Summary
In recent years, it has become clear that reactive oxygen species (ROS, which include superoxide, hydrogen peroxide and other metabolites) are produced in biological systems. Rather than being simply a byproduct of aerobic metabolism, it is now recognized that specific enzymes --- the Nox (NADPH-oxidase) and Duox (Dual oxidase) enzymes ---- seem to have the sole function of generating ROS in a carefully regulated manner, and key roles in signal transduction, immune function, hormone biosynthesis and other normal biological functions are being uncovered. The prototypical Nox is the respiratory burst oxidase or phagocyte oxidase, which generates large amounts of superoxide and other reactive species in the phagosomes of neutrophils and macrophages, playing a central role in innate immunity by killing microbes. This enzyme system has been extensively studied over the past two decades, and provides a basis for comparison with the more recently described Nox and Duox enzymes, which generate ROS in a variety of cells and tissues. This review first considers the structure and regulation of the respiratory burst oxidase, and then reviews recent studies relating to the regulation of the activity of the novel Nox/Duox enzymes. The regulation of Nox and Duox expression in tissues and by specific stimuli is also considered here. An accompanying review considers biological and pathological roles of the Nox family of enzymes.
The Respiratory Burst Oxidase of Phagocytes
a. The respiratory burst
The “respiratory burst” refers to the early observation that when professional phagocytes such as neutrophils and macrophages are exposed to microbes, they consume large amounts of oxygen. Unexpectedly, this oxygen consumption was not inhibited by cyanide, an inhibitor of mitochondrial electron transport. This observation led to a more than 25 year search for the enzymatic origin of the respiratory burst and to the eventual discovery and molecular characterization of the phagocytic NADPH-oxidase or “respiratory burst oxidase”. The phagocyte oxidase generates superoxide via the one electron-reduction of oxygen by NADPH, with secondary production of hydrogen peroxide, HOCl and other activated forms of oxygen. Together, these reactive oxygen species (ROS) participate in host defense by killing invading microbes.
b. gp91phox, the catalytic moiety of the respiratory burst oxidase
The phagocytic NADPH-oxidase consists of a membrane-localized glycosylated, catalytic subunit, gp91phox (which has also come to be known as Nox2, a terminology that will be used in this review), along with a second membrane-associated subunit, p22phox, both depicted in Fig. 1. Nox2 and p22phox stabilize one another in a tightly associated heterodimer which is referred to as flavocytochrome b558. The C-terminal half of Nox2 forms a domain that is homologous to known flavoprotein dehydrogenases, and contains a bound FAD and an NADPH binding site. The N-terminal half of Nox2 consists of six predicted transmembrane α-helices containing four histidine residues that are absolutely conserved among all known Nox enzymes (T. Kawahara and D. Lambeth, unpublished). These histidine residues, located in a-helices 3 and 5, provide the axial and distal ligands for binding to the irons of two nonidentical [1] hemes (red in Fig. 1), as demonstrated by mutational analysis [2]. Molecular models [e.g., [3]] of the locations of the conserved histidine residues in the transmembrane helices indicate that the two hemes are located approximately in the inner and outer leaflets of the membrane bilayer, with the hemes oriented perpendicular to the surface of the membrane, providing a conduit for electrons to pass from the cytosolic NADPH, through FAD and across the membrane via the hemes to reduce molecular oxygen to form superoxide. The reaction with oxygen is thought to occur from the edge of the second heme in an outer sphere reaction rather than directly from the heme iron [4], accounting for lack of inhibition by cyanide and carbon monoxide.
Fig. 1. Schematic Structure of Nox2 and p22phox.
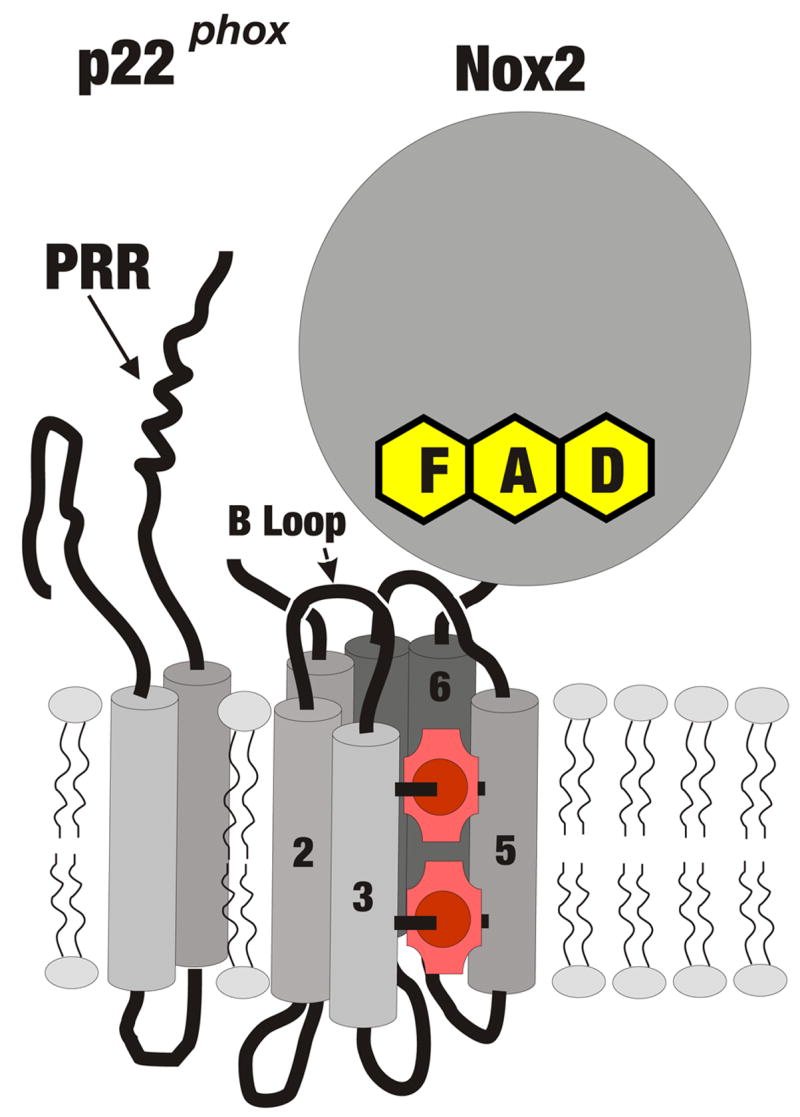
Indicated are Nox2 and p22phox, with predicted α-helical transmembrane regions depicted as cylinders, with the cytosol-facing B loop connecting helices 2 and 3, and hemes indicated in red, with conserved histidine residues in helices 3 and 5 providing axial and distal ligands to the heme irons (red circles). Helix 6 is connected to the predicted globular flavoprotein domain containing flavin adenine nucleotide (FAD) and the NADPH binding site, located on the cytosolic side. Nox2 is associated in the membrane with p22phox, which has 2 predicted α-helical transmembrane segments and whose C-terminus contains a proline-rich region (PRR) which is the target for binding by the bis-SH3 domain of p47phox.
The interaction of the membrane components with cytosolic regulatory subunits is important for the activation of electron flow. p22phox plays a central role in this process, via interaction of its proline-rich domain (PRD in Fig. 1) with p47phox, as described in detail below. An additional region of importance is the B-loop on Nox2 (Fig. 1), a polybasic sequence that also participates in the interaction with p47phox [5].
c. Regulatory subunits for the Nox2 system
Early attempts to reconstitute the NADPH- oxidase activity demonstrated that proteins in the cytosolic as well as the membrane were required for activity [6–8]. Subsequently, p47phox, p67phox, and the small GTPase Rac1 [9] or Rac2 [10] were identified as essential regulatory subunits. p47phox and p67phox were demonstrated to be important for NADPH oxidase activity, based on impairment of ROS generation in forms of chronic granulomatous disease in which these components were missing or mutated [11–14]. Using knockout mice, it was found that Rac2 was needed for optimal activation of the respiratory burst in neutrophils [15, 16], but Rac1 also functions in vitro [17] and in other cell types such as macrophages [9]. p40phox was later discovered as an additional member of the complex that translocates from cytosol to membrane to activate Nox2 [18]. In resting cells, these subunits reside in at least two cytosolic complexes: one containing p47phox, p67phox and p40phox [18, 19] and a second containing Rac and its inhibitory protein RhoGDI (GDP Dissociation Inhibitor for Rho) [9, 20, 21]. For the phagocyte system, all except RhoGDI assemble in a membrane complex with the flavocytochrome following cell activation, resulting in activation of electron transfer reactions within the complex. Neither p47phox nor p40phox are absolutely required for in vitro activity [22, 23]. However, p47phox markedly enhances the binding of the other regulatory subunits [22, 24] and it is an essential protein in intact cells [13]. In activated neutrophils, p47phox is phosphorylated at 7–8 sites in its C terminus [25–28] releasing autoinhibitory internal interactions and allowing its binding to p22phox [29–31]. By simultaneously binding to p67phox, p47phox helps to organize the regulatory subunits in the active complex, leading to the concept that p47phox functions as an “adapter” or “organizer protein” [22]. In addition, p40phox also functions to increase the interaction of other regulatory subunits [32, 33], although its in vivo role does not appear to be as critical as that of p47phox since no mutations in p40phox have been detected in chronic granulomatous disease. Cell activation causes Rac-GDP to separate from RhoGDI, as a result of the phosphorylation of GDI and through the action of lipid mediators [34, 35], and this is accompanied by exposure to guanine nucleotide exchange factors which catalyze the formation of Rac-GTP. Rac-GTP translocates to the plasma membrane independently of the phox components [20, 36–38], where it interacts with p67phox and wth flavocytochrome b558. Thus, activation involves an assembly of regulatory subunits with the catalytic subunit, resulting in the activation electron flow through the prosthetic groups of the catalytic subunit Nox2.
Because both hemes as well as FAD remain mostly in the oxidized form during catalytic turnover of the activated flavocytochrome [39–41], the rate-determining step in the active enzyme is the reduction of FAD by NADPH [41]. An “activation domain” was identified in p67phox (Fig. 2) that regulates the hydride transfer step from NADPH → FAD to form NADP+ plus FADH2 without affecting NADPH binding [41]; this sequence is highly conserved among p67phox homologs in multiple species, pointing to its general importance (T. Kawahara and D. Lambeth, unpublished studies). In addition, Rac, which is modified at its C-terminus with the hydrophobic geranyl-geranyl membrane anchoring moiety [42, 43], binds in its active GTP-associated form simultaneously to membrane and to p67phox [44–46], tethering p67phox to the membrane and probably simultaneously to the cytochrome [47]. To date, it has not been possible to replace the function of Rac by using other methods to tether p67phox to the membrane, leading to the proposal that Rac has a specific role in Nox2 catalysis. Two theories that need not be mutually exclusive have been advanced for a specific role for Rac. First, Rac binding induces a conformational change in p67phox [48] which may permit the activation domain of p67phox to act on Nox2. Second, studies using a diaphorase electron acceptor to intercept electrons from the FAD support the view that Rac plays a direct role in stimulating electron transfers that is independent of p67phox [49].
Fig. 2. Regulatory Subunits for Nox2.
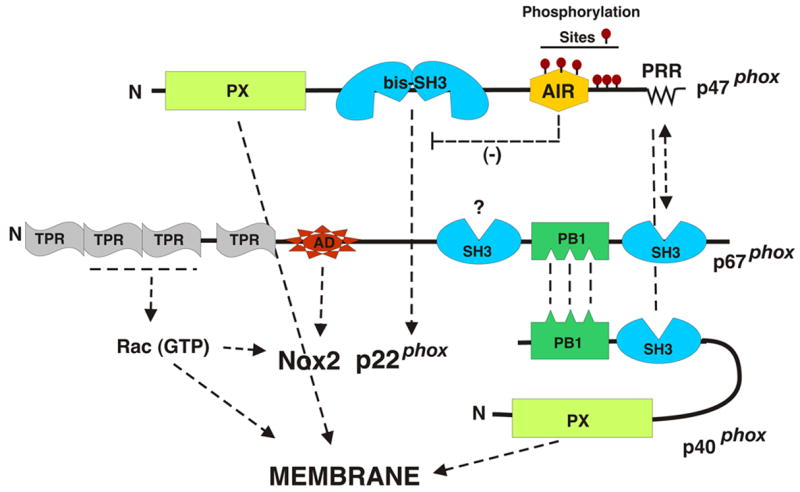
Indicated are domains of the cytosolic factors required for Nox2 activation along with arrows indicating the domains or components with which they interact. Abbreviations are: AD, activation domain; TPR, tetratricopeptide repeats; SH3, Src Homology 3 domain; bis-SH3 refers to the tandem SH3 domains that function as a single domain; PX, phox domain; PB1, a domain originally described in p47phox and Bem1p; AIR, autoinhibitory region.
d. Domain structures of the phox regulatory subunits
Binding and structural investigations have been used in combination with mutational approaches to understand the domain structure of the regulatory subunits. These interactions have been summarized in detail recent excellent reviews [50–52] and are considered in less detail briefly here.
i. p47phox
Phosphorylation of p47phox serves as one of several triggers for the assembly of an active oxidase. As shown in Fig. 2, this protein contains a polybasic C-terminal autoinhibitory region (AIR) that contains multiple serine residues that are the targets for phosphorylation [30, 53]. In the resting cell, p47phox is dephosphorylated and is maintained in an inactive conformation by an intramolecular interaction between the AIR and the centrally-located adjacent SH3 domains [31] (referred to as the bis-SH3 domain, Fig. 2), which function together to form a single binding site for its target proline-rich regions (PRR) in the AIR. Phosphorylation of p47phox releases this binding, freeing the bis-SH3 domain to bind to the PRR of p22phox, thereby triggering the initial phase of oxidase assembly. Although less well understood, another, autoinhibitory intramolecular interaction occurs in the non-phosphorylated p47phox that prevents the N-terminally located PX domain [54] from binding to membrane lipids [55]. Phosphorylation of p47phox releases this inhibition, allowing the PX domain to interact with membrane via a site that binds to the 3-phosphorylated phosphatidylinositol PtdIns (3,4)P2 [56, 57] and a second site that binds to phosphatidic acid [58]. Phosphatidyl inositol 3,4,5-trisphosphate (PIP3) is generated in activated phagocytes through the action of PI 3-kinase, serving as a second type of “trigger” for oxidase assembly. The C-terminus of p47phox also utilizes a PRR to bind to the C-terminal SH3 domain of p67phox in a “tail-to-tail” interaction (Fig. 2) [59, 60], “taking it along for the ride” when it binds to p22phox and membrane lipids. While the other regions of p47phox are highly conserved, this tail-to-tail mode of binding p47phox to p67phox has not been stringently conserved during evolution, with various isologs of p47phox and p67phox utilizing a variety of different modular interactions to accomplish the same interaction (T. Kawahara and D. Lambeth, unpublished).
ii. p67phox
In addition to the activation domain discussed above, p67phox contains four tetratricopeptide repeats (TPR) that together form a binding site for Rac-GTP (Fig. 2) [61, 62]. p67phox also contains a C-terminal SH3 domain for binding to the PRR of p47phox (see above), a second centrally located SH3 domain of unknown function, and a PB1 domain located between the SH3 domains. A PB1 domain is also found in p40phox. This domain, was originally described in p47phox, Bem1p proteins and various eukaryotic cytoplasmic signaling proteins, and is thought to mediate protein-protein interactions with other proteins containing a PB1 counterpart; reviewed in [63]. The PB1 domain of p67phox forms a complex with the PB1 domain of p40phox, thereby mediating the binding of p40phox to the phox regulatory protein complex [64].
iii. p40phox
p40phox was identified as a co-purifying component present in a 250 kDa cytosolic complex that also contained p67phox and p47phox [18]. In addition to its PB1 domain mentioned above, p40phox contains a PX domain and an SH3 domain [54, 65] (Fig. 2). The SH3 domain of p40phox can compete with the C-terminal SH3 domain of p67phox for binding to the proline rich region of p47phox [66, 67], which suggests some sort of rearrangement of binding partners must occur upon assembly and activation. When cells are activated, p40phox becomes phosphorylated [68] and translocates along with the p47phox-p67phox complex to the membrane [32, 33]. The PX domain of p40phox binds to phosphatidylinositol-3 phosphate and other 3-phosphorylated lipids in the membrane [56, 58, 69], helping to anchor the other phox regulatory components to the membrane in cells in which PI 3-kinase has been activated [32].
Identification and Domain Structure of Novel NADPH oxidases
a. Reactive oxygen in non-phagocytic cells
During the 1990s, probably as a result of more sensitive methods for detection of H2O2 and O2−, several groups reported the occurrence of ROS in a wide variety of cell types other than professional phagocytes [70–76]. Although frequently attributed to mitochondrial respiration, in many cases ROS generation was inhibited by diphenylene iodonium (DPI), an inhibitor of the phagocyte NADPH oxidase and some other flavoprotein dehydrogenases [77]. However, although phox regulatory subunits were sometimes detected in these tissues, gp91phox (Nox2) was usually absent. Based on the assumption that that ROS originated from a homolog of gp91phox, Lambeth and colleagues [78] identified a homolog of gp91phox, now called Nox1 (originally Mox1), and this group and others subsequently and independently identified the additional homologs Nox3, Nox4, Nox5, Duox1 and Duox2 in humans and other species [79–85]. A large number of isologs have now been catalogued in species ranging from plants, fungi, amoeba, nematodes, insects, and vertebrates. Based on sequence homology of the catalytic regions (i.e., excluding non-catalytic domains, see below), these can be classified into 7 subfamilies (T. Kawahara and D. Lambeth, unpublished), and the regulated generation of ROS by these enzymes performs a range of biological functions in addition to innate immunity, considered in the accompanying article. Interestingly, no Nox isologs have been identified in bacteria, indicating that defense against ROS evolved earlier than enzymes that deliberately generate ROS.
b. Domain structure of the mammalian Nox enzymes
The mammalian Nox enzymes can classified into three groups, based on the presence of domains in addition to the Nox flavocytochrome domain (i.e., the transmembrane region containing 2 hemes plus the flavoprotein domain). Nox1, Nox2, Nox3, and Nox4 all contain the flavocytochrome catalytic moiety depicted in Fig. 1 and are ~65–66 kDa in size. Nox enzymes 1–4 all require p22phox for stabilization and/or function [51, 86–88]. In addition to Nox enzymes 1–4, a short form of Nox5 encoded by a short mRNA splice form conforms to the same domain structure [82].
A long form of Nox5 is the single member of the second group which has, in addition to the basic Nox catalytic moiety, an amino-terminal calmodulin-like domain that contains four calcium binding EF-hand structures [83]. While present in most mammals, a Nox5 isolog is absent in rodents where its function may have been taken over by another Nox or Duox.
A third group of Nox’s are the “dual oxidases” or Duox, so-named because they contain not only an NADPH-oxidase domain but also a domain that is homologous to heme-containing peroxidases such as myeloperoxidase and lactoperoxidase [79, 81, 89]. These enzymes are comprised of the basic Nox5-like structure fused at the N-terminus with an additional transmembrane α-helix that is linked at its N-terminus to the peroxidase-like domain. Topologically, the arrangement of transmembrane sequences places the peroxidase domain on the opposite side of the membrane from the FAD domain, immediately adjacent to the site of ROS generation at the second heme group. The Duox peroxidase domain has been proposed [90] to utilize H2O2 generated by its Nox catalytic moiety (presumably formed via the dismutation of 2 superoxide molecules, although only H2O2 has thus far been detected [89, 91, 92]). The Duox peroxidase domain of C. elegans and insects contains a histidine at the homologous position as the axial histidine in myeloperoxidase, and is therefore assumed to function in these species as a typical peroxidase. Indeed, the expressed peroxidase domain of the nematode Duox peroxidase domain shows peroxidase type activities including tyrosine cross-linking [90]. While the expressed human Duox1 peroxidase domain also catalyzes peroxidase reactions [90] and [Inoue and Lambeth unpublished], the activity and heme binding of this subunit is more controversial because of the absence of a histidine at the position homologous to the axial heme iron-binding histidine of myeloperoxidase. Supporting the view of mammalian Duox enzymes as peroxidases, it was recently reported that treatments that increased or decreased Duox2 levels in airway epithelial cells resulted in parallel increases and decreases in peroxidase activity [93]. Countering this view is the suggestion that the peroxidase-like domain could serve as a heterodimerization site for another peroxidase (M. Geiszt, personal communication). A catalytic or other role for the peroxidase domain of the human Duox enzymes remains an area for further investigation.
c. Homologs of phox regulatory subunits
Several groups [94–97] reported homologs of p47phox and p67phox, whose structures are summarized in Fig. 3. Like p47phox, NOXO1 (referring to Nox organizer protein 1) contains an N-terminal PX domain, a central bis-SH3 domain and a proline-rich region at its C-terminus. The PX domain of NOXO1, like that of p47phox, binds lipids, although its specificity favors the 4- and 5- monophosphorylated forms of phosphatidylinositol, species that are present in membranes without cell activation. This domain mediates the localization of NOXO1 to the plasma membrane [97]. The bis-SH3 domain binds like that of p47phox to the PRR of p22phox [95], although with some subtle differences in binding specificity [87]. NOXO1, however, lacks the polybasic autoinhibitory region and regulatory phosphorylation sites that are present in p47phox, and this is thought to explain the association with membrane in the absence of cell activation [97].
Fig. 3. Regulatory Subunits for Nox1.
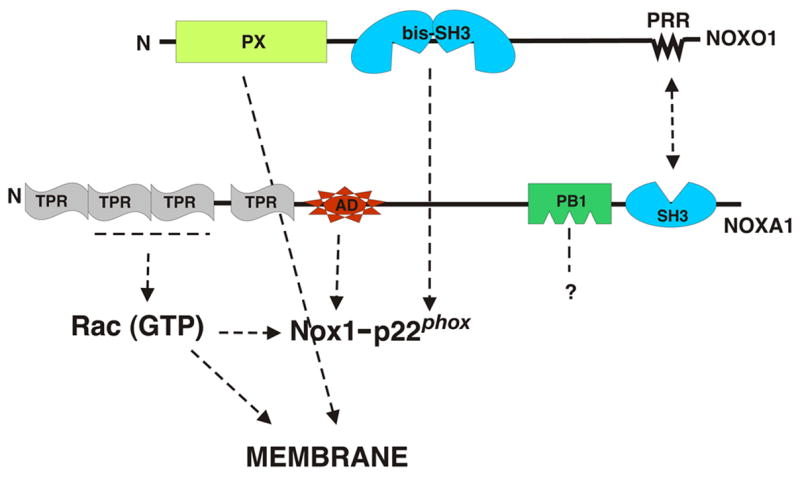
Indicated are domains of the regulatory factors required for Nox1 activation along with arrows indicating the domains or components with which they interact. Abbreviations are as in Fig. 2.
NOXA1 (referring to Nox Activator Protein 1) has a domain structure that is similar in most respects to p67phox. The N-terminal TPR repeat in NOXA1, like that in p67phox, functions to bind to Rac [95, 98]. In addition, there is a conserved activation domain that is critical for Nox1 activation [99], a C-terminal SH3 domain that binds to NOXO1 [95], and a PB1 domain of unknown function. NOXA1 differs from p67phox primarily in that it lacks the first SH3 domain that is present in the phox protein. The significance of this structural difference is not known, but might relate to association of the phox complex with other cellular proteins.
To date, no homologs of p40phox or of p22phox have been reported. However, it should be noted that the presence of a PB1 domain in NOXA1 suggests the existence of an unknown dimerization partner. The PB1-like domain in NOXA1 lacks a conserved lysine residue that is present in p67phox, and does not bind to p40phox [95].
Regulation of the Activity of Non-phagocytic Nox/Duox Enzymes
A summary of the known modes of regulation of Nox and Duox enzymes is shown in Table 1, and is detailed below.
Table 1.
Summary of major mechanisms for regulating the activity of Human Nox and Duox enzymes.
| Nox or Duox | p22phox-Requiring | Mode of Regulation: Additional Protein Subunits |
|---|---|---|
| Nox1 | Yes | Regulatory Subunits: NOXO1, NOXA1, Rac1 |
| Nox2 | Yes | Regulatory Subunits: p47phox, p67phox, p40phox, Rac2/1 |
| Nox3 | Yes | Regulatory Subunits: NOXO1 |
| Nox4 | Yes* | Constitutively active; unknown activator(s) |
| Nox5 | No | Calcium, phosphorylation |
| Duox1 | No | Calcium |
| Duox2 | No | Calcium |
PRR not required.
a. Regulation of Nox 1
Nox1 forms a complex with p22phox [86], and its activity requires p22phox, since depletion of p22phox using RNA interference inhibits Nox1 activity [87]. NOXO1 and NOXA1 are co-expressed in colon epithelium along with Nox1 [94], and co-transfection studies demonstrated that these subunits can indeed activate Nox1 in a manner that is dependent on the activation domain of NOXA1 [98]. Activation does not absolutely require phorbol esters, although phorbol esters typically increase activity by up to 2-fold in many cell systems [97, 98]. The considerable constitutive activation of Nox1 in the absence of cell stimulation is explained in part by the absence of regulatory phosphorylation sites on NOXO1 and the ability of NOXO1 to localize to the resting cell membrane via its PX domain [97]. NOXO1 must also bind to the PRR of p22phox, because mutation of the PRR prevents NOXO1-dependent activation of Nox1 [87]. Like NOXO1, NOXA1 is also constitutively located in the plasma membrane, in a manner dependent on membrane localization of either NOXO1, or, when NOXO1 is absent, the activated form of Rac1 [100]. As implied by the Rac-binding TPR repeat domain of NOXA1, Nox1 is regulated by Rac1-GTP [98, 100, 101]. Unlike the Nox2-p47phox-p67phox-Rac system that requires protein phosphorylation, lipid phosphorylation and guanine nucleotide exchange on Rac, the Nox1 system seems to have relatively few regulatory “triggers”. Currently only guanine nucleotide exchange on Rac1 is convincingly documented as a regulatory trigger [98], although as mentioned above, phorbol esters exert a modest stimulatory effect, which points to phosphorylation of an unknown component in modulating activity. While Nox2 is completely dependent upon active Rac, Nox1 activation by Rac1 is less stringent and is obvious only when cellular levels of NOXO1 and NOXA1 are low [98]. Immunoprecipitation studies have demonstrated an active complex containing Nox1, NOXO1, NOXA1 and Rac1, and direct associations of Nox1-p22phox have been shown with each of the individual regulatory subunits (i.e., these interactions are independent of the presence of the other subunits) [98].
In addition to NOXO1 and NOXA1, Nox1 shows a modest capacity to be activated when NOXO1 is expressed in cells along with p67phox or when p47phox is combined with NOXA1 [99]. In a similar manner, heterologous combinations of regulatory subunits are capable of activating Nox2 [99]. While heterologously regulated Nox1 and Nox2 show lower activity than homologously regulated Nox enzymes, these observations raise the possibility of some flexibility for Nox1 and Nox2 regulation. While the in vivo significance is unclear, this may explain studies in p47phox (−/−) mice that demonstrate p47phox-dependent regulation of ROS production in some tissues where Nox2 does not appear to be expressed, e.g., see [102, 103].
b. Regulation of Nox3
Nox3-dependent ROS production requires p22phox, based on co-transfection and RNA interference studies [87]. Nox3 forms a complex with p22phox, based in co-immunoprecipitation [87, 104]. While Nox3 may produce a small amount of ROS without co-expressed regulatory subunits [104, 105], subunits markedly increase activity. Subunit regulation differs depending on species: human NOXO1 dramatically stimulates Nox3 activity in absence of NOXA1 or p67phox [99]. However, mouse Nox3 activity requires both NOXO1 and NOXA1 [105]. The human Nox3 can also be regulated in transfected cell models by the combination of p47phox and p67phox [99]. In the major site of Nox3 expression, the inner ear, NOXO1 is the physiologically relevant regulator of Nox3, based on the impairment of Nox3-dependent otoconia formation in a mouse harboring an inactivating mutation in NOXO1 [106]. A possible role for Rac1 in regulation of Nox3 has been investigated by several groups. Neither the dominant negative nor the constitutively active forms of Rac1 affected Nox3-dependent activity in transfected CHO and HeLa cell models [101, 104]. On the other hand, a constitutively active form of Rac1 stimulated the activity of Nox3 alone or when NOXA1 was co-expressed with Nox3 [100]. Thus, it appears that Nox3 has a quantitatively less signficant requirement for Rac compared with Nox1 or Nox2.
c. Regulation of Nox4
Amino acid sequence comparisons show that Nox1 and Nox3 are both closely related to Nox2, while Nox4 is more distantly related [79]. Nox4 is unique among the human Nox and Duox enzymes in that it shows high constitutive activity when expressed in cell models in the absence of activators such as PMA [80, 87, 88] or calcium (T. Kawahara and D. Lambeth, unpublished). In addition, Nox4 activity was not affected by co-expression any of the known regulatory subunits including p47phox, p67phox, NOXO1, NOXA1 and Rac [87, 88]. Nox4 does, however, require p22phox for activity, since depletion of p22phox inhibits Nox4 activity without affecting Nox4 protein level [87]. Unlike Noxes1–3, Nox4 activity is independent of the PRR of p22phox [87], consistent with the lack of a requirement for p47phox or NOXO1. Based on co-immunoprecipitation, Nox4 forms a complex with p22phox [86, 88], and Nox4 co-localizes with p22phox in human kidney tissue [87]. While these observations indicate that the Nox4-p22phox complex can produce ROS without the aid of any known regulatory subunits, other studies indicate that Nox4 activity can be acutely regulated in some cells by unknown mechanisms. For example, insulin activates Nox4-dependent ROS generation within 5 min [107], a response that is too rapid to be accounted for by increased Nox4 protein expression. Lipopolysaccharide also activates Nox4 via a TLR receptor signaling cascade within 30 min [108]. Thus, it seems likely that unknown mechanisms acutely regulate Nox4 activity, and that these mechanisms do not funtion in transfected cell systems wherein overexpressed Nox4 is constitutively active. This remains an important unresolved area for future exploration.
d. Regulation of Nox5
In addition to the basic Nox catalytic moiety of Nox1–4, Nox5 encodes the N-terminal domain containing four calcium-binding EF-hand motifs. Calcium-dependent ROS production by Nox5 has been shown in Nox5-transfected cell lines [83]. Binding of calcium changes the conformation of the EF-hand domain and allows it to bind to the catalytic domain, which is proposed to activate electron transfer from NADPH to oxygen to form superoxide [109]. Nox5, at least the full-length form, does not form a functional complex with p22phox, because depletion of p22phox does not affect the Nox5 activity [87] and because no complex between the two proteins can be detected by co-immunoprecipitation (T. Kawahara and D. Lambeth, unpublished). One puzzling aspect of the calcium activation was that higher than biologically realistic concentrations of calcium were required to activate the enzyme. However, a recent study showed that PMA triggers phosphorylation of T494 and S498 of Nox5, and that this increases the sensitivity of the enzyme for calcium, allowing activation at levels of calcium that are seen in the cytosol following agonist stimulation of cells [110]. Thus, the regulation of Nox5 seems to be more complex than originally appreciated and involves synergy between calcium-dependent and phosphorylation-dependent pathways.
e. Regulation of Duox enzymes
Human Duox enzymes, like Nox5, contain a calcium-binding domain, although only two, rather than four canonical calcium-binding EF-hands are present. The presence of the calcium-binding domain probably explains the observation that calcium ion triggers ROS production in thyroid cells and human bronchial epithelial cells, both of which express abundant Duox proteins [92]. Duox2-transfected HEK293 cells also produce ROS in response to calcium ion using a cell membrane preparation [111]. Characterization of the function and regulation of Duox proteins has been hindered because the ectopically expressed Duox protein failed to become properly glycosylated and was retained in the endoplasmatic reticulum. Recently, proteins that permit the proper post-translational processing and trafficking of Duox1 and Duox2 (DuoxA1 and DuoxA2, respectively) were identified [112]. These maturation proteins allow the Duox enzymes to be expressed in their appropriate location at the plasma membrane. Interestingly, the chromosomal location of each of the DuoxA genes is adjacent to the respective Duox, and operon-like co-regulation of the enzyme and its maturation factor has been proposed. Human Duox2 protein reportedly co-immunoprecipitates with p22phox [111], but there is little or no effect of p22phox on Duox enzymatic activity. Since p22phox is needed for the proper glycosylation and localization of Nox2, DuoxA proteins may serve a function analogous to that of p22phox for Duox enzymes [112].
Regulation of Nox/Duox expression
Although the activity of Nox and Duox enzymes is acutely regulated by subunits or calcium, the maximum capacity of a cell to generate ROS will be determined not only by the activation state, but also by the protein expression of Nox/Duox enzymes and their regulatory subunits. Misregulation of Nox protein expression is a component of certain disease states and stress responses, as discussed in the accompanying review. Therefore, the transcriptional and perhaps translational regulation of Nox family proteins is an important part of the consideration of Nox regulation.
a. Regulation of Nox1 expression
Nox1 expression is normally highest in the colon, but it is also seen in a variety of other tissues, including vascular smooth muscle. Nox1 transcript is up-regulated by growth factors and growth-related agonists including angiotensin II, urokinase plasminogen activator, platelet-derived growth factor, bone morphogenic protein 4, prostaglandin F2, keratinocyte growth factor-α, phorbol ester, and mutationally activated K-Ras (RasG12V) (see Table 2). Nox1 is also induced by inflammatory mediators such as interferon gamma (INF-γ) [113, 114] and pathogen-associated bacterial molecules including a flagella filament of Salmonella enteritidis, and lipopolysaccharide (LPS) of Helicobacter pylori. Interestingly H. pylori LPS and IFN-γ also induce expression of NOXO1. Vitamin D3, which induces differentiation of colon epithelium, also induces Nox1. Hypoxia leads to increased Nox1 expression in a human pulmonary epithelial cell line, and up-regulation of Nox1 also correlates with activation of hypoxia-inducible factor 1 (HIF-1α)-dependent pathways, but it is not clear whether HIF-1α induces Nox1 expression.
Table 2.
Regulation of Nox and Duox Expression by Agonists and Other Stimuli.
| Inducer | Nox/Duox | Tissues or cells | References |
|---|---|---|---|
| Angiotensin II | Nox1, Nox4 | r-Aortic vascular smooth muscle cells | [131] |
| Urokinase plasminogen activator | Nox1, Nox4 | r-Aortic vascular smooth muscle cells | [132] |
| Platelet-derived growth factor | Nox1 | r-Aortic vascular smooth muscle cells | [133] (7) |
| Bone morphologenic protein 4 | Nox1 | m-Aortic endothelial cells | [134] |
| Prostaglandin F2α | Nox1 | r-Aortic vascular smooth muscle cells | [135] |
| Phorbol ester | Nox1 | r-Aortic vascular smooth muscle cells | [133] |
| K-Ras G12V | Nox1 | r-, m-fibroblast cell line | [136] |
| Vitamin D3 | Nox1 | h-Colon caner cell line | [114] |
| Interferon-γ | Nox1 | h-Colon caner cell line | [113, 114] |
| Flagellin (S. enteritidis) | Nox1 | h-Colon caner cell line | [137] |
| Lipopolysaccharide (H. pylori) | Nox1 | gp-Gastric mucosal cells | [138] |
| Hypoxia | Nox1 | h-Pulmonary epithelial cell line | [139] |
| Lipopolysaccharide (E. coli) | Nox2 | h-Macrophage, h-Granulocyte | [140] |
| IFN-γ; TNF-γ; GM-CSF | Nox2 | h-Macrophage, h-Granulocyte | [140] |
| M-CSF | Nox2 | h-Macrophage | [140] |
| TGF-β1 | Nox4 | h-Cardiac fibroblast cells | [141] |
| TNFα | Nox4 | h-Aortic vascular smooth muscle cells | [142] |
| Low pH | Nox5-short | h-Barret esophagus adenocarcinoma | |
| Th-2 cytokines (IL-4, IL-13) | Duox1 | h-Tracheobronchial epithelial cells | [130] |
| Th-1 cytolikes (IFN-γ, IL-1) | Duox2 | h-Tracheobronchial epithelial cells | [130] |
The abbreviations used are: h, human; r, rat; m, mouse, gp, guinea pig.
Several groups recently reported studies of the promoter of human Nox1. GATA transcription factors (GATA-4 to 6) are regulators of intestine-specific gene expression and participate in intestinal proliferation and differentiation [115]. Nox1 expression in the human colon cancer cell line Caco2 was mainly driven by elements in the −306-bp proximal promoter region in the 5′ flanking sequence [116]. This region contains two GATA-binding sites, and GATA-6 was implicated (Fig. 4). The IFN-γ-responsive region of the human Nox1 gene was localized in the range between −4331 and −2552 in the 5′ flanking sequence, and regulation was found to be due to a single γ-activated sequence (GAS) element (−3818 to −3810 bp) [113] (Fig. 4). This region is regulated by the transcription factor STAT1. Mouse Nox1 transcripts consist of three splice variants of 5′ untranslated regions [117]. The murine colon expresses the a-type that possesses a short UTR. Tow other types (f- and c-types) have longer 5′ UTRs, which are specifically expressed in smooth muscle. As in human, all three mouse transcripts are driven by the proximal promoter region, but the responsible cellular transcription factor(s) is not clear.
Fig. 4. Promoter regions of human Nox1 and Nox2.
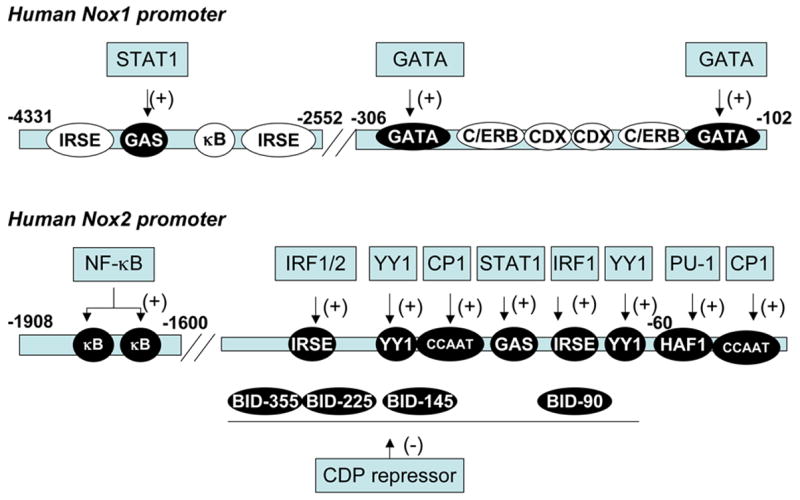
Shown are schematic drawings of promoter regions of human Nox1 and Nox2. White letters in a filled circle indicate the region identified as regulating responsible gene expression. Black letters in an open circle indicate the region containing a consensus sequence for a given transcription factor. Boxes indicate transcription factors. The abbreviations of responsive element in promoter regions are: IRSE, IFN-γ-responsive stimulated elements; GAS, γ-activated sequence; C/ERB, CCAAT/enhancer binding protein; CDX, caudal-related homeobox; HAF-1, hematopoietic-associated factor 1; CCAAT, CCAAT box binding, BID, binding increased during differentiation.
b. Regulation of Nox2 expression
Nox2 expression is transcriptionally up-regulated during the maturation of myeloid cells and in response to pro-inflammatory cytokines such as IFNγ. The principal cis-regulatory elements are clustered in the proximal promoter region of the gene between positions −450 and + 12 bp (Fig. 4). Several transcription factors bind these regions. A transcriptional repressor, CCAAT displacement protein (CDP), binds to fivesites in the proximal promoter and constitutively suppresses Nox2 expression in immature cells. CDP protein expression decreases during maturation of monocytes and macrophages resulting in increased expression of Nox2. In addition, CCAAT-binding factor CP1 also binds to the proximal promoter region in the absence of the suppressor CDP [119]. PU.1 is a key transcription factor for Nox2. PU.1 binds to the hematopoietic-associated factor (HAF)-1-binding element. The importance of PU.1 in Nox2 regulation was demonstrated with the discovery of a patient with chronic granulomatous disease harboring four single-base mutations within PU.1 binding sites [118]. These mutations inhibited the binding of PU.1 to the promoter, markedly decreasing Nox2 expression. Binding of the transcription factor YY1 to two YY1-binding sites in the proximal promoter was detected and shown to increase Nox2 promoter activity in vitro. However, the function of YY1 during myeloid cell development is not clear because YY1 protein levels do not correlate with differentiation and Nox2 expression [120]. Co-treatment with IFN-γ and LPS strongly activates Nox2 expression in phagocytes. Interferon regulatory factor (IRF) and STAT1 have been identified as important transcription factors mediating the IFN-γ response. Both IRF1 and STAT1 also activate the Nox2 promoter through two interferon-stimulated response elements (ISRE) interacting with IRF, and the single γ-activated sequence (GAS) element interacting with STAT1 in the absence of CDP binding. NF-κB was also recently identified as a regulator of Nox2 expression [121]. The cis-regulatory elements are situated in the distal promoter region of the gene between positions −1908 and −1600 bp. Thus, as shown in Fig. 4, Nox2 expression is regulated in a complex manner in a phagocyte and stimulus-specific manner.
c. Regulation of Nox3 expression
Nox3 mRNA is expressed almost exclusively in the inner ear including cochlea and vestibular sensory epithelium [105, 122]. As of this writing, there are no reports examining the transcriptional regulation of Nox3 and its restriction to inner ear.
d. Regulation of Nox4 expression
Nox4 expression is most strongly observed in the kidney. Tissue-staining studies showed that human Nox4 protein expression is observed in renal distal tubules [85], whereas in situ hybridization demonstrates that murine Nox4 transcript in renal proximal tubules [80]. Moderate levels of Nox4 are seen in a wide variety of tissues and cells including vascular endothelial cells, vascular smooth muscle, murine osteoclasts, murine adipocytes, and pancreas [82]. Activators of Nox4-trancription in smooth muscle cells include urokinase, plasminogen activator, angiotensin II, transforming growth factor-β1, and TNF-α, whereas bone morphogenic protein 4 (BMP4) decreased Nox4 expression in human endothelial cells (Table 4). We are not aware of published reports focusing on the promoter structure or transcriptional factors regulating Nox4.
e. Regulation of Nox5 expression
Nox5 mRNA is expressed in human in relatively high levels spleen, testis, and vascular smooth muscle. As of this writing, no reports have characterized the active promoter region of the human Nox5 gene.
f. Regulation of Duox expression
Duox1 and Duox2 are expressed in thyroid [92], and an ascidian ortholog of Duox is expressed in the endostyle, which is regarded as the primitive homolog of the thyroid gland in vertebrates [123]. Human Duox1 is also highly expressed in the lung, pancreas, placenta, prostate, testis, and salivary gland [90]. Human Duox2 is highly expressed in the trachea, stomach, colon and rectum [90, 91, 122, 124]. The two human Duox genes are arranged head-to-head, separated by 15.8-kb. Duox1 and Duox2 expression are regulated in both thyroid and non-thyroid cells by a promoter region within −150 bp of the first exon of Duox1 and within −250 bp of Duox2 [125]. More distal promoter regions within −6.1 kb of Duox1 and −14.6 kb of Duox2 were also important, showing increased activity in non-thyroid cells, but not in thyroid cells. In dog [81]and pig [126] thyrocytes, cAMP, which is downstream of the TSH receptor, increased Duox protein and mRNA. However, stimulation of the cAMP pathway did not affect human Duox promoter activity [125], in agreement with the absence of a significant cAMP effect on Duox mRNA expression in human thyrocytes [125, 127]. Thyroid transcription factor-1 (TTF-1) and Pax8 control the expression of genes involved in differentiated thyroid function, such as thyroglobulin or thyroid peroxidase [128]. Interestingly, a competition assay using transfection of dominant negative form of TTF-1 or Pax8 displayed no reduction in transcriptional activity of Duox1/2 genes in the differentiated thyroid cell line PCCl3 [129], indicating that regulation of human Duox1/2 gene expressions is not responsive to these thyroid-specific transcription factors. Duox1 expression in airway epithelium was strongly induced by the Th-2 dominant cytokines including IL-4 and IL-13 [130]. In contrast, Duox2 expression was regulated by the Th-1 dominant cytokine IL-1α, IL-1β and IFN-γ.
Conclusions
This review of the mechanism and regulation of ROS generation by Nox and Duox enzymes makes evident that ROS levels in cells and tissues are carefully controlled, both by tissue- and stimulus-specific expression of Nox and Duox proteins and their regulatory subunits, and by acute regulation via calcium, protein phosphorylation, guanine nucleotide exchange on Rac and the assembly of regulatory subunits. Both the expression and the acute activation are regulated by complex mechanisms, and much remains to be explored, particularly with regard to the non-phagocytic Nox and Duox enzymes. Despite incomplete knowledge, it is clear that these regulatory mechanisms provide a variety of cell types with the capacity to carefully regulate ROS generation, a capacity that evolved with eukaryotic cells. As will be discussed in the accompanying review, this ROS generation is beneficial for a variety of physiological processes. However, biology’s “choice” to use the chemical properties of ROS in signaling and other biological processes has proven to be a double edged sword.
Acknowledgments
Supported by NIH Grants CA105116 and CA084138.
List of Abbreviations
- Nox
NADPH oxidase
- Duox
Dual oxidase
- ROS
reactive oxygen species
- phox
phagocyte oxidase
- LPS
lipopolysaccharide
- IFN-γ
interferon gamma
- NOXO1
Nox organizer protein 1
- NOXA1
Nox activator protein 1
- FAD
flavin adenine dinucleotide
- NADPH
nicotinamide adenine dinucleotide phosphate, reduced form
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cross A, Rae J, Curnutte J. Cytochrome b-245 of the neutrophil superoxide-generating system contains two nonidentical hemes. Journal of Biological Chemistry. 1995;270:17075–17077. doi: 10.1074/jbc.270.29.17075. [DOI] [PubMed] [Google Scholar]
- 2.Bibersine-Kinkade KJ, DeLeo FR, Epstein RI, LeRoy BA, Bauseef WM, Dinauer MC. Heme-ligating histidines in flavocytochrome b558. Identification of specific histidines in gp91phox. Journal of Biological Chemistry. 2001;276:31105–31112. doi: 10.1074/jbc.M103327200. [DOI] [PubMed] [Google Scholar]
- 3.Mankelow TJ, Henderson LM. Proton conduction through full-length gp91phox requires histidine 115. Protoplasma. 2003;221:101–108. doi: 10.1007/s00709-002-0056-1. [DOI] [PubMed] [Google Scholar]
- 4.Isogai Y, Iizuka T, Shiro Y. The mechanism of electron donation to molecular oxygen by phagocytic cytochrome b558. J Biol Chem. 1995;270:7853–7857. doi: 10.1074/jbc.270.14.7853. [DOI] [PubMed] [Google Scholar]
- 5.Biberstine-Kinkade B, Yu L, Dinauer M. Mutagenesis of an arginine- and lysine-rich domain in the gp91phox subunit of the phagocyte NADPH-oxidase flavocytochrome b558. Journal of Biological Chemistry. 1999;274:10451–10457. doi: 10.1074/jbc.274.15.10451. [DOI] [PubMed] [Google Scholar]
- 6.Bromberg Y, Pick E. Unsaturated fatty acids stimulate NADPH-dependent superoxide production by cell-free system derived from macrophages. Cellular Immunology. 1984;88:213–221. doi: 10.1016/0008-8749(84)90066-2. [DOI] [PubMed] [Google Scholar]
- 7.McPhail LC, Shirley PS, Clayton CC, Snyderman R. Activation of the respiratory burst enzyme from human neutrophils in a cell-free system: Evidence for a soluble cofactor. Journal of Clinical Investigation. 1985;75:1735–1739. doi: 10.1172/JCI111884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Curnutte JT. Activation of human neutrophil nicotinamide adenine dinucleotide phosphate, reduced (triphosphopyridine nucleotide, reduced) oxidase by arachidonic acid in a cell-free system. Journal of Clinical Investigation. 1985;75:1740–1743. doi: 10.1172/JCI111885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abo A, Pick E, Hall A, Totty N, Teahan CG, Segal AW. Activation of the NADPH oxidase involves the small GTP-binding protein p21rac1. Nature. 1991;353:668–670. doi: 10.1038/353668a0. [DOI] [PubMed] [Google Scholar]
- 10.Knaus UG, Heyworth PG, Kinsella BT, Curnutte JT, Bokoch GM. Purification and characterization of Rac 2: A cytosolic GTP-binding protein that regulates human neutrophil NADPH oxidase. Journal of Biological Chemistry. 1992;267:23575–23582. [PubMed] [Google Scholar]
- 11.Nunoi H, Rotrosen D, Gallin JI, Malech HL. Two forms of autosomal chronic granulomatous disease lack distinct neutrophil cytosol factors. Science. 1988;242:1298–1301. doi: 10.1126/science.2848319. [DOI] [PubMed] [Google Scholar]
- 12.Clark RA, Malech HL, Gallin JI, Nunoi H, Volpp BD, Pearson DW, Nauseef WM, Curnutte JT. Genetic variants of chronic granulomatous diseaseGenetic variants of chronic granulomatous disease: Prevalence of deficiencies of two cytosolic components of the NADPH oxidase system. The New England Journal of Medicine. 1989;321:647–652. doi: 10.1056/NEJM198909073211005. [DOI] [PubMed] [Google Scholar]
- 13.Lomax KJ, Leto TL, Nunoi H, Gallin JI, Malech HL. Recombinant 47-kilodalton cytosol factor restores NADPH oxidase in chronic granulomatous disease. Science. 1989;245:409–412. doi: 10.1126/science.2547247. [DOI] [PubMed] [Google Scholar]
- 14.Leto TL, Lomax KJ, Volpp BD, Nunoi H, Sechler JMG, Nauseef WM, Clark RA, Gallin JI, Malech HL. Cloning of a 67-kD neutrophil oxidase factor with similarity to a noncatalytic region of p60c-src. Science. 1990;248:727–730. doi: 10.1126/science.1692159. [DOI] [PubMed] [Google Scholar]
- 15.Roberts AW, Kim C, Zhen L, Lowe JB, Kapur R, Petryniak B, Spaetti A, Pollock JD, Borneo JB, Bradford GB, Atkinson SJ, Dinauer MC, Williams DA. Deficiency of the hematopoietic cell-specific Rho family GTPase Rac2 is characterized by abnormalities in neutrophil function and host defense. Immunity. 1999;10:183–196. doi: 10.1016/s1074-7613(00)80019-9. [DOI] [PubMed] [Google Scholar]
- 16.Kim C, Dinauer MC. Rac2 is an essential regulator of neutrophil nicotinamide adenine dinucleotide phosphate oxidase activation in response to specific signaling pathways. J Immunol. 2001;166:1223–1232. doi: 10.4049/jimmunol.166.2.1223. [DOI] [PubMed] [Google Scholar]
- 17.Kreck ML, Uhlinger DJ, Tyagi SR, Inge KL, Lambeth JD. Participation of the small molecular weight GTP-binding protein Rac1 in cell-free activation and assembly of the respiratory burst oxidase: Inhibition by a carboxyl terminal Rac peptide. Journal of Biological Chemistry. 1994;269:4161–4168. [PubMed] [Google Scholar]
- 18.Wientjes FB, Hsuan JJ, Totty NF, Segal AW. p40phox, a third cytosolic component of the activation complex of the NADPH oxidase to contain src homology 3 domains. Biochem J. 1993;296(Pt 3):557–561. doi: 10.1042/bj2960557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iyer S, Pearson D, Nauseef W, Clark R. Evidence for a readily dissociable complex of p47phox and p67phox in cytosol of unstimulated human neutrophils. Journal of Biological Chemistry. 1994;269:22405–22411. [PubMed] [Google Scholar]
- 20.Heyworth P, Bohl B, Bokoch G, Curnutte J. Rac translocates independently of the neutrophil NADPH oxidase components p47phox. Evidence for its interaction with flavocytochrome b558. Journal of Biological Chemistry. 1994;269:30749–30752. [PubMed] [Google Scholar]
- 21.Ando S, Kaibuchi K, Sasaki T, Hiraoka K, Nishiyama T, Mizuno T, Asada M, Nunoi H, Matsuda I, Matsuura Y, Polakis P, McCormick F, Takai Y. Post-translational processing of rac p21s is important both for their interaction with the GDP/GTP exchange proteins and for their activation of NADPH oxidase. Journal of Biological Chemistry. 1992;267:25709–25713. [PubMed] [Google Scholar]
- 22.Freeman JL, Lambeth JD. NADPH oxidase activity is independent of p47phox in vitro. J Biol Chem. 1996;271:22578–22582. doi: 10.1074/jbc.271.37.22578. [DOI] [PubMed] [Google Scholar]
- 23.Koshkin V, Lotan O, Pick E. The cytosolic component p47phox is not a Sine Qua Non participant in the activation of NADPH oxidase but is required for optimal superoxide production. Journal of Biological Chemistry. 1996;271:30326–30329. doi: 10.1074/jbc.271.48.30326. [DOI] [PubMed] [Google Scholar]
- 24.Uhlinger D, Taylor K, Lambeth JD. p67-phox enhances the binding of p47-phox to the human neutrophil respiratory burst oxidase complex. Journal of Biological Chemistry. 1994;269:22095–22098. [PubMed] [Google Scholar]
- 25.Bolscher BGJM, van Zwieten R, Kramer IM, Weening RS, Verhoeven AJ, Roos DA. phosphoprotein of Mr 47,000, defective in autosomal chronic granuloamatous disease, copurifies with one of two soluble components required for NADPH:O2 oxidoreductase activity in human neutrophils. Journal of Clinical Investigation. 1989;83:757–763. doi: 10.1172/JCI113954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ambruso DR, Bolscher BGJM, Stokman PM, Verhoeven AJ, Roos D. Assembly and activation of the NADPH:O2 oxidoreductase in human neutrophils after stimulation with phorbol myristate acetate. Journal of Biological Chemistry. 1990;265:924–930. [PubMed] [Google Scholar]
- 27.El Benna J, Faust L, Babior B. The phosphorylation of the respiratory burst oxidase component p47phox during neutrophil activation. Phosphorylation of sites recognized by protein kinase C and by proline-directed kinases. Journal of Biological Chemistry. 1994;269:23431–23436. [PubMed] [Google Scholar]
- 28.El Benna J, Faust L, Johnson J, Babior B. Phosphorylation of the respiratory burst oxidase subunit p47phox as determined by two-dimensional phosphopeptide mapping. Journal of Biological Chemistry. 1996;271:6374–6378. doi: 10.1074/jbc.271.11.6374. [DOI] [PubMed] [Google Scholar]
- 29.Ogura K, Nobuhisa I, Yuzawa S, Takeya R, Torikai S, Saikawa K, Sumimoto H, Inagaki F. NMR solution structure of the tandem Src homology 3 domains of p47phox complexed with a p22phox-derived proline-rich peptide. J Biol Chem. 2006;281:3660–3668. doi: 10.1074/jbc.M505193200. [DOI] [PubMed] [Google Scholar]
- 30.Yuzawa S, Suzuki NN, Fujioka Y, Ogura K, Sumimoto H, Inagaki F. A molecular mechanism for autoinhibition of the tandem SH3 domains of p47phox, the regulatory subunit of the phagocyte NADPH oxidase. Genes Cells. 2004;9:443–456. doi: 10.1111/j.1356-9597.2004.00733.x. [DOI] [PubMed] [Google Scholar]
- 31.Yuzawa S, Ogura K, Horiuchi M, Suzuki NN, Fujioka Y, Kataoka M, Sumimoto H, Inagaki F. Solution structure of the tandem Src homology 3 domains of p47phox in an autoinhibited form. J Biol Chem. 2004;279:29752–29760. doi: 10.1074/jbc.M401457200. [DOI] [PubMed] [Google Scholar]
- 32.Ueyama T, Tatsuno T, Kawasaki T, Tsujibe S, Shirai Y, Sumimoto H, Leto TL, Saito N. A Regulated Adaptor Function of p40phox: Distinct p67phox Membrane Targeting by p40phox and by p47phox. Mol Biol Cell. 2007;18:441–454. doi: 10.1091/mbc.E06-08-0731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cross A. p40phox participates in the activation of NADPH oxidase by increasing the affinity of p47phox for flavocytochrome b558. Biochem J. 2000;349:113–117. doi: 10.1042/0264-6021:3490113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abo A, Webb MR, Grogan A, Segal A. Activation of NADPH oxidase involves the dissociation of p21rac from its inhibitory GDP/GTP exchange protein (rhoGDI) followed by its translocation to the plasma membrane. Biochemical Journal. 1994;298:585–591. doi: 10.1042/bj2980585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.DerMardirossian C, Bokoch GM. GDIs: central regulatory molecules in Rho GTPase activation. Trends Cell Biol. 2005;15:356–363. doi: 10.1016/j.tcb.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Uhlinger DJ, Tyagi SR, Inge KL, Lambeth JD. The respiratory burst oxidase of human neutrophils: Guanine nucleotides and arachidonate regulate the assembly of a multicomponent complex in a semirecombinant cell-free system. Journal of Biological Chemistry. 1993;268:8624–8631. [PubMed] [Google Scholar]
- 37.Quinn MT, Evans T, Loetterle LR, Jesaitis AJ, Bokoch GM. Translocation of Rac correlates with NADPH oxidase activation: Evidence for equimolar translocation of oxidase components. Journal of Biological Chemistry. 1993;268:20983–20987. [PubMed] [Google Scholar]
- 38.Kleinberg ME, Malech HL, Mital DA, Leto TL. p21rac does not participate in the early interaction between p47-phox and cytochrome b558 that leads to phagocyte NADPH oxidase activation in vitro. Biochemistry. 1994;33:2490–2495. doi: 10.1021/bi00175a018. [DOI] [PubMed] [Google Scholar]
- 39.Cross AR, Parkinson JF, Jones OTG. Mechanism of the superoxide-producing oxidase of neutophils: O2 is necessary for the fast reduction of cytochrome b-245 by nadph. Biochemical Journal. 1985;226:881–884. doi: 10.1042/bj2260881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cross AR, Curnutte JT. The cytosolic activating factors p47phox and p67phox have distinct roles in the regulation of electron flow in NADPH oxidase. J Biol Chem. 1995;270:6543–6548. doi: 10.1074/jbc.270.12.6543. [DOI] [PubMed] [Google Scholar]
- 41.Nisimoto Y, Motalebi S, Han C-H, Lambeth JD. The p67phox activation domain regulates electron transfer flow from NADPH to flavin in flavocytochrome b558. Journal of Biological Chemistry. 1999;274:22999–23005. doi: 10.1074/jbc.274.33.22999. [DOI] [PubMed] [Google Scholar]
- 42.Hori Y, Kikuchi A, Isomura M, Katayama M, Miura Y, Fujioka H, Kaibuchi K, Takai Y. Post-translational modifications of the C-terminal region of the rho protein are important for its interaction with membranes and the stimulatory and inhibitory GDP/GTP exchange proteins. Oncogene. 1991;6:515–522. [PubMed] [Google Scholar]
- 43.Hoffman GR, Nassar N, Cerione RA. Structure of the Rho family GTP-binding protein Cdc42 in complex with the multifunctional regulator RhoGDI. Cell. 2000;100:345–356. doi: 10.1016/s0092-8674(00)80670-4. [DOI] [PubMed] [Google Scholar]
- 44.Diekmann D, Abo A, Johnson C, Segal A, Hall A. Interaction of Rac with p67phox and regulation of phagocytic NADPH oxidase activity. Science. 1994;265:531–533. doi: 10.1126/science.8036496. [DOI] [PubMed] [Google Scholar]
- 45.Dorseuil O, Reibel L, Bokoch G, Camonis J, Gacon G. The Rac target NADPH oxidase p67phox interacts preferentially with Rac2 rather than Rac1. Journal of Biological Chemistry. 1996;271:83–88. doi: 10.1074/jbc.271.1.83. [DOI] [PubMed] [Google Scholar]
- 46.Nisimoto Y, Freeman J, Motalebi S, Hirshberg M, Lambeth JD. Rac binding to p67phox. Journal of Biological Chemistry. 1997;272:18834–18841. doi: 10.1074/jbc.272.30.18834. [DOI] [PubMed] [Google Scholar]
- 47.Freeman JL, Abo A, Lambeth JD. Rac “Insert Region” is a Novel Effector Region that is Implicated in the Activation of NADPH Oxidase, but not PAK65. Journal of Biological Chemistry. 1996;271:19794–19801. doi: 10.1074/jbc.271.33.19794. [DOI] [PubMed] [Google Scholar]
- 48.Sarfstein R, Gorzalczany Y, Mizrahi A, Berdichevsky Y, Molshanski-Mor S, Weinbaum C, Hirshberg M, Dagher MC, Pick E. Dual role of Rac in the assembly of NADPH oxidase, tethering to the membrane and activation of p67phox: a study based on mutagenesis of p67phox-Rac1 chimeras. J Biol Chem. 2004;279:16007–16016. doi: 10.1074/jbc.M312394200. [DOI] [PubMed] [Google Scholar]
- 49.Diebold BA, Bokoch GM. Molecular basis for Rac2 regulation of phagocyte NADPH oxidase. Nat Immunol. 2001;2:211–215. doi: 10.1038/85259. [DOI] [PubMed] [Google Scholar]
- 50.Vignais PV. The superoxide-generating NADPH oxidase: structural aspects and activation mechanism. Cell Mol Life Sci. 2002;59:1428–1459. doi: 10.1007/s00018-002-8520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 52.Sumimoto H, Miyano K, Takeya R. Molecular composition and regulation of the Nox family NAD(P)H oxidases. Biochem Biophys Res Commun. 2005;338:677–686. doi: 10.1016/j.bbrc.2005.08.210. [DOI] [PubMed] [Google Scholar]
- 53.Ago T, Nunoi H, Ito T, Sumimoto H. Mechanism for phosphorylation -induced activation of the phagocyte NADPH oxidase protein p47phox. Journal of Biological Chemistry. 1999;274:33644–33653. doi: 10.1074/jbc.274.47.33644. [DOI] [PubMed] [Google Scholar]
- 54.Ponting CP. Novel domains in NADPH oxidase subunits, sorting nexins and PtdIns 3-kinases, binding partners of SH3 domains? Protein Sci. 1996;5:2353–2357. doi: 10.1002/pro.5560051122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hiroaki H, Ago T, Ito T, Sumimoto H, Kohda D. Solution structure of the PX domain, a target of the SH3 domain. Nat Struct Biol. 2001;8:526–530. doi: 10.1038/88591. [DOI] [PubMed] [Google Scholar]
- 56.Kanai F, Liu H, Field SJ, Akbary H, Matsuo T, Brown GE, Cantley LC, Yaffe MB. The PX domains of p47phox and p40phox bind to lipid products of PI(3)K. Nat Cell Biol. 2001;3:675–678. doi: 10.1038/35083070. [DOI] [PubMed] [Google Scholar]
- 57.Ago T, Kuribayashi F, Hiroaki H, Takeya R, Ito T, Kohda D, Sumimoto H. Phosphorylation of p47phox directs phox homology domain from SH3 domain toward phosphoinositides, leading to phagocyte NADPH oxidase activation. Proc Natl Acad Sci U S A. 2003;100:4474–4479. doi: 10.1073/pnas.0735712100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stahelin RV, Burian A, Bruzik KS, Murray D, Cho W. Membrane binding mechanisms of the PX domains of NADPH oxidase p40phox and p47phox. J Biol Chem. 2003;278:14469–14479. doi: 10.1074/jbc.M212579200. [DOI] [PubMed] [Google Scholar]
- 59.de Mendez I, Homayounpour N, Leto TL. Specificity of p47phox SH3 domain interactions in NADPH oxidase assembly and activation. Mol Cell Biol. 1997;17:2177–2185. doi: 10.1128/mcb.17.4.2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hata K, Takeshige K, Sumimoto H. Roles for proline-rich regions of p47phox and p67phox in the phagocyte NADPH oxidase activation in vitro. Biochem Biophys Res Commun. 1997;241:226–231. doi: 10.1006/bbrc.1997.7807. [DOI] [PubMed] [Google Scholar]
- 61.Koga H, Terasawa H, Nunoi H, Takeshige K, Inagaki F, Sumimoto H. Tetratricopeptide repeat (TPR) motifs of p67phox participate in interaction with the small GTPase Rac and activation of the phagocyte NADPH oxidase. Journal of Biological Chemistry. 1999;274:25051–25060. doi: 10.1074/jbc.274.35.25051. [DOI] [PubMed] [Google Scholar]
- 62.Lapouge K, Smith SJ, Walker PA, Gamblin SJ, Smerdon SJ, Rittinger K. Structure of the TPR domain of p67phox in complex with Rac.GTP. Mol Cell. 2000;6:899–907. doi: 10.1016/s1097-2765(05)00091-2. [DOI] [PubMed] [Google Scholar]
- 63.Moscat J, Diaz-Meco MT, Albert A, Campuzano S. Cell signaling and function organized by PB1 domain interactions. Mol Cell. 2006;23:631–640. doi: 10.1016/j.molcel.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 64.Kuribayashi F, Nunoi H, Wakamatsu K, Tsunawaki S, Sato K, Ito T, Sumimoto H. The adaptor protein p40(phox) as a positive regulator of the superoxide-producing phagocyte oxidase. Embo J. 2002;21:6312–6320. doi: 10.1093/emboj/cdf642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fuchs A, Dagher MC, Vignais PV. Mapping the domains of interaction of p40phox with both p47phox and p67phox of the neutrophil oxidase complex using the two-hybrid system. J Biol Chem. 1995;270:5695–5697. doi: 10.1074/jbc.270.11.5695. [DOI] [PubMed] [Google Scholar]
- 66.Ito T, Nakamura R, Sumimoto H, Takeshige K, Sakaki Y. An SH3 domain-mediated interaction between the phagocyte NADPH oxidase factors p40phox and p47phox. FEBS Letters. 1996;385:229–232. doi: 10.1016/0014-5793(96)00387-0. [DOI] [PubMed] [Google Scholar]
- 67.Wientjes F, Panayotou G, Reeves E, Segal A. Interactions between cytosolic components of the NADPH oxidase: p40phox interacts with both p67phox and p47phox. Biochem J. 1996;317:919–924. doi: 10.1042/bj3170919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bouin A-P, Grandvaux N, Vignais P, Fuchs A. p40phox is phosphorylated on threonine 154 and serine 315 during activation of the phagocyte NADPH oxidase. Journal of Biological Chemistry. 1998;273:30097–30103. doi: 10.1074/jbc.273.46.30097. [DOI] [PubMed] [Google Scholar]
- 69.Zhan Y, Virbasius JV, Song X, Pomerleau DP, Zhou GW. The p40phox and p47phox PX domains of NADPH oxidase target cell membranes via direct and indirect recruitment by phosphoinositides. J Biol Chem. 2002;277:4512–4518. doi: 10.1074/jbc.M109520200. [DOI] [PubMed] [Google Scholar]
- 70.Meier B, Cross AR, Hancock JT, Kaup FJ, Jones TG. Identification of a superoxide-generating NADPH oxidase system in human fibroblasts. Biochemical Journal. 1991;275:241–245. doi: 10.1042/bj2750241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cross AR, Jones OTG. Enzymic mechanisms of superoxide production. Biochimica et Biophysica Acta. 1991;1057:281–298. doi: 10.1016/s0005-2728(05)80140-9. [DOI] [PubMed] [Google Scholar]
- 72.Maly FE, Cross AR, Jones OTG, Wolf-Vorbeck G, Walker C, Dahinden CA, De Weck AL. The superoxide generating system of B cell lines: Structural homology with the phagocytic oxidase and triggering via surface Ig. Journal of Immunology. 1988;140:2334–2339. [PubMed] [Google Scholar]
- 73.Jones OTG, Jones SA, Hancock JT, Topley N. Composition and organization of the NADPH oxidase of phagocytes and other cells. Biochem Soc Trans. 1993;21:343–346. doi: 10.1042/bst0210343. [DOI] [PubMed] [Google Scholar]
- 74.Burdon R. Superoxide and hydrogen peroxide in relation to mammalian cell proliferation. Free Radical Biology and Medicine. 1995;18:775–794. doi: 10.1016/0891-5849(94)00198-s. [DOI] [PubMed] [Google Scholar]
- 75.Sundaresan M, Yu Z-X, Ferrans VJ, Sulciner DJ, Gutkind JS, Irani K, Goldschmidt-Clermont PJ, Finkel T. Regulation of reactive-oxygen-species generation in fibroblasts by Rac 1. Biochem J. 1996;318:379–382. doi: 10.1042/bj3180379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sankarapandi S, Zweier JL, Mukherjee G, Quinn MT, Huso DL. Measurement and characterization of superoxide generation in microglial cells: evidence for an NADPH oxidase-dependent pathway. Arch Biochem Biophys. 1998;353:312–321. doi: 10.1006/abbi.1998.0658. [DOI] [PubMed] [Google Scholar]
- 77.Cross A, Jones O. The effect of the inhibitor diphenylene iodonium on the superoxide-generating system of neutrophils. Biochemical Journal. 1986;237:111–116. doi: 10.1042/bj2370111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Suh Y-A, Arnold RS, Lassegue B, Shi J, Xu X, Sorescu D, Chung AB, Griendling KK, Lambeth JD. Cell transformation by the superoxide-generating oxidase Mox1. Nature. 1999;401:79–82. doi: 10.1038/43459. [DOI] [PubMed] [Google Scholar]
- 79.Lambeth JD, Cheng G, Arnold RS, Edens WE. Novel homologs of gp91phox. TIBS. 2000;25:459–461. doi: 10.1016/s0968-0004(00)01658-3. [DOI] [PubMed] [Google Scholar]
- 80.Geiszt M, Kopp JB, Varnai P, Leto TL. Identification of renox, an NAD(P)H oxidase in kidney. Proc Natl Acad Sci U S A. 2000;97:8010–8014. doi: 10.1073/pnas.130135897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.De Deken X, Wang D, Many MC, Costagliola S, Libert F, Vassart G, Dumont JE, Miot F. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. Journal of Biological Chemistry. 2000;275:23227–23233. doi: 10.1074/jbc.M000916200. [DOI] [PubMed] [Google Scholar]
- 82.Cheng G, Cao Z, Xu X, van Meir EG, Lambeth JD. Homologs of gp91phox: cloning and tissue expression of Nox3, Nox4, and Nox5. Gene. 2001;269:131–140. doi: 10.1016/s0378-1119(01)00449-8. [DOI] [PubMed] [Google Scholar]
- 83.Banfi B, Molinar G, Maturana A, Steger K, Hegedus B, Demaurex N, Krause K-H. A Ca2+-activated NADPH oxidase in testis, spleen and lymph nodes. Journal of Biological Chemistry. 2001;276:37594–37601. doi: 10.1074/jbc.M103034200. [DOI] [PubMed] [Google Scholar]
- 84.Yang S, Madyashtha P, Bingel S, Ries W, Key L. A new superoxide-generating oxidase in murine osteoclasts. Journal of Biological Chemistry. 2001;276:5452–5458. doi: 10.1074/jbc.M001004200. [DOI] [PubMed] [Google Scholar]
- 85.Shiose A, Kuroda J, Tsuruya K, Hirai M, Hirakata H, Naito S, Hattori M, Sakaki Y, Sumimoto H. A novel superoxide-producing NAD(P)H oxidase in kidney. J Biol Chem. 2001;276:1417–1423. doi: 10.1074/jbc.M007597200. [DOI] [PubMed] [Google Scholar]
- 86.Ambasta RK, Kumar P, Griendling KK, Schmidt HH, Busse R, Brandes RP. Direct interaction of the novel Nox proteins with p22phox is required for the formation of a functionally active NADPH oxidase. J Biol Chem. 2004 doi: 10.1074/jbc.M406486200. [DOI] [PubMed] [Google Scholar]
- 87.Kawahara T, Ritsick D, Cheng G, Lambeth JD. Point mutations in the proline-rich region of p22phox are dominant inhibitors of Nox1- and Nox2-dependent reactive oxygen generation. J Biol Chem. 2005;280:31859–31869. doi: 10.1074/jbc.M501882200. [DOI] [PubMed] [Google Scholar]
- 88.Martyn KD, Frederick LM, von Loehneysen K, Dinauer MC, Knaus UG. Functional analysis of Nox4 reveals unique characteristics compared to other NADPH oxidases. Cellular Signalling. 2006;18:69–82. doi: 10.1016/j.cellsig.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 89.Dupuy C, Ohayon R, Valent A, Noe-Hudson M, Dee D, Virion A. Purification of a novel flavoprotein involved in the thyroid NADPH oxidase. Journal of Biological Chemistry. 1999;274:37265–37269. doi: 10.1074/jbc.274.52.37265. [DOI] [PubMed] [Google Scholar]
- 90.Edens WA, Sharling L, Cheng G, Shapira R, Kinkade JM, Edens HA, Tang X, Flaherty DB, Benian G, Lambeth JD. Tyrosine cross-linking of extracellullar matrix is catalyzed by Duox, a multidomain oxidase/peroxidase with homology to the phagocyte oxidase subunit gp91phox. Journal of Cell Biology. 2001;154:879–891. doi: 10.1083/jcb.200103132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Geiszt M, Witta J, Baffi J, Lekstrom K, Leto TL. Dual oxidases represent novel hydrogen peroxide sources supporting mucosal surface host defense. Faseb J. 2003;17:1502–1504. doi: 10.1096/fj.02-1104fje. [DOI] [PubMed] [Google Scholar]
- 92.De Deken X, Wang D, Dumont JE, Miot F. Characterization of ThOX proteins as components of the thyroid H(2)O(2)-generating system. Exp Cell Res. 2002;273:187–196. doi: 10.1006/excr.2001.5444. [DOI] [PubMed] [Google Scholar]
- 93.Harper RW, Xu C, McManus M, Heidersbach A, Eiserich JP. Duox2 exhibits potent heme peroxidase activity in human respiratory tract epithelium. FEBS Lett. 2006;580:5150–5154. doi: 10.1016/j.febslet.2006.08.048. [DOI] [PubMed] [Google Scholar]
- 94.Geiszt M, Lekstrom K, Witta J, Leto TL. Proteins Homologous to p47phox and p67phox Support Superoxide Production by NAD(P)H Oxidase 1 in Colon Epithelial Cells. J Biol Chem. 2003;278:20006–20012. doi: 10.1074/jbc.M301289200. [DOI] [PubMed] [Google Scholar]
- 95.Takeya R, Ueno N, Kami K, Taura M, Kohjima M, Izaki T, Nunoi H, Sumimoto H. Novel human homologues of p47phox and p67phox participate in activation of superoxide-producing NADPH oxidases. J Biol Chem. 2003;278:25234–25246. doi: 10.1074/jbc.M212856200. [DOI] [PubMed] [Google Scholar]
- 96.Banfi B, Clark RA, Steger K, Krause KH. Two novel proteins activate superoxide generation by the NADPH oxidase NOX1. J Biol Chem. 2003;278:3510–3513. doi: 10.1074/jbc.C200613200. [DOI] [PubMed] [Google Scholar]
- 97.Cheng G, Lambeth JD. NOXO1, Regulation of Lipid Binding, Localization, and Activation of Nox1 by the Phox Homology (PX) Domain. J Biol Chem. 2004;279:4737–4742. doi: 10.1074/jbc.M305968200. [DOI] [PubMed] [Google Scholar]
- 98.Cheng G, Diebold BA, Hughes Y, Lambeth JD. Nox1-dependent reactive oxygen generation is regulated by Rac1. J Biol Chem. 2006;281:17718–17726. doi: 10.1074/jbc.M512751200. [DOI] [PubMed] [Google Scholar]
- 99.Cheng G, Ritsick DR, Lambeth JD. Nox3 regulation by NOXO1, p47phox and p67phox. J Biol Chem. 2004 doi: 10.1074/jbc.M400660200. [DOI] [PubMed] [Google Scholar]
- 100.Ueyama T, Geiszt M, Leto TL. Involvement of Rac1 in activation of multicomponent Nox1- and Nox3-based NADPH oxidases. Mol Cell Biol. 2006;26:2160–2174. doi: 10.1128/MCB.26.6.2160-2174.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Miyano K, Ueno N, Takeya R, Sumimoto H. Direct involvement of the small GTPase Rac in activation of the superoxide-producing NADPH oxidase Nox1. Journal of Biological Chemistry. 2006;281:21857–21868. doi: 10.1074/jbc.M513665200. [DOI] [PubMed] [Google Scholar]
- 102.Lavigne MC, Malech HL, Holland SM, Leto TL. Genetic demonstration of p47phox-dependent superoxide anion production in murine vascular smooth muscle cells. Circulation. 2001;104:79–84. doi: 10.1161/01.cir.104.1.79. [DOI] [PubMed] [Google Scholar]
- 103.Brandes RP, Miller FJ, Beer S, Haendeler J, Hoffmann J, Ha T, Holland SM, Gorlach A, Busse R. The vascular NADPH oxidase subunit p47phox is involved in redox-mediated gene expression. Free Radic Biol Med. 2002;32:1116–1122. doi: 10.1016/s0891-5849(02)00789-x. [DOI] [PubMed] [Google Scholar]
- 104.Ueno N, Takeya R, Miyano K, Kikuchi H, Sumimoto H. The NADPH oxidase Nox3 constitutively produces superoxide in a p22phox-dependent manner: its regulation by oxidase organizers and activators. Journal of Biological Chemistry. 2005;280:23328–23339. doi: 10.1074/jbc.M414548200. [DOI] [PubMed] [Google Scholar]
- 105.Banfi B, Malgrange B, Knisz J, Steger K, Dubois-Dauphin M, Krause KH. NOX3, a superoxide-generating NADPH oxidase of the inner ear. J Biol Chem. 2004;279:46065–46072. doi: 10.1074/jbc.M403046200. [DOI] [PubMed] [Google Scholar]
- 106.Kiss PJ, Knisz J, Zhang Y, Baltrusaitis J, Sigmund CD, Thalmann R, Smith RJ, Verpy E, Banfi B. Inactivation of NADPH oxidase organizer 1 results in severe imbalance. Curr Biol. 2006;16:208–213. doi: 10.1016/j.cub.2005.12.025. [DOI] [PubMed] [Google Scholar]
- 107.Mahadev K, Motoshima H, Wu X, Ruddy JM, Arnold RS, Cheng G, Lambeth JD, Goldstein BJ. The NAD(P)H oxidase homolog Nox4 modulates insulin-stimulated generation of H2O2 and plays an integral role in insulin signal transduction. Mol Cell Biol. 2004;24:1844–1854. doi: 10.1128/MCB.24.5.1844-1854.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Park HS, Jung HY, Park EY, Kim J, Lee WJ, Bae YS. Cutting edge: direct interaction of TLR4 with NAD(P)H oxidase 4 isozyme is essential for lipopolysaccharide-induced production of reactive oxygen species and activation of NF-kappa B. Journal of Immunology. 2004;173:3589–3593. doi: 10.4049/jimmunol.173.6.3589. [DOI] [PubMed] [Google Scholar]
- 109.Banfi B, Tirone F, Durussel I, Knisz J, Moskwa P, Molnar GZ, Krause KH, Cox JA. Mechanism of Ca2+ activation of the NADPH oxidase 5 (NOX5) J Biol Chem. 2004;279:18583–18591. doi: 10.1074/jbc.M310268200. [DOI] [PubMed] [Google Scholar]
- 110.Jagnandan D, Church JE, Banfi B, Stuehr DJ, Marrero MB, Fulton DJ. Novel mechanism of activation of NADPH oxidase 5(NOX5): Calcium-sensitization via phosphorylation. J Biol Chem. 2006 doi: 10.1074/jbc.M608966200. [DOI] [PubMed] [Google Scholar]
- 111.Ameziane-El-Hassani R, Morand S, Boucher JL, Frapart YM, Apostolou D, Agnandji D, Gnidehou S, Ohayon R, Noel-Hudson MS, Francon J, Lalaoui K, Virion A, Dupuy C. Dual oxidase-2 has an intrinsic Ca2+-dependent H2O2-generating activity. J Biol Chem. 2005;280:30046–30054. doi: 10.1074/jbc.M500516200. [DOI] [PubMed] [Google Scholar]
- 112.Grasberger H, Refetoff S. Identification of the maturation factor for dual oxidase. Evolution of an eukaryotic operon equivalent. J Biol Chem. 2006;281:18269–18272. doi: 10.1074/jbc.C600095200. [DOI] [PubMed] [Google Scholar]
- 113.Kuwano Y, Kawahara T, Yamamoto H, Teshima-Kondo S, Tominaga K, Masuda K, Kishi K, Morita K, Rokutan K. Interferon-gamma activates transcription of NADPH oxidase 1 gene and upregulates production of superoxide anion by human large intestinal epithelial cells. Am J Physiol Cell Physiol. 2006;290:C433–443. doi: 10.1152/ajpcell.00135.2005. [DOI] [PubMed] [Google Scholar]
- 114.Geiszt M, Lekstrom K, Brenner S, Hewitt SM, Dana R, Malech HL, Leto TL. NAD(P)H Oxidase 1, a Product of Differentiated Colon Epithelial Cells, Can Partially Replace Glycoprotein 91(phox) in the Regulated Production of Superoxide by Phagocytes. J Immunol. 2003;171:299–306. doi: 10.4049/jimmunol.171.1.299. [DOI] [PubMed] [Google Scholar]
- 115.Burch JB. Regulation of GATA gene expression during vertebrate development. Seminars in Cell & Developmental Biology. 2005;16:71–81. doi: 10.1016/j.semcdb.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 116.Brewer AC, Sparks EC, Shah AM. Transcriptional regulation of the NADPH oxidase isoform, Nox1, in colon epithelial cells: role of GATA-binding factor(s) Free Radical Biology & Medicine. 2006;40:260–274. doi: 10.1016/j.freeradbiomed.2005.08.022. [DOI] [PubMed] [Google Scholar]
- 117.Arakawa N, Katsuyama M, Matsuno K, Urao N, Tabuchi Y, Okigaki M, Matsubara H, Yabe-Nishimura C. Novel transcripts of Nox1 are regulated by alternative promoters and expressed under phenotypic modulation of vascular smooth muscle cells. Biochemical Journal. 2006;398:303–310. doi: 10.1042/BJ20060300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Newburger PE, Skalnik DG, Hopkins PJ, Eklund EA, Curnutte JT. Mutations in the promoter region of the gene for gp91-phox in X-linked chronic granulomatous disease with decreased expression of cytochrome b558. Journal of Clinical Investigation. 1994;94:1205–1211. doi: 10.1172/JCI117437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Skalnik DG, Strauss EC, Orkin SH. CCAAT displacement protein as a repressor of the myelomonocytic-specific gp91-phox gene promoter. Journal of Biological Chemistry. 1991;266:16736–16744. [PubMed] [Google Scholar]
- 120.Jacobsen BM, Skalnik DG. YY1 binds five cis-elements and trans-activates the myeloid cell-restricted gp91(phox) promoter. Journal of Biological Chemistry. 1999;274:29984–29993. doi: 10.1074/jbc.274.42.29984. [DOI] [PubMed] [Google Scholar]
- 121.Anrather J, Racchumi G, Iadecola C. NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. Journal of Biological Chemistry. 2006;281:5657–5667. doi: 10.1074/jbc.M506172200. [DOI] [PubMed] [Google Scholar]
- 122.Krause KH. Tissue distribution and putative physiological function of NOX family NADPH oxidases. Jpn J Infect Dis. 2004;57:S28–29. [PubMed] [Google Scholar]
- 123.Hiruta J, Mazet F, Ogasawara M. Restricted expression of NADPH oxidase/peroxidase gene (Duox) in zone VII of the ascidian endostyle. Cell Tissue Res. 2006;326:835–841. doi: 10.1007/s00441-006-0220-6. [DOI] [PubMed] [Google Scholar]
- 124.El Hassani RA, Benfares N, Caillou B, Talbot M, Sabourin JC, Belotte V, Morand S, Gnidehou S, Agnandji D, Ohayon R, Kaniewski J, Noel-Hudson MS, Bidart JM, Schlumberger M, Virion A, Dupuy C. Dual oxidase2 is expressed all along the digestive tract. Am J Physiol Gastrointest Liver Physiol. 2005;288:G933–942. doi: 10.1152/ajpgi.00198.2004. [DOI] [PubMed] [Google Scholar]
- 125.Pachucki J, Wang D, Christophe D, Miot F. Structural and functional characterization of the two human ThOX/Duox genes and their 5′-flanking regions. Mol Cell Endocrinol. 2004;214:53–62. doi: 10.1016/j.mce.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 126.Morand S, Chaaraoui M, Kaniewski J, Deme D, Ohayon R, Noel-Hudson MS, Virion A, Dupuy C. Effect of iodide on nicotinamide adenine dinucleotide phosphate oxidase activity and Duox2 protein expression in isolated porcine thyroid follicles. Endocrinology. 2003;144:1241–1248. doi: 10.1210/en.2002-220981. [DOI] [PubMed] [Google Scholar]
- 127.De Deken X, Wang D, Many MC, Costagliola S, Libert F, Vassart G, Dumont JE, Miot F. Cloning of two human thyroid cDNAs encoding new members of the NADPH oxidase family. Journal of Biological Chemistry. 2000;275:23227–23233. doi: 10.1074/jbc.M000916200. [DOI] [PubMed] [Google Scholar]
- 128.Damante G, Tell G, Di Lauro R. A unique combination of transcription factors controls differentiation of thyroid cells. Progress in Nucleic Acid Research and Molecular Biology. 2001;66:307–356. doi: 10.1016/s0079-6603(00)66033-6. [DOI] [PubMed] [Google Scholar]
- 129.Christophe-Hobertusa C, Christophe D. Human Thyroid Oxidases genes promoter activity in thyrocytes does not appear to be functionally dependent on Thyroid Transcription Factor-1 or Pax8. Molecular and Cellular Endocrinology. 2007;264:157–163. doi: 10.1016/j.mce.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 130.Harper RW, Xu C, Eiserich JP, Chen Y, Kao CY, Thai P, Setiadi H, Wu R. Differential regulation of dual NADPH oxidases/peroxidases, Duox1 and Duox2, by Th1 and Th2 cytokines in respiratory tract epithelium. FEBS Letters. 2005;579:4911–4917. doi: 10.1016/j.febslet.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 131.Wingler K, Wunsch S, Kreutz R, Rothermund L, Paul M, Schmidt HH. Upregulation of the vascular NAD(P)H-oxidase isoforms Nox1 and Nox4 by the renin-angiotensin system in vitro and in vivo. Free Radic Biol Med. 2001;31:1456–1464. doi: 10.1016/s0891-5849(01)00727-4. [DOI] [PubMed] [Google Scholar]
- 132.Menshikov M, Plekhanova O, Cai H, Chalupsky K, Parfyonova Y, Bashtrikov P, Tkachuk V, Berk BC. Urokinase plasminogen activator stimulates vascular smooth muscle cell proliferation via redox-dependent pathways. Arterioscler Thromb Vasc Biol. 2006;26:801–807. doi: 10.1161/01.ATV.0000207277.27432.15. [DOI] [PubMed] [Google Scholar]
- 133.Katsuyama M, Fan C, Arakawa N, Nishinaka T, Miyagishi M, Taira K, Yabe-Nishimura C. Essential role of ATF-1 in induction of NOX1, a catalytic subunit of NADPH oxidase: involvement of mitochondrial respiratory chain. Biochem J. 2005;386:255–261. doi: 10.1042/BJ20041180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sorescu GP, Song H, Tressel SL, Hwang J, Dikalov S, Smith DA, Boyd NL, Platt MO, Lassegue B, Griendling KK, Jo H. Bone morphogenic protein 4 produced in endothelial cells by oscillatory shear stress induces monocyte adhesion by stimulating reactive oxygen species production from a nox1-based NADPH oxidase. Circ Res. 2004;95:773–779. doi: 10.1161/01.RES.0000145728.22878.45. [DOI] [PubMed] [Google Scholar]
- 135.Katsuyama M, Fan C, Yabe-Nishimura C. NADPH oxidase is involved in prostaglandin F2alpha-induced hypertrophy of vascular smooth muscle cells: induction of NOX1 by PGF2alpha. J Biol Chem. 2002;277:13438–13442. doi: 10.1074/jbc.M111634200. [DOI] [PubMed] [Google Scholar]
- 136.Mitsushita J, Lambeth JD, Kamata T. The superoxide-generating oxidase Nox1 is functionally required for Ras oncogene transformation. Cancer Research. 2004;64:3580–3585. doi: 10.1158/0008-5472.CAN-03-3909. [DOI] [PubMed] [Google Scholar]
- 137.Kawahara T, Kuwano Y, Teshima-Kondo S, Takeya R, Sumimoto H, Kishi K, Tsunawaki S, Hirayama T, Rokutan K. Role of nicotinamide adenine dinucleotide phosphate oxidase 1 in oxidative burst response to Toll-like receptor 5 signaling in large intestinal epithelial cells. J Immunol. 2004;172:3051–3058. doi: 10.4049/jimmunol.172.5.3051. [DOI] [PubMed] [Google Scholar]
- 138.Kawahara T, Kohjima M, Kuwano Y, Mino H, Teshima-Kondo S, Takeya R, Tsunawaki S, Wada A, Sumimoto H, Rokutan K. Helicobacter pylori lipopolysaccharide activates Rac1 and transcription of NADPH oxidase Nox1 and its organizer NOXO1 in guinea pig gastric mucosal cells. Am J Physiol Cell Physiol. 2005;288:C450–457. doi: 10.1152/ajpcell.00319.2004. [DOI] [PubMed] [Google Scholar]
- 139.Goyal P, Weissmann N, Grimminger F, Hegel C, Bader L, Rose F, Fink L, Ghofrani HA, Schermuly RT, Schmidt HH, Seeger W, Hanze J. Upregulation of NAD(P)H oxidase 1 in hypoxia activates hypoxia-inducible factor 1 via increase in reactive oxygen species. Free Radic Biol Med. 2004;36:1279–1288. doi: 10.1016/j.freeradbiomed.2004.02.071. [DOI] [PubMed] [Google Scholar]
- 140.Newburger PE, Dai Q, Whitney C. In vitro regulation of human phagocyte cytochrome b heavy and light chain gene expression by bacterial lipopolysaccharide and recombinant human cytokines. Journal of Biological Chemistry. 1991;266:16171–16177. [PubMed] [Google Scholar]
- 141.Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res. 2005;97:900–907. doi: 10.1161/01.RES.0000187457.24338.3D. [DOI] [PubMed] [Google Scholar]
- 142.Moe KT, Aulia S, Jiang F, Chua YL, Koh TH, Wong MC, Dusting GJ. Differential upregulation of Nox homologues of NADPH oxidase by tumor necrosis factor-alpha in human aortic smooth muscle and embryonic kidney cells. J Cell Mol Med. 2006;10:231–239. doi: 10.1111/j.1582-4934.2006.tb00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


