antibody and adsorption of the primary antibody with 10 μM antigen to verify specificity.
Homologous Competitive Endothelin Receptor Binding Assay
Binding assays were performed in triplicate in 96-well microtiter plates pretreated with 0.1% BSA. Aliquots of the membrane preparation (2 μg protein/well) were incubated with 12 different concentrations of EDN1 or EDN3 and 0.1 nM [125I]EDN1 or [125I]EDN3, respectively. After a 3-h incubation at 25°C, unbound ligands were separated from bound by vacuum filtration using glass-fiber filter strips in a PHD cell harvester (Cambridge Technology, Watertown, MA). Filters were washed three times with saline (1 ml each) and the radioactivity was measured in a Wallac gamma counter (PerkinElmer Life Sciences, Boston, MA). Nonspecific binding was determined in the presence of 1 μM EDN1 or EDN3. Maximum binding (Bmax, fmol/μg protein) and affinity (Kd) were computed by nonlinear regression [6] using GraphPad Prism (GraphPad Software, San Diego, CA). EDN1 binds to both 0.1 nM) whereas EDNRA and EDNRB with the same potency (IC50 = 0.1 nM) than for binding has a 2000-fold greater potency for EDNRB (IC50 = 200 nM) [7]. Homologous EDN3 competition determined the EDNRA (IC50 = Bmax for EDNRB, homologous EDN1 competition represented binding of both receptors, and the difference determined the Bmax for EDNRA.
Statistical Analyses
Results are presented as mean ± SEM. Statistical comparisons among multiple groups were made using an analysis of variance followed by a post hoc Newman-Keuls test. When comparisons were made between two groups, a t-test was used. All statistical tests were two-tailed and results were considered statistically significant at P < 0.05.
RESULTS
Placenta
EDN1 mRNA expression (as a percent of Actb) was 65% greater in hypoxic rat placenta (P < 0.01) than in normoxic (Fig. 1). In contrast, the expression of Ednra and Ednrb mRNA in the placenta was not influenced by hypoxia (Fig. 1). Compared with normoxic controls, hypoxic animals expressed similar amounts of mRNA for each receptor. Corresponding with the RT-PCR data, the Western blot data showed no difference in protein expression of EDNRA or EDNRB in the placenta between normoxic control rats and hypoxic rats (Fig. 2).
FIG. 1.
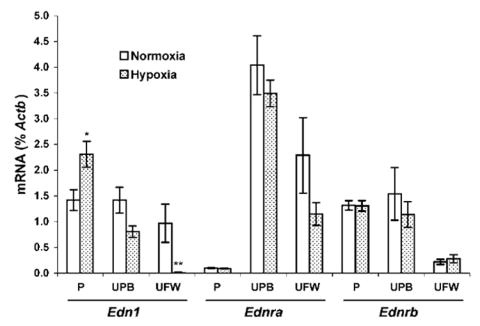
Expression of Edn1, as well as Ednra and Ednrb receptor mRNA in rat placentas (P), uterine placental beds (UPB), and uterine free wall (UFW). Comparative mRNA expression was determined by real-time quantitative RT-PCR in tissues from rats maintained in normoxic conditions (n = 6) or in 12% O2 for 3 days (Gestational Days 18–21, n = 6). Results are expressed as percent of an internal control gene, cytoplasmic beta actin (Actb), and each value represents the mean ± SEM. *P < 0.05 and **P < 0.01 vs. normoxic control.
FIG. 2.
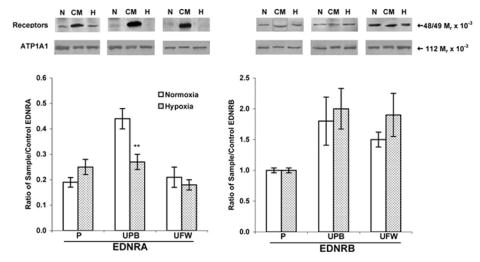
Protein expression of EDNRA (48 Mr × 10−3, left) and EDNRB (49 Mr × 10−3, right) receptors in pregnant rats. Western blots were performed on membranes isolated from placental (P), uterine placental bed (UPB), and uterine free wall (UFW) homogenates from normoxic (N; n = 6) and hypoxic (H; Gestational Days 18–21, 12% O2, n = 6) pregnant rats. Gels included control membranes (CM) from rat brain and the densitometric results are expressed as the mean ± SEM of the ratio of reproductive tissue and control receptor band densities. Na+/K+ transporting ATPase alpha 1 polypeptide (ATP1A1; 112 Mr × 10−3) was used as a control membrane protein to verify loading of equal amounts of protein into each well. **P < 0.01 vs. normoxic control.
Immunohistochemistry also demonstrated more intense staining for EDN1 in the hypoxic placenta compared with normoxic (Fig. 3). This staining localized to the fetal placental vascular membranes in the placental labyrinth. There were no differences between groups in the staining of the decidual and basal zones of the placenta, which showed no staining and moderate staining, respectively (data not shown). Labeling for EDNRA and EDNRB was present primarily in the labyrinth in a similar pattern as for EDN1, but was not different between groups (Fig. 3). When PBS replaced the primary antibody, no staining was present, and when primary antibodies were adsorbed with antigen, the staining was dramatically reduced, demonstrating the specificity of the antibodies.
FIG. 3.
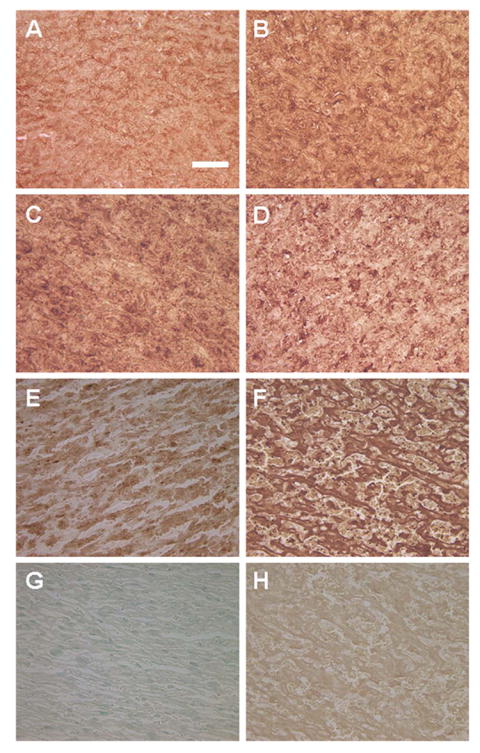
Immunohistochemistry of the rat placental labyrinth from normoxic rats (A, C, E, and G) and hypoxic rats (B, D, F, and H). Localization is shown for EDNRA (A and B), EDNRB (C and D), EDN1 (E and F), PBS control (G), and EDN1-adsorbed control (H). All photomicrographs are at the same magnification. Bar = 100 μm.
In homologous competition studies, unlabeled EDN1 or EDN3 at various concentrations was used to compete against the respective radiolabeled ligand. The Kd values were 3.12 nM for EDN1 and 0.87 nM for EDN3 in normoxic (control) placenta. Hypoxia decreased the Kd values to 23% and 15% of control values, respectively (0.73 nM for EDN1 and 0.13 nM for EDN3), indicating a larger increase in affinity of EDNRB than of EDNRA binding. The EDNRA receptor Bmax was significantly higher than EDNRB Bmax in both normoxic and hypoxic environments (2.4-fold and 2-fold, P < 0.01 and P < 0.05, normoxic and hypoxic, respectively), indicating greater abundance of EDNRA than EDNRB available to bind ligand on cells in these tissues (Fig. 4). Bmax for both receptors was sharply downregulated in hypoxic conditions (74% and 70%, P < 0.001, for EDNRA and EDNRB, respectively), indicating a significant reduction in the number of receptors available for ligand binding, despite the lack of change in receptor mRNA or protein.
FIG. 4.
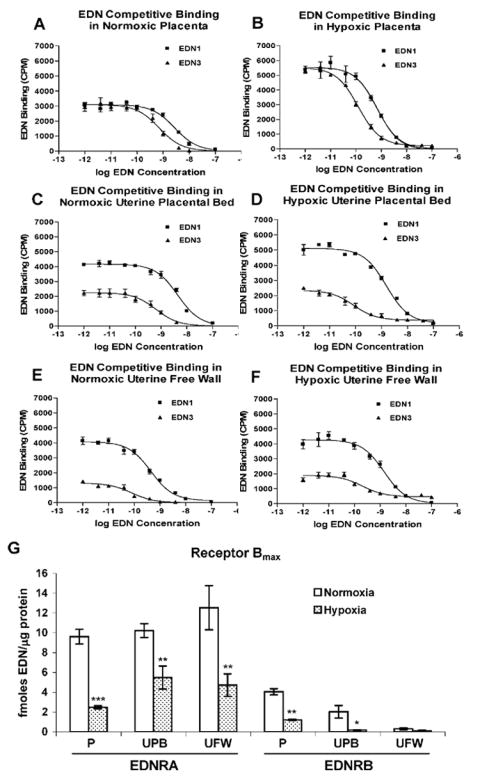
Homologous competitive binding of EDN1 and EDN3 by EDNRA and EDNRB receptors in rat placenta (P), uterine placental bed (UPB), and uterine free wall (UFW). Placental membranes were prepared from normoxic (n = 6) and hypoxic (3 days, Gestational Days 18–21, 12% O2, n = 6) pregnant rats. A, C, and E) Representative binding curves for normoxic placental, uterine placental bed, and uterine free wall membrane preparations (A, C, and E, respectively). B, D, and F) Representative binding curves for hypoxic placental, uterine placental bed, and uterine free wall membrane preparations (B, D, and F, respectively). Each experiment was performed in triplicate. G) The maximum binding coefficient, Bmax, for both receptors is expressed as fmol endothelin/μg protein and each value represents the mean ± SEM. EDN in captions and axis labels refers to both EDN1 and EDN3. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. normoxic control.
Uterine Placental Bed
EDN1 mRNA expression in the uterine placental bed did not differ significantly in response to hypoxia compared with controls (Fig. 1). Correspondingly, placental bed Ednra and Ednrb mRNA expression was not significantly affected by hypoxia, compared with control (Fig. 1). The EDNRA protein expression in hypoxic uterine placental bed decreased 39% (P < 0.01) compared with normoxic controls (Fig. 2), whereas EDNRB protein expression remained unchanged.
Competition studies in normoxic placental bed resulted in Kd values of 3.33 nM for EDN1 and 0.50 nM for EDN3, similar to those in placenta. Hypoxia decreased these values to 2.09 nM for EDN1 and 0.04 nM for EDN3, the greater change for EDN3 indicating a larger increase in EDNRB binding affinity than in EDNRA binding affinity. Placental bed EDNRA receptor Bmax was significantly higher than EDNRB Bmax in both normoxic and hypoxic environments (5-fold and 30-fold, P < 0.001 and P < 0.01, normoxic and hypoxic, respectively), indicating a greater abundance of EDNRA than EDNRB receptors available for ligand binding in this tissue (Fig. 4). Bmax for both receptors was sharply downregulated in hypoxic conditions (46% and 91%, P < 0.01 and P < 0.05 for EDNRA and EDNRB, respectively), indicating a decrease of the number of receptors available for binding in hypoxia.
Uterine Free Wall
EDN1 mRNA expression decreased 98% in response to hypoxia (Fig. 1) in the uterine free wall. Neither Ednra nor Ednrb receptor mRNA expression were significantly decreased (Fig. 1). Western blots showed no difference in either EDNRA or EDNRB protein expression in response to hypoxia (Fig. 2).
EDN1, EDNRA, and EDNRB were localized by immunohistochemistry to the vascular lining in both arterioles and venules in the uterine wall. The staining patterns were the same as reported by many others for EDN1 and its receptors; therefore, the data are not shown. Labeling for all of these antigens was present on only some of the vessels in any particular section, and no difference was observed between groups.
Competition studies in normoxic uterine free wall resulted in Kd values of 5.50 nM for EDN1 and 0.23 nM for EDN3. Hypoxia decreased these values to 2.74 nM for EDN1 and 0.11 nM for EDN3. The EDNRA receptor was responsible for most of the binding of EDN1 in the uterine free wall (Fig. 4), the EDNRA Bmax being 40-fold higher than EDNRB Bmax (P < 0.001 and P < 0.05, normoxia and hypoxia, respectively). Though EDNRB activity remained unchanged in response to hypoxia, EDNRA binding decreased 62% (P < 0.01).
DISCUSSION
In a previous study, we established that EDN1 plays a primary role in the pathophysiology of hypoxia-induced fetal growth restriction in the rat by demonstrating that EDNRA antagonism in the setting of hypoxia produces normal fetal growth [5]. We also demonstrated that hypoxia causes a decrease in placental perfusion, which is normalized by administration of an EDNRA antagonist [8]. The purpose of the present study was to evaluate the effects of chronic maternal hypoxia on the expression of endothelin and its receptors in the uterus and placenta of the rat in order to elucidate more fully the role of EDN1 in hypoxia-induced fetal growth restriction. The results of this study demonstrate that the placenta is the primary source of EDN1 in hypoxia-induced fetal growth restriction.
Expression of EDN1 in response to hypoxia is tissue-dependent. Although the placenta responds to hypoxia with a dramatic upregulation of EDN1 production, the uterus responds by downregulation of EDN1. The downregulation of EDN1 in the uterus may be a compensatory response that maintains uteroplacental perfusion. Upregulation of EDN1 appears to be a pathologic response to hypoxia in the placenta. Alternatively, hypoxia-induced upregulation of EDN1 in the placenta may also be a compensatory response that could lead to increased trophoblast proliferation and invasion, as has been demonstrated in the first trimester in humans [1], with resultant improved placental function. This effect, however, is out-weighed by the adverse impact on placental perfusion of EDN1-induced vasoconstriction.
Receptor mRNA expression and protein levels in the placenta and uterus were essentially unaffected by hypoxia, although receptor binding capacity (Bmax) for both EDNRA and EDNRB was significantly decreased. In all tissues the decrease in the number of receptors available for EDN1 binding was accompanied with an increase in receptor affinity (Kd). The decreased binding capacity along with increased affinity may be a consequence of posttranslational modifications such as receptor phosphorylation or sequestration of receptors intracellularly in recycling endosomes, both of which are well-established mechanisms for cellular regulation of G-protein coupled receptor activity. EDNRA has been shown to have 15 phosphorylation and 5 palmitoylation sites; EDNRB has 13 phosphorylation and 4 palmitoylation sites [9], providing ample potential for regulation. Desensitization and decreased activity have been shown to result from phosphorylation of both receptors [10]. Deglycosylation has been shown to reduce Bmax of both EDNRA and EDNRB by decreasing low-affinity sites while leaving high-affinity sites unaffected, thereby increasing the net receptor affinity [11]. Similarly, arachidonate decreases adenosine A1 receptor Bmax and increases receptor affinity to both agonists and antagonists [12]. Further, recycling endosomes are preserved during crude membrane preparation [13], which would prevent sequestered receptors from binding ligand, resulting in binding that is representative only of cell surface receptors. The fact that receptor binding is consistently downregulated by hypoxia in all three tissues suggests that receptor modification or sequestration/recycling may be methods by which these cells regulate their response to EDN1, an area of investigation that was not the focus of the current study. However, even if decreased receptor availability is a primary protective response, it is insufficient to prevent hypoxia-induced fetal growth restriction. Administration of an EDNRA receptor antagonist is necessary to prevent the pathophysiologic effects of EDN1 activity in this model [5].
Others have previously evaluated the expression of EDN1 and its receptors in the uterus and placenta in the rat. EDN1 is expressed in cytotrophoblasts and trophoblastic giant cells of the basal zone as well as in endothelial cells [14]. In the rat uterus, EDN1 mRNA and EDN1 have been localized to the endometrial glandular and myometrial cells [15]. Horwitz et al. [16] demonstrated placental expression of EDN1 in the labyrinthine and basal zones, and suggested that EDN1 may exert paracrine effects on the placenta or uterus in the rat. Our findings are in agreement with that opinion, particularly under pathophysiologic conditions. Notably, they observed upregulation of placental EDN1 expression as gestation continued. Our results reveal an additional significant increase in placental EDN1 production in response to hypoxia and identify the labyrinth as the most likely source of the increased EDN1. Endothelin receptors have been well-characterized in the rat placenta and uterus. EDNRA is most abundant in the decidual tissue and vascular wall, whereas EDNRB has been localized to the cytotrophoblasts and trophoblastic giant cells of the basal zone [14]. Both receptors are equally represented in the labyrinth. Rat myometrial EDN1 binding sites have been shown to be primarily EDNRA [17]. Our finding that EDNRA is the predominant EDN1 receptor type in the uterine placental bed and free wall is consistent with that conclusion.
In the human uterus and placenta, both EDN1 and its receptors are expressed. In uterine tissue, placental site biopsies have demonstrated EDNRA primarily on the endometrial stromal cells and EDNRB on glandular epithelium [18]. In the placenta, both EDN1 and EDN3 are produced in stem villi vessels—the primary site of placental vascular resistance [19]. Both EDNRA and EDNRB are expressed in the placenta, with EDNRB predominating, particularly in first, but also in third, trimester placentas [20, 21]. EDNRA increases nearer to term and predominates in stem villi vessels, whereas EDNRB expression does not change and predominates in more peripheral vessels, as well as in the decidua [20-22]. Vasoconstriction from increased EDN1 acting on EDNRA in the placenta may contribute to the placental ischemia often associated with fetal growth restriction in humans, just as we have demonstrated in this model of hypoxia-induced fetal growth restriction in the rat. These findings suggest that EDN1 plays an important role in human uteroplacental tissues, as it does in the rat.
Our results as well as those reported by others [14] show that EDNRA and EDNRB are both expressed with neither being heavily predominant in the rat placenta. In contrast, in the human placenta EDNRB is the more abundant receptor [20, 21]. Although the predominance of, and possibly the location of, receptors may vary between species, the function of the receptors remains the same. EDNRA plays a role in regulating placental vascular resistance, causing vasoconstriction particularly in the placental stem villi [22]. EDNRB, on the other hand, produces vasodilation by EDNRB-mediated release of nitric oxide [3] in the distal vessels of the villi as well as in deciduas. EDNRB is also a clearance receptor for excess endothelins [4, 20]. Additionally, EDNRB may function in the regulation of trophoblast replication and invasion early in gestation [23]. Antagonism of EDNRA during the third trimester in the normal rat has no effect on pregnancy outcome, including fetal growth and mortality rate. Conversely, EDNRB antagonism in the normal rat results in decreased placental perfusion, fetal growth restriction, and a high rate of fetal death [24]. Therefore, the selectivity of receptor antagonists has significant implications for pregnancy outcome.
The role of endothelin in the pathophysiology of hypoxia-induced fetal growth restriction in the rat is mediated by increased EDN1 expression in the placenta and by regulation of receptor activity in both the uterus and placenta. Taken in the context of our prior study results demonstrating prevention of hypoxia-induced fetal growth restriction with EDNRA antagonism, these findings describe the interaction of EDN1 and its receptors in the pathophysiology of this model of fetal growth restriction in the rat. The extent to which EDN1 plays a similar role in human pregnancy disorders remains to be more fully evaluated.
Acknowledgments
The authors thank Ms. Karyn Brasky, Mr. Dan Hutchins, Ms. LynAriane Lucas, and Ms. Erica Schwartz at Evanston Northwestern Healthcare for their technical assistance. We also thank Drs. Jinshyun R. Wu-Wong and William Chiou at Abbott Laboratories for their expertise and assistance with the binding experiments.
Footnotes
Supported by National Institutes of Health grants HD34777 and HD01484 (L.T.).
References
- 1.Cervar M, Puerstner P, Kainer F, Desoye G. Endothelin-1 stimulates the proliferation and invasion of first trimester trophoblastic cells in vitro — a possible role in the etiology of pre-eclampsia? J Investig Med. 1996;44:447–453. [PubMed] [Google Scholar]
- 2.Shichiri M, Kato H, Marumo F, Hirata Y. Endothelin-1 as an autocrine/paracrine apoptosis survival factor for endothelial cells. Hypertension. 1997;30:1198–1203. doi: 10.1161/01.hyp.30.5.1198. [DOI] [PubMed] [Google Scholar]
- 3.De Nucci G, Thomas R, D’Orleans-Juste P, Antunes E, Walder C, Warner TD, Vane JR. Pressor effects of circulating endothelin are limited by its removal in the pulmonary circulation and by the release of prostacyclin and endothelium-derived relaxing factor. Proc Natl Acad Sci U S A. 1988;85:9797–9800. doi: 10.1073/pnas.85.24.9797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukuroda T, Fujikawa T, Ozaki S, Ishikawa K, Yano M, Nishikibe M. Clearance of circulating endothelin-1 by ETB receptors in rats. Biochem Biophys Res Commun. 1994;199:1461–1465. doi: 10.1006/bbrc.1994.1395. [DOI] [PubMed] [Google Scholar]
- 5.Thaete LG, Neerhof MG, Caplan MS. Endothelin receptor A antagonism prevents hypoxia-induced intrauterine growth restriction in the rat. Am J Obstet Gynecol. 1997;176:73–76. doi: 10.1016/s0002-9378(97)80014-2. [DOI] [PubMed] [Google Scholar]
- 6.Motulsky H, Christopoulos A. Fitting Models to Biological Data using Linear and Nonlinear Regression. San Diego, CA: GraphPad Software, Inc; 2003. pp. 222–232. [Google Scholar]
- 7.Opgenorth TJ. Endothelin receptor antagonism. Adv Pharmacol. 1995;33:1–65. doi: 10.1016/s1054-3589(08)60665-1. [DOI] [PubMed] [Google Scholar]
- 8.Thaete LG, Dewey ER, Neerhof MG. Endothelin and the regulation of uterine and placental perfusion in hypoxia-induced fetal growth restriction. J Soc Gynecol Investig. 2004;11:16–21. doi: 10.1016/j.jsgi.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 9.Stannard C, Lehenkari P, Godovac-Zimmermann J. Functional diversity of endothelin pathways in human lung fibroblasts may be based on structural diversity of the endothelin receptors. Biochemistry. 2003;42:13909–13918. doi: 10.1021/bi0354132. [DOI] [PubMed] [Google Scholar]
- 10.Freedman NJ, Ament AS, Oppermann M, Stoffel RH, Exum ST, Lefkowitz RJ. Phosphorylation and desensitization of human endothelin A and B receptors Evidence for G protein-coupled receptor kinase specificity. J Biol Chem. 1997;272:17734–17743. doi: 10.1074/jbc.272.28.17734. [DOI] [PubMed] [Google Scholar]
- 11.Shraga-Levine Z, Sokolovsky M. Functional role for glycosylated subtypes of rat endothelin receptors. Biochem Biophys Res Commun. 1998;246:495–500. doi: 10.1006/bbrc.1998.8646. [DOI] [PubMed] [Google Scholar]
- 12.Cunha RA, Constantino MD, Fonseca E, Ribeiro JA. Age-dependent decrease in adenosine A1 receptor binding sites in the rat brain. Effect of cis unsaturated free fatty acids. Eur J Biochem. 2001;268:2939–2947. doi: 10.1046/j.1432-1327.2001.02183.x. [DOI] [PubMed] [Google Scholar]
- 13.Smart EJ, Ying Y-S, Mineo C, Anderson RGW. A detergent-free method for purifying caveolae membrane from tissue culture cells. Proc Natl Acad Sci U S A. 1995;92:10104–10108. doi: 10.1073/pnas.92.22.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shigematsu K, Nakatani A, Kawai K, Moriuchi R, Katamine S, Miyamoto T, Niwa M. Two subtypes of endothelin receptors and endothelin peptides are expressed in differential cell types of the rat placenta: in vitro receptor autoradiographic and in situ hybridization studies. Endocrinol. 1996;137:738–748. doi: 10.1210/endo.137.2.8593825. [DOI] [PubMed] [Google Scholar]
- 15.Kajihara T, Tomioka Y, Hata T, Ghazizadeh M, Asano G. Synthesis of endothelin-1 in rat uterus during pregnancy. J Histochem Cytochem. 1996;44:953–957. doi: 10.1177/44.9.8773560. [DOI] [PubMed] [Google Scholar]
- 16.Horwitz MJ, Clarke MR, Kanbour-Shakir A, Amico JA. Developmental expression and anatomical localization of endothelin-1 messenger ribonucleic acid and immunoreactivity in the rat placenta: a Northern analysis and immunohistochemistry study. J Lab Clin Med. 1995;125:713–718. [PubMed] [Google Scholar]
- 17.Sakamoto S, Obayashi S, Aso T, Sato J, Hamasaki H, Azuma H. The mechanism of myometrial contractions induced by endothelin-1 in rat. Mol Human Reprod. 1997;3:1029–1035. doi: 10.1093/molehr/3.12.1029. [DOI] [PubMed] [Google Scholar]
- 18.Kohnen G, Campbell S, Irvine GA, Church HJ, MacLachlan F, Titterington M, Cameron IT. Endothelin receptor expression in human decidua. Mol Human Reprod. 1998;4:185–193. doi: 10.1093/molehr/4.2.185. [DOI] [PubMed] [Google Scholar]
- 19.Bourgeois C, Robert B, Rebourcet R, Mondon F, Mignot T-M, Duc-Goiran P, Ferré F. Endothelin-1 and ETA receptor expression in vascular smooth muscle cells from human placenta: a new ETA receptor messenger ribonucleic acid is generated by alternative splicing of exon 3. J Clin Endocrinol Metab. 1997;82:3116–3123. doi: 10.1210/jcem.82.9.4209. [DOI] [PubMed] [Google Scholar]
- 20.Mondon F, Anouar A, Ferré F. Endothelin receptor subtypes in the microvillous trophoblastic membrane of early gestation and term human placentas. Eur J Endocrinol. 1998;139:231–237. doi: 10.1530/eje.0.1390231. [DOI] [PubMed] [Google Scholar]
- 21.Cervar M, Huppertz B, Barth S, Hahn T, Weiss U, Kaufmann P, Desoye G. Endothelin A and B receptors change their expression levels during development of human placental villi. Placenta. 2000;21:536–546. doi: 10.1053/plac.2000.0542. [DOI] [PubMed] [Google Scholar]
- 22.Kohnen G, Mackenzie F, Collett GP, Campbell S, Davenport AP, Cameron AD, Cameron IT. Differential distribution of endothelin receptor subtypes in placentae from normal and growth-restricted pregnancies. Placenta. 1997;18:173–180. doi: 10.1016/s0143-4004(97)90090-4. [DOI] [PubMed] [Google Scholar]
- 23.Chakraborty C, Barbin YP, Chakrabarti S, Chidiac P, Dixon SJ, Lala PK. Endothelin-1 promotes migration and induces elevation of [Ca2+]i and phosphorylation of MAP kinase of a human extravillous trophoblast cell line. Mol Cell Endocrinol. 2003;201:63–73. doi: 10.1016/s0303-7207(02)00431-8. [DOI] [PubMed] [Google Scholar]
- 24.Madsen KM, Neerhof MG, Wessale JL, Thaete LG. Influence of ETB receptor antagonism on pregnancy outcome in rats. J Soc Gynecol Investig. 2001;8:239–244. doi: 10.1016/s1071-5576(01)00120-4. [DOI] [PubMed] [Google Scholar]


