Abstract
Background
Evolutionary dynamics plays a central role in facilitating the mechanisms of species divergence among pathogenic and saprophytic mycobacteria. The ability of mycobacteria to colonize hosts, to proliferate and to cause diseases has evolved due to its predisposition to various evolutionary forces acting over a period of time. Mycobacterium indicus pranii (MIP), a taxonomically unknown ‘generalist’ mycobacterium, acts as an immunotherapeutic against leprosy and is approved for use as a vaccine against it. The large-scale field trials of this MIP based leprosy vaccine coupled with its demonstrated immunomodulatory and adjuvant property has led to human clinical evaluations of MIP in interventions against HIV-AIDS, psoriasis and bladder cancer. MIP, commercially available as ‘Immuvac’, is currently the focus of advanced phase III clinical trials for its antituberculosis efficacy. Thus a comprehensive analysis of MIP vis-à-vis evolutionary path, underpinning its immanent immunomodulating properties is of the highest desiderata.
Principal Findings
Genome wide comparisons together with molecular phylogenetic analyses by fluorescent amplified fragment length polymorphism (FAFLP), enterobacterial repetitive intergenic consensus (ERIC) based genotyping and candidate orthologues sequencing revealed that MIP has been the predecessor of highly pathogenic Mycobacterium avium intracellulare complex (MAIC) that did not resort to parasitic adaptation by reductional gene evolution and therefore, preferred a free living life-style. Further analysis suggested a shared aquatic phase of MAIC bacilli with the early pathogenic forms of Mycobacterium, well before the latter diverged as ‘specialists’.
Conclusions/Significance
This evolutionary paradigm possibly affirms to marshal our understanding about the acquisition and optimization of virulence in mycobacteria and determinants of boundaries therein.
Introduction
Genus Mycobacterium represents some of man's most potent microbiological adversaries like M. tuberculosis (MTB), the tuberculosis (TB) causing bacterium that is responsible for the loss of more than 50,000 human lives every week, globally. Also, leprosy caused by M. leprae, is still a major public health problem [1] whereas M. avium and other ‘opportunist’ mycobacteria are the major cause of disease and death in immune compromised hosts, including HIV patients. Moreover, the advent of XDR [2] and MDR [3] strains of MTB coupled to prevalence of HIV co-infection and the emergence of TB-IRIS (Immune Responsive Inflammatory Syndrome) [4], has exacerbated the situation. Despite better insights into the molecular basis of disease pathogenesis, substantial gaps persist in our understanding of the evolution of the soil-derived mycobacterial progenitors into ‘seasoned’ pathogens, and their effective prophylaxis.
Mycobacterium indicus pranii (MIP), formerly Mycobacterium w [5], is an atypical saprophytic bacterium that was listed in Runyon Group IV, along with M. fortuitum, M. smegmatis, M. chelonae and M. vaccae, based on its growth and metabolic properties [6]. MIP, in an extended phase III clinical trial in India, was used as an adjunct to the standard multidrug therapy with multibacillary leprosy patients and showed a significantly enhanced bacillary clearance, thus, shortening the full recovery time [6], [7]. MIP, commercially available as “Immuvac” vaccine, also exhibited immunoprophylactic benefits in household contacts of leprosy patients in the largest ever clinical trial in India [8]. It not only shares antigens with M. leprae and M. tuberculosis but also provides protection to both BCG responder and non-responder genetic strains of mice against M. tuberculosis H37Rv infection [9]. Based on a strong indication of its immuno-therapeutic role in category II tuberculosis patients [10], large-scale phase III trials are currently in progress to evaluate its anti-tuberculosis efficacy. MIP is also under clinical trials as an immuno-modulator and adjuvant, based on encouraging findings about its role in HIV [11], bladder cancer [12] and psoriasis [13].
To optimally harness the therapeutic potential of MIP, it is imperative to understand how MIP shaped its exceptional immunomodulating properties akin to a philanthropic vaccine strain without embracing the dreadful pathogenic attributes of MTB. Conventionally, evolutionary and comparative genomic studies have been Rosetta stone to gain insights into mycobacterial divergence and acquisition of virulence therein. The epistemological studies involving extensive genomic characterization of MIP using several molecular tools and markers along with the comparative genomic studies with its whole genome data reveal that MIP has been the predecessor of MAIC bacilli and shared a common aquatic phase with early pathogenic forms of mycobacteria thus, presenting a holistic picture of Mycobacterium evolution.
Results
MIP belongs to MAIC
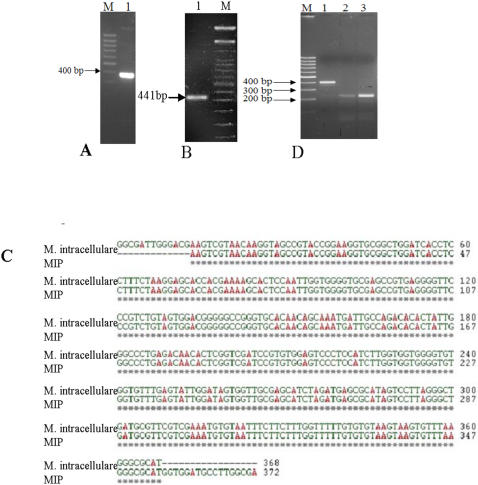
MIP genomic DNA was subjected to different diagnostic PCRs targeted at signature sequences such as internal transcribed spacer (ITS) region between rrn16 and rrn23 genes and 65 kDa heat shock protein (hsp65) gene (Figure 1). The amplicons corresponding to these loci were sequenced and aligned to evaluate sequence similarity of MIP with other mycobacterial bacilli. The ITS region matched perfectly well with the corresponding region of M. intracellulare (Figure 1C) pointing to the possible genetic affinity of MIP to MAIC complex. In hsp65, all the four nucleotide substitutions, reported as MIP signatures, were identified at the right locations, thereby confirming the identity of MIP used in this study [14]. The IS900 specific PCR, considered as a signature for MAPC (M. avium subsp. paratuberculosis complex) bacilli, however, did not yield PCR product specific to MAPC (Figure 1), thereby excluding an evolutionary link between MIP and MAPC. The three principle genetic groups (PGG) based on the single nucleotide polymorphisms of katG 463CTG (Leu) and gyrA 95ACC (Thr) (15) showed that MIP and MAIC bacilli belong to group 1 (primitive) that includes pathogenic branch members like M. marinum, M. ulcerans and MTB W-Beijing strain.
Figure 1. Confirmation of the genetic signatures of MIP (A) PCR with ITS region sequences showing a ∼350 bp amplicon, M-100 bp marker (B) MIP strain confirmation PCR with hsp65 gene region, M-100 bp marker.
This 441 bp amplicon was having all the 4 unique substitutions known for MIP (C) Sequence alignment of ITS sequence of MIP with MAIC organism (D) IS900 PCR to rule out MAPC lineage-M: marker, Lane 1: M. paratuberculosis control PCR, lanes 2 and 3: MIP.
Phylogenetic placement
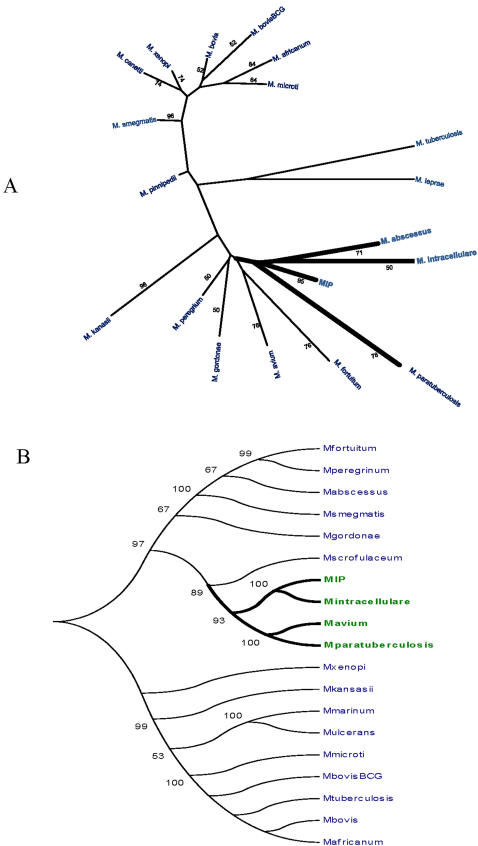
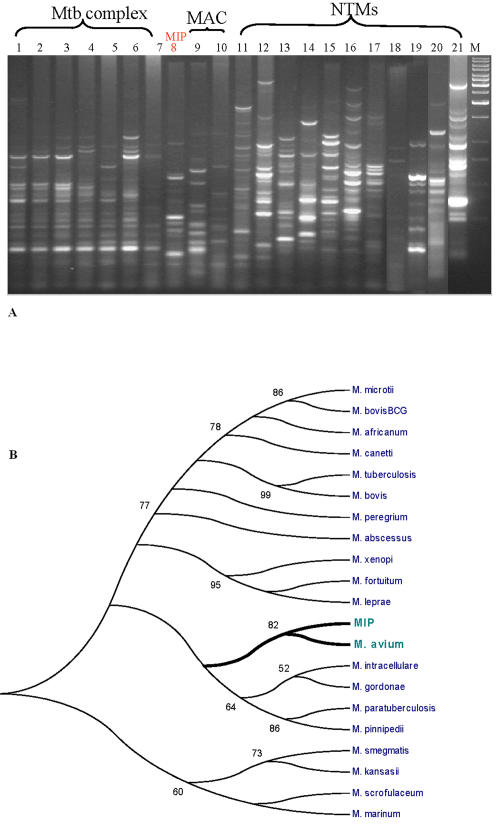
FAFLP [16] and ERIC [17] are the whole genome based cardinal genotyping approaches that complement the piecemeal studies with candidate genes that may be influenced by horizontal gene transfer events in closely related species. FAFLP analysis (Figure 2A) as well as ERIC based molecular typing revealed considerable genetic similarity of MIP with MAIC (Figure 3). MIP, along with the members of MAIC, formed a separate group. This method did not reveal any significant genetic similarity between the bacilli belonging to M. tuberculosis complex (MTBC) and MIP. All the pathogenic species, including M. leprae, formed a separate cluster. MIP was found to be genetically linked to M. intracellulare and M. abcessus. All the candidate gene sequences upon alignment with known databank sequences of MTBC, MAPC, MAIC and other non-tuberculous mycobacteria (NTMs), revealed substantial sequence similarities between MIP and MAC as well as MAIC bacilli. Construction of a phylogenetic tree based on concatenated multigene “super locus” comprising of sequences derived from rrn16, sodA, hsp65, rpoB and recA eventually placed MIP into MAIC cluster [18]. MIP DNA sequences corresponding to candidate orthologues have been deposited to Genbank (DQ437715-437722).
Figure 2. Phylogenetic trees based on FAFLP and multigene sequence analyses. A. Polymorphic fragments were subjected to allele calling in Genotyper (Applied Biosystems, USA) and allele scoring was recorded in a binary format.
This binary data were used to construct a phylogenetic tree (please see methods). B. Phylogenetic tree constructed based on consensus multigene alignment which involved concatenation of individual gene sequences corresponding to rpoB, recA, sodA, rrn16 and hsp65. Bootstrap values conveying significance of the internal branch topology are clearly marked near each branch. Values above 50 were deemed to be significant to convey correct topology of the internal branches.
Figure 3. ERIC analysis of MIP, other tubercle bacilli and NTMs.
A. ERIC based fingerprinting. B. Phylogenetic analysis. Polymorphic ERIC fragments were subjected to allele calling in Quantity 1 software (Biorad, USA) and scoring was recorded in a binary format. These binary data were used to construct a phylogenetic tree developed using bootstrapping methods in MEGA software. Bootstrap values for the internal branch topology are clearly marked near each branch. Values above 50 were assumed as significant to convey acceptable topology of the internal branches.
The phylogeny with gyrB [19] and 32 kDa protein genes [20], which are crucial to infer evolutionary relationship in closely related species also confirmed the above findings (Figure S1). The 32 kDa protein coding gene, despite having an identical sequence in strains belonging to M. tuberculosis complex (M. tuberculosis, M. bovis, M. bovis BCG and M. microti), considerably differs at the nucleotide level in case of M. avium and M. intracellulare [20]. Likewise, gyrB gene is a house-keeping gene, found in almost all bacteria and does not appear to be frequently horizontally transmitted [21]. The alignment of gyrB of MAIC bacilli and MIP showed just one substitution in MIP sequence with respect to M. intracellulare as compared to 119 of MAP (Figure S2). This almost 100% conservation of gyrB between MIP and M. intracellulare is surprising since gyrB evolves rapidly due to its faster evolution rate. In case of 32 kDa protein coding gene, similar analysis of MIP with that of M. avium complex revealed 98% similarity with M. intracellulare as compared to 93% of MAP (Figure S3), thus firmly establishing phylogenetic proximity of MIP to Mycobacterium intracellulare.
Evolution of mycobacterial ‘generalists’ versus ‘specialists’
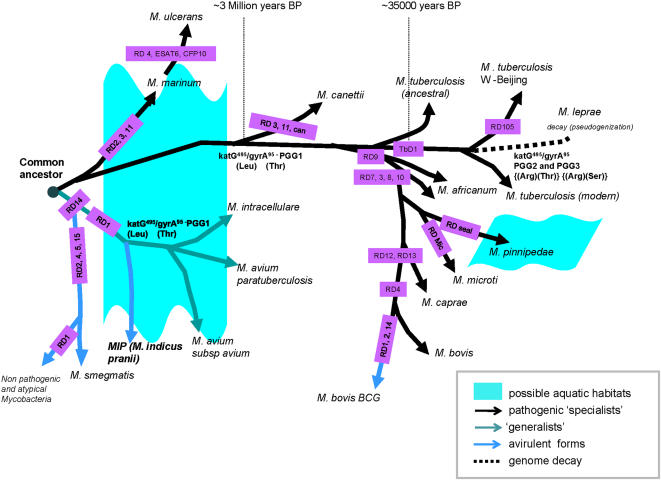
The comparative genome analyses have been employed to dissect the intricate mechanisms of ancient and contemporary dissemination and evolution of mycobacteria [22], [23]. Considering their broadly clonal population structure, it has been hypothesized that a single strain may not have a sufficiently divergent genetic material to qualify for a species status and, therefore, speciation in Mycobacterium most likely has arisen due to specific deletion events [22]. Lately, several genomic regions of deletions (RDs), considered as an important evolutionary paradigm in mycobacteria, have been shown to be associated with the attenuation of virulence [23], [24]. The analyses revealed that MIP has shown a congruent pattern of RD's with respect to the members of MAIC and a similar pattern with that of early pathogenic members of MTBC like M. marinum and M. ulcerans. The RD1 locus, encoding ESAT6 and CFP10 proteins putatively associated with virulence, was absent in MIP and MAIC bacilli, but present in M. marinum. While RD4 is absent in M. ulcerans, it is only partially deleted in M. marinum. In M. ulcerans, ESAT6 and CFP10, known to enhance DNA transfer, are deleted [25]. Acquisition of plasmids in the early group of bacilli, with some deficient in RD1, appears to be a faster mode to gain novel functions for diversification as exemplified by similar synonymous substitution frequencies for plasmid and chromosomal encoded genes in M. ulcerans [26]. Likewise, RD3 and RD11 are absent in MIP and MAIC as well as in M. marinum, M. ulcerans and M. canetti, but RD2 is present in M. canetti (Table 1). Similarly, MIP and MAIC bacilli can be typified by the absence of RD6 region. It is noteworthy that RD6 constitutes IS1532 transposase integrations while RD3 and RD11 represents phiRv phage based integrations into the genomes that may be helpful to generate antigenic diversities. The presence of these regions in the subsequent MTBC members, however, suggests that these were deleted only in some lineages of tubercle bacilli. Alternatively, these regions might have been regained via phiRv phage based transductions in recently evolved MTBC members.
Table 1. Distribution of Regions of deletion (RDs) across the Mycobacterial ‘specialists’ and ‘generalists’.
| Region | M. marinum | M. ulcerans | M. canetti | M. africanum | M. microti | M. bovis | M. bovis BCG | M.tb H37Rv | M. smegmatis | MIP | MAP | MAA |
| RD1 | Present | Partially present | Present | Present | Present | Present | Absent | Present | Present | Absent | Absent | Absent |
| RD2 | Partially present | Partially present | Present | Present | Present | Present | Absent | Present | Absent | Partially present | Partially present | Partially present |
| RD3 | Absent | Absent | Absent | Absent | Present | Absent | Present | Present | Absent | Absent | Absent | Absent |
| RD4 | Partially present | Absent | Present | Present | Present | Absent | Absent | Present | Absent | Partially present | Partially present | Partially present |
| RD5 | Present | Present | Present | Present | Absent | Absent | Absent | Present | Absent | Partially present | Partially present | Partially present |
| RD6 | Absent | Absent | Present | Present | Absent | Absent | Absent | Present | Absent | Absent | Absent | Absent |
| RD7 | Present | Present | Present | Present | Absent | Absent | Absent | Present | Partially present | Partially present | Partially present | Partially present |
| RD8 | Present | Present | Present | Present | Absent | Absent | Absent | Present | Partially present | Present | Present | Present |
| RD9 | Present | Present | Present | Absent | Absent | Absent | Absent | Present | present | Present | Present | Present |
| RD10 | Present | Present | Present | Present | Absent | Absent | Absent | Present | Present | Present | Present | Present |
| RD11 | Absent | Absent | Absent | Present | Present | Absent | Present | Present | Absent | Absent | Absent | Absent |
| RD12 | Partially present | Partially present | Present | Present | Present | Absent | Present | Present | Partially present | Partially present | Partially present | Partially present |
| RD13 | Present | Present | Present | Present | Present | Absent | Present | Present | Present | Present | Present | Present |
| RD14 | Partially present | Partially present | Present | Present | Present | Present | Present | Present | Absent | Absent | Absent | Absent |
| RD15 | Partially present | Partially present | Not studied | Not studied | Not studied | Absent | Present | Present | Absent | Partially present | Partially present | Partially present |
| RD16 | Present | Present | Not studied | Not studied | Not studied | Absent | Present | Present | Present | Present | Present | Present |
Furthermore, the evaluation of MIP, based on whole genome data at 10X coverage (Saini et al, unpublished), revealed that its genome content is ∼20% in excess to that of M. avium. The phylogenetic data in light of genome size evaluation placed MIP way ahead of MAIC bacilli on an evolutionary time scale (Figure 4). Also, M. marinum, in pathogenic branch, and M. smegmatis together with MIP, within the ‘generalist’ branch, appear as the immediate descendants of the ancient mycobacterial progenitor (Figure 4).
Figure 4. Landscape of genome evolution across the ‘generalist’ and ‘specialist’ mycobacterial lineages.
Various RDs refer to genomic deletion points as discussed elsewhere [24]. Divergence of M. tuberculosis W-Beijing was deduced based on the RD105 [41]. The inferred time points of some important lineage divergences mentioned as years-before-present (years BP) on the basis of the conventions established earlier [15], [24], [42]. For further details please refer to manuscript text.
Discussion
The saprophytic mycobacteria are believed to play a protective role in chronic infections like asthma and TB [27]. However, clinical trials with saprophytic M. vaccae didn't benefit TB patients and led to local adverse reactions in recipients [28]. Contrarily, MIP, besides giving protection against TB and AIDS infections, elicits immune responses even in the heat inactivated form [29], [30]. Considering variable BCG efficacy and increasing HIV/TB pandemic, alternate strategies involving MIP are pertinent to confront this deadly duo that has caused nearly 150 million deaths since World War I-more total deaths than in all wars in the last 2000 years [31].
The phylogenetic link of saprophytic MIP to the non-tubercle MAIC bacilli came as a surprise especially as all the members of this ilk are slow growers that are only second to MTBC bacilli in terms of their ability to infect immune compromised humans and are classically termed as ‘pathogens’. Besides, they are believed to be responsible for Crohn's disease in humans and John's disease in ruminants, especially dairy cattle, causing extensive economic loses to farmers. MAIC bacilli, though formally divided into M. avium subsp avium (MAA), M. avium subsp paratuberculosis (MAP) and M. intracellulare, remain a challenge to mycobacterial taxonomy due to high heterogeneity within their constituent species. Although the evolution of highly niche adapted parasitic forms by genomic downsizing is an accepted norm in MTB [24], [32], the details remain obscure for MAIC bacilli. The adaptation of an organism to parasitic lifestyle in a particular host renders various metabolic gene functions redundant due to non-functionalization or pseudogenization of some genes or deletion of the large genomic chunks and the host machinery is utilized to cater these metabolic needs. M. leprae, Shigella flexneri and Salmonella typhi have undergone similar miniaturization by extensive genome reduction [33]. The genome content could, as per this analogy, be inversely proportional to the fitness of the organism in animal hosts. When we look at the genetic similarity of MAIC or MAPC bacilli to MIP in the light of its genome size, it is evident that the former are highly pathogenic and evolved organisms that have shed genes detrimental to pathogenic lifestyle in particular niches.
Since mycobacteria have a restricted synonymous polymorphism rate [15], this implies that deletion or acquisition of the genes might be a more important mechanism than point mutations for generating niche specific evolutionary novelties. The evidence has emerged for lateral gene acquisitions unique to MAC, absent in MTB and MAP, facilitating its intracellularization in protozoic hosts [34]. Similarly, a significant chunk (∼4%) of MAP genome is reportedly shaped up by lateral gene acquisitions [35]. Interestingly, MIP and MAA harbor about 60% and 75% (E<10−5) of the genes reported to be laterally acquired in MAP, most of them proteobacterial in origin (Saini et al, unpublished). These findings are consistent with the ability of MAIC bacilli to survive in an extensive range of habitats including soil and water despite their extreme genetic homogeneity and a low rate of recombination. The presence of laterally acquired gene homolog of rsbR, a possible sigma B regulator, in MIP and MAIC might be seen as one of the plausible mechanisms to overcome stress as this gene has been found to be a positive regulator of sigB under stress conditions in B. subtilis [36]. On the other hand although MTBC bacilli appear to be relatively non-amenable to lateral gene acquisition [37], yet only M. marinum and M. ulcerans, the early pathogenic branch members (“specialists”), were found to possess significant proportions of these genes reported to be laterally acquired in MAP (13% and 7.5%, respectively, data not shown). The proteobacterial origins of such major chunk of genome in MIP and MAIC bacilli and a similar RD profile of these bacilli with respect to early pathogenic lineage of MTBC envisage plausible similarities in early life style of the ‘generalist’ and ‘specialist’ groups. Since lateral gene transfers are influenced by physical proximity, it is tempting to speculate that earliest pathogenic and non-pathogenic members of Mycobacterium might have shared a common aquatic phase before diversification [38] (Figure 4).
Our findings, thus, in the light of above discussion provide a novel perspective of mycobacterial evolution and we hypothesize that the progenitors of MAIC and MTBC could have been soil dwelling microbes like MIP that preferred water bodies, probably due to nutritional needs and shared a common aquatic phase in their early life history. These bacilli subsequently augmented their fitness for niche specific adaptations by tuning their genomic repertoires with a constant genetic flux aided by extensive and selective lateral gene acquisitions counter balanced by a directional genome decay concomitant with their wide host range. Also, in the backdrop of MIP's congruent RD profile with opportunistic MAIC, it appears that immunomodulation in MIP, unlike M. bovis BCG, may not be due to selective deletion events but rather it is the selective acquisition of genes that shaped up its antigenic repertoire. It, therefore, becomes perceptible that MIP existed in nature much before the divergence of MAIC and MAPC and, therefore, could well be the ancestor of the MAIC bacilli that did not parasitize human or animal niches. It has not escaped our notice that the genetic similarity between MIP and MAP immediately suggests a plausible advantage for therapeutic intervention against Crohn's and Johne's diseases.
Materials and Methods
Bacterial cultures and DNA samples
MIP stock culture was a kind gift from Rajani Rani, National Institute of Immunology, New Delhi, India. The bacteria were streaked onto Middlebrook (MB) 7H11 agar plate supplemented with 1xOADC. Culture was also streaked onto LB agar to check for any contaminating bacteria. Once the purity of the culture was confirmed [14], MIP genomic DNA was isolated from culture grown in Middlebrook (MB) 7H9 liquid medium supplemented with 0.5% glycerol, 0.2% Tween-80, 1xADC with constant shaking (200 rpm) at 37°C. The genomic DNA of MTB was obtained from the RIVM, Bilthoven, Netherlands and the DNA of several other non-tuberculous mycobacteria (NTMs) such as M. avium, M. intracellulare, M. smegmatis, M. xenopi, M. abscessus, M. fortuitum/peregrinum, M. scrofulaceum, M. fortuitum, M. gordonae, M. paratuberculosis, M. marinum and M. kansasii were a gift from Leonardo A. Sechi, Dept. of Biomedical Sciences, University of Sassari, Italy.
DNA fingerprinting and genotyping
DNA fingerprinting by fluorescent amplified fragment analysis was performed as described previously [16], [39]. Briefly, the profiling of whole genome micro-restriction fingerprints with EcoRI/MseI enzymes using fluorescence tagged primer pairs EcoRI+A/MseI+0 and EcoRI+G or A / MseI+0 was performed for all the strains. The PCR amplified fragments for each of the strains were then subjected to electrophoretic separation on a 5% acrylamide gel and scoring of the fluorescent markers was done using an automated DNA analysis workstation (ABI Prism 3100 DNA sequencer).
ERIC based genotyping was carried out by PCR using primers ERIC1R (5-ATGTAAGCTCCTGGGGATTCAC) and ERIC2 (5-AAGTAAGTGACTGGGGTGAGCG). Amplification reactions were performed in a total volume of 20 µl containing 50 ng of DNA, 1X PCR buffer (Applied Biosystems), 2 mM MgCl2 (Applied Biosystems), 200 mM deoxynucleoside triphosphate, 20 picomoles of each primer and 2 U of Taq DNA polymerase (Applied Biosystems, USA). The reaction mixtures were incubated in GeneAmp PCR system 9700 for 2 min at 94°C, followed by 35 cycles of 94°C for 45 s, 52°C for 1 min, and 70°C for 10 min and a final extension at 70°C for 20 min as described previously [17].
Comprehensive phylogeny and comparative genomics
A comprehensive genomic characterization of MIP was carried out based on several molecular signatures: i) FAFLP based high resolution fingerprinting [16], ii) ERIC typing [17], iii) sequencing of candidate orthologues corresponding to rrn16, hsp65, sodA, rpoB, gyrB, recA, ITS and the 32 kDa protein coding gene [18], [19], [20] iv) presence/absence of RDs [24], [32], and, v) katG and gyrA polymorphisms [15]. With respect to these features, MIP was compared against 9 genome sequences of mycobacteria including M. marinum, M. ulcerans, M. tuberculosis H37Rv, M. tuberculosis CDC1551, M. bovis, M. bovis BCG, M. avium subsp paratuberculosis, M. smegmatis and M. avium subsp avium. The genomes were downloaded from NCBI (ftp://ftp.ncbi.nih.gov/genomes/Bacteria/). Phylogenetic trees based on the candidate gene polymorphisms in either individual genes or their concatenated ‘super loci’ were obtained by using the neighbour joining method with Kimura-2 parameter (K2P) and a distance correction model with 1000 bootstrap replicates [in MEGA3.1; 40]. In case of FAFLP data, the trees were constructed using the binary data by minimum evolution method with topology validated through an interior branch test, a t-test, which is computed using the bootstrap procedure. ERIC based phylogenetic trees were also obtained based on binary data, but by using the neighbor joining method with nucleotide p distance model with 100 bootstrap replicates [in MEGA3.1; 40]. Internal branch topology was validated via the interior branch test.
Whole-genome sequencing
The shotgun data for MIP genome were generated from paired end sequences from whole genome shot gun libraries with an average insert size of 2–3 kb and 4–5 kb using BDT (big dye terminator) technology on ABI3700 DNA sequencers. The data were subsequently assembled and analyzed using Phred/Phrap/Consed package available from University of Washington, U.S.A.
Supporting Information
Phylogenetic trees based on comparison of the DNA sequences corresponding to gyrB gene (A) and 32kDa protein gene (B) of MIP and other mycobacteria. Sequence alignment was performed in Clustal W software and phylogenetic trees were developed in MEGA3.1 using bootstrapping method.
(0.11 MB PPT)
Alignment of gyrB of MIP with other members of MAIC complex (M. avium and M. intracellulare)
(0.15 MB PPT)
Alignment of 32kDa protein gene of MIP with other members of MAIC complex (M. avium and M. intracellulare). The identities are depicted by dots only.
(0.12 MB PPT)
Footnotes
Competing Interests: The authors have declared that no competing interests exist.
Funding: This work was supported by a research grant (BT/PR4127/MED/12/165/2003) to NA, JPK, AKT, AKT and SEH from the Department of Biotechnology of the Indian Ministry of Science and Technology. VS acknowledges CSIR, India for research studentship (CSIR JRF/SRF).
References
- 1.Cole ST, Eiglmeier K, Parkhill J, James KD, Thomson NR, et al. Massive gene decay in the leprosy bacillus. Nature. 2001;409(6823):1007–1011. doi: 10.1038/35059006. [DOI] [PubMed] [Google Scholar]
- 2.Raviglione MC, Smith IM. XDR tuberculosis–implications for global public health. N Engl J Med. 2007;356(7):656–659. doi: 10.1056/NEJMp068273. [DOI] [PubMed] [Google Scholar]
- 3.Siddiqi N, Shamim M, Jain NK, Rattan A, Amin A, et al. Molecular genetic analysis of multi-drug resistance in Indian isolates of Mycobacterium tuberculosis. Mem Inst Oswaldo Cruz. 1998;93(5):589–594. doi: 10.1590/s0074-02761998000500006. [DOI] [PubMed] [Google Scholar]
- 4.French MA, Price P, Stone SF. Immune restoration disease after antiretroviral therapy. Aids. 2004;18(12):1615–1627. doi: 10.1097/01.aids.0000131375.21070.06. [DOI] [PubMed] [Google Scholar]
- 5.Talwar GP, Ahmed N, Saini V. The use of the name Mycobacterium w for the leprosy immunotherapeutic bacillus creates confusion with M. tuberculosis-W (Beijing strain): A suggestion. Infect Genet Evol doi:10.1016/j.meegid.2007.07.009. 2007 doi: 10.1016/j.meegid.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 6.Zaheer SA, Mukherjee R, Ramkumar B, Misra RS, Sharma AK, et al. Combined multidrug and Mycobacterium w vaccine therapy in patients with multibacillary leprosy. J Infect Dis. 1993;167(2):401–410. doi: 10.1093/infdis/167.2.401. [DOI] [PubMed] [Google Scholar]
- 7.Yadava A, Suresh NR, Zaheer SA, Talwar GP, Mukherjee R. T-cell responses to fractionated antigens of Mycobacterium w, a candidate anti-leprosy vaccine, in leprosy patients. Scand J Immunol. 1991;34(1):23–31. doi: 10.1111/j.1365-3083.1991.tb01517.x. [DOI] [PubMed] [Google Scholar]
- 8.Sharma P, Mukherjee R, Talwar GP, Sarathchandra KG, Walia R, et al. Immunoprophylactic effects of the anti-leprosy Mw vaccine in household contacts of leprosy patients: clinical field trials with a follow up of 8–10 years. Lepr Rev. 2005;76(2):127–143. [PubMed] [Google Scholar]
- 9.Singh IG, Mukherjee R, Talwar GP, Kaufmann SH. In vitro characterization of T cells from Mycobacterium w-vaccinated mice. Infect Immun. 1992;60(1):257–263. doi: 10.1128/iai.60.1.257-263.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Patel N, Deshpande MM, Shah M. Effect of an immunomodulator containing Mycobacterium w on sputum conversion in pulmonary tuberculosis. J Indian Med Assoc. 2002;100(3):191–193. [PubMed] [Google Scholar]
- 11.Kharkar R. Immune recovery in HIV with Mycobacterium W. J Indian Med Assoc. 2002;100(9):578–579. [PubMed] [Google Scholar]
- 12.Chaudhuri P, Mukhopadhyay S. Bladder preserving approach for muscle invasive bladder cancer–role of mycobacterium w. J Indian Med Assoc. 2003;101(9):559–560. [PubMed] [Google Scholar]
- 13.Rath N, Kar HK. Efficacy of intradermal heat-killed Mycobacterium w in psoriasis: a pilot study. Int J Dermatol. 2003;42(9):756–757. doi: 10.1046/j.1365-4362.2003.01962.x. [DOI] [PubMed] [Google Scholar]
- 14.Reddi PP, Amin AG, Khandekar PS, Talwar GP. Molecular definition of unique species status of Mycobacterium w; a candidate leprosy vaccine strain. Int J Lepr Other Mycobact Dis. 1994;62(2):229–236. [PubMed] [Google Scholar]
- 15.Sreevatsan S, Pan X, Stockbauer KE, Connell ND, Kreiswirth BN, et al. Restricted structural gene polymorphism in the Mycobacterium tuberculosis complex indicates evolutionarily recent global dissemination. Proc Natl Acad Sci U S A. 1997;94(18):9869–9874. doi: 10.1073/pnas.94.18.9869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmed N, Alam M, Abdul Majeed A, Asad Rahman S, Cataldi A, et al. Genome sequence based, comparative analysis of the fluorescent amplified fragment length polymorphisms (FAFLP) of tubercle bacilli from seals provides molecular evidence for a new species within the Mycobacterium tuberculosis complex. Infect Genet Evol. 2003;2(3):193–199. doi: 10.1016/s1567-1348(02)00100-4. [DOI] [PubMed] [Google Scholar]
- 17.Sechi LA, Zanetti S, Dupre I, Delogu G, Fadda G. Enterobacterial repetitive intergenic consensus sequences as molecular targets for typing of Mycobacterium tuberculosis strains. J Clin Microbiol. 1998;36(1):128–132. doi: 10.1128/jcm.36.1.128-132.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devulder G, Perouse de Montclos M, Flandrois JP. A multigene approach to phylogenetic analysis using the genus Mycobacterium as a model. Int J Syst Evol Microbiol. 2005;55(Pt 1):293–302. doi: 10.1099/ijs.0.63222-0. [DOI] [PubMed] [Google Scholar]
- 19.Yamamoto S, Harayama S. PCR amplification and direct sequencing of gyrB genes with universal primers and their application to the detection and taxonomic analysis of Pseudomonas putida strains. Appl Environ Microbiol. 1995;61(3):1104–1109. doi: 10.1128/aem.61.3.1104-1109.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soini H, Bottger EC, Viljanen MK. Identification of mycobacteria by PCR-based sequence determination of the 32-kilodalton protein gene. J Clin Microbiol. 1994;32(12):2944–2947. doi: 10.1128/jcm.32.12.2944-2947.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamamoto S, Harayama S. Phylogenetic analysis of Acinetobacter strains based on the nucleotide sequences of gyrB genes and on the amino acid sequences of their products. Int J Syst Bacteriol. 1996;46(2):506–511. doi: 10.1099/00207713-46-2-506. [DOI] [PubMed] [Google Scholar]
- 22.Mahairas GG, Sabo PJ, Hickey MJ, Singh DC, Stover CK. Molecular analysis of genetic differences between Mycobacterium bovis BCG and virulent M. bovis. J Bacteriol. 1996;178(5):1274–1282. doi: 10.1128/jb.178.5.1274-1282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Behr MA, Wilson MA, Gill WP, Salamon H, Schoolnik GK, et al. Comparative genomics of BCG vaccines by whole-genome DNA microarray. Science. 1999;284(5419):1520–1523. doi: 10.1126/science.284.5419.1520. [DOI] [PubMed] [Google Scholar]
- 24.Brosch R, Gordon SV, Marmiesse M, Brodin P, Buchrieser C, et al. A new evolutionary scenario for the Mycobacterium tuberculosis complex. Proc Natl Acad Sci U S A. 2002;99(6):3684–3689. doi: 10.1073/pnas.052548299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Flint JL, Kowalski JC, Karnati PK, Derbyshire KM. The RD1 virulence locus of Mycobacterium tuberculosis regulates DNA transfer in Mycobacterium smegmatis. Proc Natl Acad Sci U S A. 2004;101(34):12598–12603. doi: 10.1073/pnas.0404892101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stinear TP, Jenkin GA, Johnson PD, Davies JK. Comparative genetic analysis of Mycobacterium ulcerans and Mycobacterium marinum reveals evidence of recent divergence. J Bacteriol. 2000;182(22):6322–6330. doi: 10.1128/jb.182.22.6322-6330.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rook GA, Hamelmann E, Brunet LR. Mycobacteria and allergies. Immunobiology. 2007;212(6):461–473. doi: 10.1016/j.imbio.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 28.de Bruyn G, Garner P. Mycobacterium vaccae immunotherapy for treating tuberculosis. Cochrane Database Syst Rev. 2003;(1):CD001166. doi: 10.1002/14651858.CD001166. [DOI] [PubMed] [Google Scholar]
- 29.Talwar GP, Zaheer SA, Mukherjee R, Walia R, Misra RS, et al. Immunotherapeutic effects of a vaccine based on a saprophytic cultivable mycobacterium, Mycobacterium w in multibacillary leprosy patients. Vaccine. 1990;8(2):121–129. doi: 10.1016/0264-410x(90)90134-8. [DOI] [PubMed] [Google Scholar]
- 30.Talwar GP. An immunotherapeutic vaccine for multibacillary leprosy. Int Rev Immunol. 1999;18(3):229–249. doi: 10.3109/08830189909043027. [DOI] [PubMed] [Google Scholar]
- 31.Kaufmann SH, McMichael AJ. Annulling a dangerous liaison: vaccination strategies against AIDS and tuberculosis. Nat Med. 2005;11(4 Suppl):S33–44. doi: 10.1038/nm1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rao KR, Kauser F, Srinivas S, Zanetti S, Sechi LA, et al. Analysis of genomic downsizing on the basis of region-of-difference polymorphism profiling of Mycobacterium tuberculosis patient isolates reveals geographic partitioning. J Clin Microbiol. 2005;43(12):5978–5982. doi: 10.1128/JCM.43.12.5978-5982.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dagan T, Blekhman R, Graur D. The “domino theory” of gene death: gradual and mass gene extinction events in three lineages of obligate symbiotic bacterial pathogens. Mol Biol Evol. 2006;23(2):310–316. doi: 10.1093/molbev/msj036. [DOI] [PubMed] [Google Scholar]
- 34.Danelishvili L, Wu M, Stang B, Harriff M, Cirillo S, et al. Identification of Mycobacterium avium pathogenicity island important for macrophage and amoeba infection. Proc Natl Acad Sci U S A. 2007;104(26):11038–11043. doi: 10.1073/pnas.0610746104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marri PR, Bannantine JP, Paustian ML, Golding GB. Lateral gene transfer in Mycobacterium avium subspecies paratuberculosis. Can J Microbiol. 2006;52(6):560–569. doi: 10.1139/w06-001. [DOI] [PubMed] [Google Scholar]
- 36.Akbar S, Kang CM, Gaidenko TA, Price CW. Modulator protein RsbR regulates environmental signalling in the general stress pathway of Bacillus subtilis. Mol Microbiol. 1997;24(3):567–578. doi: 10.1046/j.1365-2958.1997.3631732.x. [DOI] [PubMed] [Google Scholar]
- 37.Rosas-Magallanes V, Deschavanne P, Quintana-Murci L, Brosch R, Gicquel B, et al. Horizontal transfer of a virulence operon to the ancestor of Mycobacterium tuberculosis. Mol Biol Evol. 2006;23(6):1129–1135. doi: 10.1093/molbev/msj120. [DOI] [PubMed] [Google Scholar]
- 38.Matte-Tailliez O, Brochier C, Forterre P, Philippe H. Archaeal phylogeny based on ribosomal proteins. Mol Biol Evol. 2002;19(5):631–639. doi: 10.1093/oxfordjournals.molbev.a004122. [DOI] [PubMed] [Google Scholar]
- 39.Cousins DV, Bastida R, Cataldi A, Quse V, Redrobe S, et al. Tuberculosis in seals caused by a novel member of the Mycobacterium tuberculosis complex: Mycobacterium pinnipedii sp. nov. Int J Syst Evol Microbiol. 2003;53(Pt 5):1305–1314. doi: 10.1099/ijs.0.02401-0. [DOI] [PubMed] [Google Scholar]
- 40.Kumar S, Tamura K, Nei M. MEGA: Molecular Evolutionary Genetics Analysis software for microcomputers. Comput Appl Biosci. 1994;10(2):189–191. doi: 10.1093/bioinformatics/10.2.189. [DOI] [PubMed] [Google Scholar]
- 41.Kong Y, Cave MD, Zhang L, Foxman B, Marrs CF, et al. Population-based study of deletions in five different genomic regions of Mycobacterium tuberculosis and possible clinical relevance of the deletions. J Clin Microbiol. 2006;44(11):3940–3946. doi: 10.1128/JCM.01146-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gutierrez MC, Brisse S, Brosch R, Fabre M, Omaïs B, et al. Ancient Origin and Gene Mosaicism of the Progenitor of Mycobacterium tuberculosis. PLoS Pathog. 2005;1(1):e5 doi:10.1371/journal.ppat.0010005. doi: 10.1371/journal.ppat.0010005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Phylogenetic trees based on comparison of the DNA sequences corresponding to gyrB gene (A) and 32kDa protein gene (B) of MIP and other mycobacteria. Sequence alignment was performed in Clustal W software and phylogenetic trees were developed in MEGA3.1 using bootstrapping method.
(0.11 MB PPT)
Alignment of gyrB of MIP with other members of MAIC complex (M. avium and M. intracellulare)
(0.15 MB PPT)
Alignment of 32kDa protein gene of MIP with other members of MAIC complex (M. avium and M. intracellulare). The identities are depicted by dots only.
(0.12 MB PPT)