Abstract
Clinical binge eating runs a protracted course. The etiology of binge-eating remains perplexing in part because, in humans, it is difficult to isolate and assess the independent and aggregate impact of various contributing variables. Using rats, we found that footshock stress and a history of caloric restriction (S+R), combine synergistically to induce binge-eating. Stress and dieting are also strong antecedents and relapse factors in human eating disorders. Here we report further behavioral and physiological parallels to human binge-eating. Like the protracted course of human binge-eating, young female Sprague-Dawley rats continued to binge-eat after 23 restriction/stress cycles (7 months) and this despite experiencing no significant weight loss during the restriction phases. Stress alone reduced adiposity by 35% (p<0.001) but S+R rats had no significant fat-loss. An endocrine profile of normal plasma leptin and insulin levels but marked elevation of plasma corticosterone levels was found only in the binge-eating (S+R) rats (p<0.01), also paralleling endocrine profiles reported in clinical binge-eating studies. These behavioral and physiological similarities between this animal model and clinical binge-eating increase its utility in understanding binge-eating. Importantly, our findings also highlight the stubborn nature of binge-eating: once a critical experience with dieting and stress in experienced, no weight loss or food restriction is necessary to sustain it.
Keywords: corticosterone, caloric restriction, hyperphagia, weight cycling, dieting, leptin, insulin, food restriction, bulimia, overeating, palatable food, body-fat, lean mass, animal model
Binge-eating is a central feature of eating disorders, including many cases of anorexia nervosa, and, by definition, all cases of bulimia nervosa and binge-eating disorder (BED) [1]. The course of bulimia nervosa is long, typically ten years, and for BED, it can be a lifetime [1]). Binge-eating is also estimated to characterize a large percentage of the obese population, and in this population, binge-eating contributes to their obese state [2].
In the clinical literature several variables have been identified as possible “triggers” for binge-eating, including dieting, stress, and negative affect (1, 12, 14). Although a history of dieting is a common antecedent of binge-eating, bingeing can persist despite a cessation of dieting or only occasional dieting. In fact, hunger is a weak trigger of binge-eating compared to negative affect and stress [3–7]. Indeed, in individuals with bulimia, hunger can even increase after a meal [8], and a diagnostic feature of BED is eating large amounts of food when not hungry [1]. It is clear that binge-eating occurs in the absence of negative energy balance. Furthermore, most individuals with bulimia are normal weight and, as mentioned previously, those with BED are often overweight [1].
Preclinical models of binge-eating offer a means of directly testing cause-effect relationships by environmental variables and a window into the physiological underpinnings of this highly recidivistic disorder. The binge-eating rat model described here was developed in our laboratory. Only those rats with a history of cyclic caloric-restriction binge when stressed with footshock [9,10]. This simulates the proclivity for stress-induced hyperphagia, versus hypophagia, in dieters [11], and is consistent with dieting and stress as key etiological features in eating disorders [12–14]. A minimum of three restriction-refeeding cycles are needed to evoke binge-eating in the binge-eating rats [9,10]. Unlike the limited amount of time with palatable food (PF) that is integral in some models to produce binge-eating [15,16], we allow them an unrestricted time with PF. Also of clinical relevance, since many individuals with eating disorders, namely bulimia nervosa and BED, do not experience drastic weight loss, our model does not rely on dramatic levels of caloric restriction or drastic fluctuations in body weight for binge-eating to be sustained.
Here we further explore: 1) whether binge-eating in this model is sustained after a long history of restriction and stress; 2) whether once binge-eating is established, it persists despite minimal if any caloric restriction-induced weight loss; achieved with shorter cycles, and 3) whether a history of restriction and stress imposes unique changes in body composition and/or endocrine parameters. We also describe the body composition and hormonal status of the binge-eating rats with particular attention to hormones involved in the regulation of feeding and stress.
While the effect of acute total food deprivation and of environmental stressors on circulating levels of leptin, insulin, and corticosterone (CORT) has been studied in rats, little is known regarding the effect of a prolonged human-like history of dieting, alone, or coupled with stress, on these parameters. This is information that will be useful in interpreting changes in brain and behavior found with this animal model and in applying this information to better treat binge-eating. Knowledge of the status of endocrine hormones known to interact with these brain substrates of feeding, reward, and mood are needed for a more complete understanding of the physiology of binge-eating, and may raise clues as to the motivation behind the rats’ binge-eating.
Traditionally, our stress-restriction protocol used long (12 day) cycles of restriction and refeeding. Rats in the restricted groups lost up to 10% of their body weight during the restriction phase then were allowed to recover their weight prior to being stressed for the feeding test [9, 10]. Here, once binge-eating on palatable food was observed (at the end of the 4th restriction-refeeding cycle), the rats were switched to short cycles. Hence, the 5th cycle was the first short cycle. We also repeated these cycles for a total of 23 cycles to assess the duration and robustness of their binge-eating after this protracted time (7 months) with dieting and stress. Finally, we obtained measures of lean tissue, fat, and bone mass and determined plasma levels of leptin, insulin, glucose, and CORT in control rats, rats with restriction-history only, with stress only, and with the combined restriction-history and stress that results in binge-eating.
METHODS
Subjects
A total of N = 25, 70-day-old female Sprague-Dawley rats were acclimated to individual cages with ad lib chow and water in individual bedded cages under a 12:12 light/dark cycle (lights off at 1200). The rats were weight-matched and assigned to one of four groups (N = 6/7 per group): a no history of restriction/no-stress group (Control); a no history of restriction/stress group (Stress); a history of restriction/no-stress group (Restrict); and the binge-eating group, the history of restriction/stress group (S+R). The University of Alabama at Birmingham Institutional Animal Care and Use Committee approved all of the experimental procedures.
Diets
Diets used included regular rat chow and Nabisco Double-Stuf Oreo cookies. Oreo cookies served as the highly palatable food (PF) and are composed of 43% kcals from fat, 57% kcals from carbohydrate, trace protein (0.02% kcals), and contain 4.83 kcals/g (Nabisco, Hanover, NJ). Regular rat chow (Harlan-Teklad, Indianapolis, IN) is composed of 3.5% kcals from fat, 70% kcals from carbohydrate, 17% kcals from protein, 10% moisture, and contains 3.74 kcals/g.
Cyclic Caloric Restriction-Refeeding and Stress Protocol
For the first four cycles of the study the cyclic restriction-refeeding/stress procedure was followed as previously described [9,10]. Table 1 details the protocol and for the first 3 cycles, the “long cycles” were used. Briefly, during the restriction phase, rats in the Restrict and S+R groups received 5 days of a restricted amount of chow (66% of the Control’s daily chow intake prior to the start of cycling); while the Control and Stress groups received ad lib chow. On the 6th and 7th days, to simulate human-like “breaking” of a diet, all groups were allowed to refeed on ad lib PF in addition to ad lib chow, and then for 4 more days on ad lib chow but no PF. On the test day (day 12), the rats were placed in a single arm of a 4-arm radial arm maze (Coulbourn Instruments Habitest System, Allentown, PA) in which footshock stress was delivered (or not delivered for the non-stressed groups) in four 3-second bouts of 0.6 mA scrambled current with current withheld for 15 seconds between bouts. ChlorhexiDerm was used in between sessions to eliminate stress-associated scents. Rats awaited placement in radial arm maze in a separate room to minimize stress from the vocalizations made by the stressed rats. Within 20 minutes following the stress (or no stress) session and just prior to lights out, ad lib chow and PF were provided in the rats’ individual home cages. All food and spillage was measured 4 and 24 hours post-stress. After the 24 hr measure was recorded, the rats immediately began another cycle according to group assignment. Four such cycles were conducted. After these initial traditional “long” cycles (Table 1), the rats began the “short” cycle protocol (marked with * in Table 1). The short cycles were predetermined to cause minimal weight loss despite restriction and would also increase the practical use of this model by decreasing the amount of time needed to obtain binge-eating. Briefly, the short cycles differed from the long cycles in that chow was limited to 50% of the Control’s initial daily intake (vs. 66%), but for only 3 days vs. 5 days. This was followed by 1 day of ad-lib chow and PF (vs. 2 days) and then by 2 days of chow only refeeding (vs. 4 days). The feeding test was the same as in the long cycles and the rats were weighed daily throughout cycling. The rats were swiftly sacrificed without anesthesia by guillotine (IACUC approved) prior to lights off on the day following the last day of chow refeeding (Day 6) of the short, 24th cycle. Besides attempting to simulate the long-lasting course of bulimia and BED, the length of cycling also was no shorter than one used in a previous study resulting in persistent hyperphagia of palatable food in rats that underwent app. 3 months of similar restriction-refeeding cycling [17].
Table 1.
Cyclic restriction-refeeding and stress protocol for binge-eating including use of long and short restriction-refeeding phases. The long (traditional) cycles were used in cycles 1–4, and the short cycles* were used in cycles 5–23.
| Phase of Cycle: | Restriction | Refeeding with PF | Refeeding without PF | Test Day |
|---|---|---|---|---|
|
Long Cycle →
Short Cycle* → GROUPS ↓ |
Day 1–5
Day 1–3* |
Day 6–7
Day 4* |
Day 8–11
Day 5–6* |
Day 12
Day 7* |
| Control | Ad lib chow | Ad lib chow + PF | Ad lib chow | No stress |
| Stress Only | Ad lib chow | Ad lib chow + PF | Ad lib chow | Stress |
| Restriction Only | 66% of chow
*50% of chow |
Ad lib chow + PF | Ad lib chow | No stress |
| Stress +Restriction | 66% of chow*
50% of chow |
Ad lib chow + PF | Ad lib chow | Stress |
PF= palatable food (cookies); 66% and 50% of chow was determined from control groups’ mean 24 hr intake over 3 days prior to onset of the first cycle.
Body Composition Measures
Percent of body fat, lean mass, bone mass, and bone mass density were determined using dual energy x-ray absorptiometry (DXA,GE-Lunar-Prodigy, Madison, Wisconsin). Carcasses were scanned and data was analyzed using the small animal module of the encore™ 2002 software (version 6.10.029). All scans and analyses were conducted at the UAB Clinical Nutrition Research Core Laboratory by the same technician.
Endocrine Measures
Trunk blood was collected during sacrifice and assayed in the UAB Clinical Nutrition Research Core Laboratory. Glucose concentration was measured on the Analox GM7 using the glucose oxidase method (Analox USA, Lunenburg, MA). Insulin and leptin were assayed in 100ul aliquots using double-antibody RIA with reagents obtained from Linco Research Products Inc. (St. Charles, MO) according to the manufacturer’s directions. Sensitivity for the insulin assay was 0.122 ng/ml, and the mean interassay coefficient of variation was 2.77%. Sensitivity for the leptin assay was 0.5ng/ml, and the mean interassay coefficient of variation was 4.69%. CORT was assayed in 10ul aliquots using double-antibody RIA with reagents obtained from MP Biomedicals/ICN (Orangeburg, NY). Sensitivity for this assay was 11.0ng/ml, with a mean interassay coefficient of variation of 8.35%.
Statistical Analyses
Differences in chow and PF food intake, body weight, body composition, and endocrine measures were analyzed with separated analyses of variance (ANOVA) and Bonferroni post-hoc tests. Alpha was set at p<0.05. Data were expressed as group mean kcals for food intake, grams for body weight, percent body fat for fat mass, ng/ml for leptin, insulin, and CORT, and mg/dl for glucose, all ± SEM.
RESULTS
Experiment 1: Effect of Shortened Restriction-Refeeding Cycles and a Protracted History of Restriction-Refeeding and Stress on Binge-Eating Behavior
As in previous studies with this stress model of binge-eating, a minimum of 3 traditional “long” cycles produced binge-eating at 4 hrs (Fig. 1A) in the S+R rats. This was due to a greater intake of palatable food (dark bars in Fig. 1). At 24 hrs, S+R rats’ intake was still elevated (84.6 ± 3.35 total kcals) compared to the other groups (mean of 64.1 ± 3.4 kcals; p<0.01, not shown). The 5th restriction-refeeding/stress cycle is shown as it represents feeding after a “short” cycles (Fig. 1B). S+R rats consuming 52% more total kcals and 60 % more PF in 4 hrs than the mean of the other groups (Fig. 1A, p<0.001) and 61% more PF at 24hr (p<0.001; not shown). Binge-eating was also evident after 12 cycles (Fig. 1C), and 23 cycles (Fig. 1D). A novel finding was that once binge-eating was established (after the first 3 cycles), shorter restriction-refeeding cycles (as outlined in Table 1), were able to sustain binge-eating in S+R rats and to do so after many restriction-refeeding/stress cycles. Further, after the last cycle (cycle 23) and prior to sacrifice, there was no attenuation in the magnitude of binge-eating of S+R rats (Fig. 1C) compared to the earlier cycles (Fig. 1A&B). In this cycle, S+R rats’ intake of PF was 58% greater at 4hrs (Fig. 1C; p<0.001) and 32% greater at 24hrs vs. the other groups (p<0.05; not shown). Binge-eating was not a result of a negative energy-balance, as S+R rats had returned to normal bodyweight post restriction prior to stress. An additional surprising and important result was that despite the 50% restricted amount of chow given to the Restrict and S+R groups during the restriction phases of the short cycles (Table 1), it did not produce a significant decrease in the body weight of the S+R group compared to controls. In fact, the S+R rats were always within at least 95% of controls’ body weight and, during many cycles, weighed as much or even exceeded controls’ body weight. Despite this, the S+R rats binged after each of these cycles (representative examples shown in Fig. 1B–D).
Figure 1.

Chow and palatable food (PF) intake over 0–4 hrs after onset of dark of control rats (Control), of rats following footshock stress (Stress), of rats after a history of caloric restriction-refeeding (Restrict), and rats with a history of caloric restriction-refeeding after stress (S+R) following 3 traditional initial “long” cycles (A); after 5 restriction-refeeding/stress cycles, the last 2 which were “short” cycles(B); after 12 restriction-refeeding/stress cycles, the last 9 which were “short” cycles (C), and after 23 restriction-refeeding/stress cycles, the last 20 being “short” cycles (D).
Experiment 2: Effect of Restriction-Refeeding History and Stress on Body Composition
Table 2 outlines the various post-mortem body composition parameters of the groups. Only the amount of fat mass differed, with the Stress group having significantly lower fat mass than the Control and Restrict groups, p<0.001. Figure 2 depicts this difference as a mean 35% decrease of body fat from the other groups. While total carcass mass of the Stress group did not statistically differ from the other groups, it was the lowest (Table 2), a result of significant loss of fat mass. This fat loss is not apparent in the S+R rats as their % body weight as fat did not differ from the Control and Restrict group (Table 2).
Table 2.
DEXA-derived values of post-mortem body composition parameters measured after 23 cycles of either no history of restriction-refeeding and no stress (Control), stress-only (Stress), restriction-refeeding only (Restrict) or stress and restriction-refeeding that results in binge-eating (S + R).
| Groups ↓ | Total mass (g) | Lean mass (g) | Fat mass (g) | BMC (g) | BMD (g/m2) |
|---|---|---|---|---|---|
| Control | 251.0 ± 7.5 | 227.5 ± 6.7 | 23.3 ± 1.4 | 9.0 ± 0.3 | 0.20 ± 0.003 |
| Stress | 238.0 ± 7.7 | 225.0 ± 8.1 | 12.8 ± 1.4* | 9.0 ± 0.3 | 0.21 ± 0.004 |
| Restrict | 249.2 ± 3.7 | 227.8 ± 4.3 | 21.8 ± 1.7 | 8.9 ± 0.3 | 0.20 ± 0.003 |
| S + R | 241.4 ± 4.8 | 226.6 ± 7.0 | 17.7 ± 1.3 | 9.4 ± 0.3 | 0.21 ± 0.003 |
BMC = bone mass content; BMD = bone mass density;
p<0.001 Stress vs. Control and Restrict groups (no other measures differed between groups).
Figure 2.
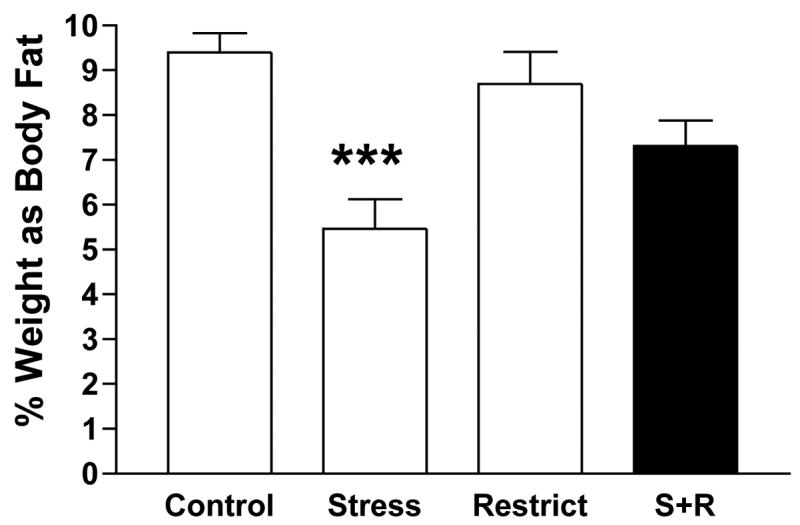
Post-sacrifice percent of body weight as fat after 23 cycles for Control rats that were never stressed or restriction-refeeding cycled, Stress rats that were only stressed, Restrict rats that were only restriction-refeeding cycled and the binge-eating S+R rats that were stressed and restriction-refeeding cycled; ***p<0.001 Stress vs. all other groups.
Experiment 3: Effect of Restriction-Refeeding History and Stress on Endocrine Hormones
Figure 3A depicts a main effect of stress to reduce plasma leptin levels; F(1,23) = 10.47, p = .004. Posthoc comparisons revealed that only the Stress group differed significantly from the Control (p<0.05) and Restriction group (p<0.01) but not the S+R group. Covariance of body fat on the ANOVA revealed that the effect of stress on leptin could be completely accounted for by a reduction in body fat (the source of leptin) of the stressed groups. However, a leptin-to-fat ratio revealed no significant differences between groups. Plasma glucose levels did not differ between the groups (Fig. 3B). Stressed groups had lower plasma insulin levels; F(1,23) = 4.55, p = 0.44 (Fig. 3C) than the non-stressed groups, but the Stress and S+R group did not differ from each other. However, as with leptin levels, covariance of body fat abolished all stress effects on insulin levels. Figure 3D graphs the plasma glucose-to-insulin ratio, revealing an absence of a diabetic-like profile in S+R rats or differences in this ratio between groups. Lastly, there was a significant interaction between stress and restriction history so that CORT levels were highest in the S+R group (Figure 4; S+R= 334.56 ± 44.7 ng/ml vs. Control = 248.9 ± 48.1, Stress = 190.73 ± 38.7, and Restrict = 132.27 ± 26.8 ng/ml; p<0.01). Correcting for body fat did not preclude the elevated CORT finding in S+R rats.
Figure 3.
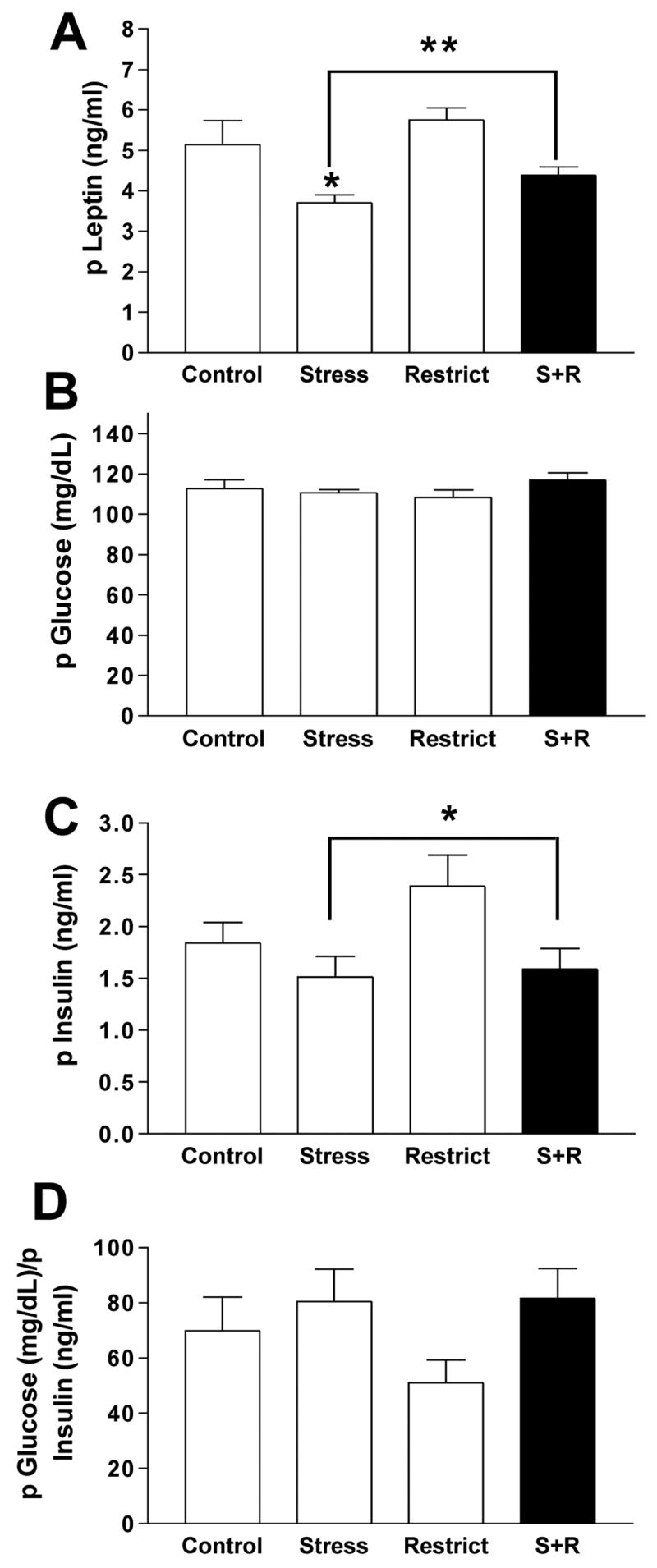
(A) Levels of plasma leptin in rats with stress and restriction-refeeding cycles (S+R; dark bar) compared to control rats (Control), rats with only stress (Stress), and rats with only a restriction-refeeding cycling (Restrict); * p<0.05 Stress vs. Control and Restrict only; ** p<0.01 main effect of stress to decrease leptin, an effect statistically accounted for by decreased body fat. (B) Plasma glucose levels for all groups; ns. (C) Plasma insulin levels for all groups; *p<0.05 main effect of stress, also statistically accounted for by decreased body fat in stressed groups. (D) Plasma glucose/insulin ratio for all groups; ns.
Figure 4.
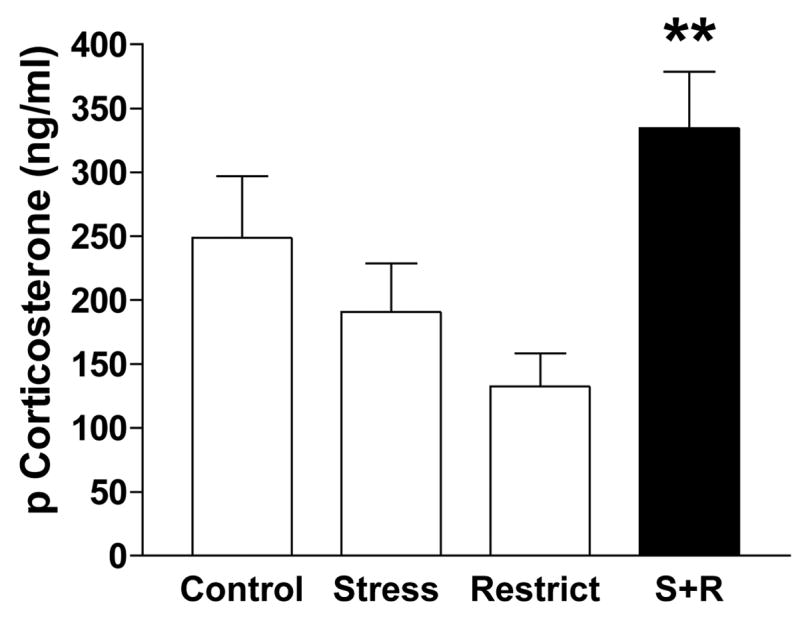
Level of plasma corticosterone in rats with stress and a history of restriction (S+R) compared to control rats (Control), rats with only stress (Stress), and rats with only a history of restriction (Restrict); ***p<0.001 S+R vs. all groups.
DISCUSSION
In this study, we determined whether binge-eating would persist in rats with a history of stress and restriction (S+R rats) after a prolonged number of stress/restriction cycles; whether minimal if any weight loss would still yield binge-eating; and lastly, whether adaptation to the combination of stress and restriction produced an endocrine profile that differed from rats exposed only to stress, only to a restriction history, or neither. We found that rats did not adapt to stress after long-term exposure to restriction-refeeding/stress cycles by normalizing their feeding response to stress. After 7 months of cycling (23 restriction/refeeding cycles), the binge-eating bouts were still robust.
We also found that binge-eating was maintained even when shorter cycles than those previously used to elicit binge-eating were used. The shorter cycles resulted in minimal weight loss during the food restriction phases compared to never-restricted control rats. During some of the cycles, S+R rats’ weights were within 100% or even over 100% of controls’ weight. This was due to the fact that with time, especially after the 6th cycle, Control rats were eating somewhat less than they initially did when they were steadily gaining weight (1st–5th cycle, not shown). Because the restricted amount of chow given to the S+R rats was tied to a percent calculated from Controls’ intake at the onset of the cycling (not at each cycle), there were times when the restricted groups (S+R and R) were eating only 1–2 grams of food less than were the non-restricted groups. In essence then, the S+R rats were not truly being deprived of food (not if operationally defined as eating less than Controls). However, the rats behaved as if they were deprived because they still overate on chow, as did the Restrict group, on the first day of ad lib feeding following the restriction phase. Increased intake of chow is a normal response to caloric deprivation. The fact that the binge-eating S+R rats were prohibited from eating freely early on in their cycling history may have been critical in shifting their threshold of caloric need upward. Because the binge-eating was always on PF and not chow, substrates linked to hedonic vs. metabolic properties of food intake may be involved in this shift. To this, we previously found that S+R rats are hyper-responsive to the anorectic actions of naloxone, an opioid-receptor antagonist, and their binge-eating was exaggerated by butorphanol, an opioid-receptor agonist (5).
Besides changes in central regulators of reward and food intake, a change in body composition and circulating hormones that regulate meal size could contribute to adaptive response to food restriction and stress. We found that stress alone decreased body fat but adding repeated food restriction (dieting) increased it. Caloric restriction by humans is intended to decrease body weight, preferably body fat, but the data from this model suggest that stress alone may achieve this goal but dieting abolishes it. The S+R group was allowed to refeed on ad lib food after restriction and to regain any lost weight, so this may have prevented loss of bone mass or bone density.
As expected, amount of body fat was tightly correlated with plasma leptin levels in the rats [18,19]. As a signal to the brain to limit food intake, leptin in abnormally low levels in the S+R rats might explain their capacity to binge eat. Although these rats had lower leptin levels, they were not significantly lower than the Control or Restrict groups. We conclude that the trend of lower leptin in the S+R rats cannot contribute to their binge-eating since lower leptin levels characterized the Stress-only rats and these rats never binged. Others using this model of binge-eating recently presented findings of greater ghrelin levels in the binge-eating rats compared the other groups of rats [20]. In their study, leptin was significantly lower in the binge-eating rats, an effect also seen in the Restrict group. It is possible that the body weight of their restricted groups was not as recovered to Control levels as ours were. In any case, greater ghrelin levels combined with decreased leptin would favor increased appetite and increased food intake [21].
Similar to the effect of stress to decrease body fat and leptin, experience with footshock stress was also associated with lower plasma insulin levels. This effect is likely linked to adipose levels as variance in fat mass accounted for the entire statistical difference in insulin levels across the groups. Indeed, insulin, like leptin, is correlated with percent body fat [22]. Regardless, we cannot attribute decreased circulating insulin to binge-eating, because a decrease in insulin was also found in the Stress rats that did not have a history of restriction and, therefore, never binged. However, as another satiety signal to the brain [18], insulin at reduced levels may render the S+R rats vulnerable to binge-eating. Glucose levels were also normal in the S+R rats and importantly, there was no evidence of an increased insulin-to-glucose ratio, ruling out the possibility that hunger induced by a diabetic-like state in S+R rats motivated their binge-eating.
While leptin and insulin levels were normal in the binge-eating rats, their plasma CORT levels were significantly elevated compared to the other groups. Footshock alone is known to elevate CORT levels [23], and we previously ascertained that CORT levels are elevated by the same intensity of footshock used here in non-cycled rats (unpublished data), but the Stress group in this study did not have elevated CORT levels relative to the non-stressed groups. This suggests that CORT levels may be attenuated by repeated experience with stress, but that adding a history of restriction reverses the attenuation. A recent study [20] replicated our results with fewer cycles, finding that only the binge-eating S+R rats had elevated CORT levels at the time they were sacrificed. Models of obesity are typically characterized by high CORT and leptin levels as leptin from increased adiposity is believed to stimulate HPA axis activity [24]. This, however, was not found here. It is possible that restriction with partial vs. daily refeeding on PF precluded obesity in this binge-eating model and, hence, precluded an association between CORT and leptin levels. However, we believe that the high CORT levels in the S+R rats contributed significantly to their binge-eating response to stress.
Several of our findings are consistent with human binge-eating, namely that binge-eating persists for long periods of time with repeated exposure to stress and that it is not contingent on extreme dieting or weight loss. The persistence of binge-eating through many cycles despite minimal, if any, weight loss, in our binge-eating rats (S+R) is analogous to the protracted course of bulimia and BED (2) and the normal to above average body weight of binge-eating patients [1], many of whom report that binge-eating persists despite absence of dieting [25,26]. These findings attest to the stubborn neuroadaptative changes to food restriction and stress that must initially occur to later sustain binge-eating. Even in overweight and obese individuals that do not binge, early attempts to lose weight through a very restrictive diet may create similar neuroadaptations to explain why it is so difficult for so many of them to lose weight and keep it off.
Also consistent with the human literature, are the body composition findings of the S+R group. Like the S+R rats, many individuals with bulimia have the same percent of body fat as healthy controls [27,28] and have no difference in bone mass density or bone mass content despite that they typically diet more frequently than individuals with BED [28]. Indeed, only bulimic patients with a history of anorexia are at higher risk for low bone mineral density, and this is attributed to lower BMI and prolonged amenorrhea [29].
A unique endocrine marker of our binge-eating rats was elevated plasma CORT levels. Increased CORT levels are associated with increased appetite and food intake in humans [30,31]. Bulimia nervosa patients have elevated CORT levels [32] and show exaggerated CORT responses to stressors [33]. More recently, a study found higher CORT levels in obese women with BED vs. obese women did not binge. When stressed with a cold pressor test, the binge-eating women reached a near significant greater elevation of CORT (p<0.057) compared to stressed non-binge-eating women. These women also reported greater hunger and desire to binge eat after the stress test [34]. Other studies using binge-eating patients and eating disordered students indicate that they perceive events as more stressful than healthy controls [35,36].
In our rats, a history of restriction may serve to increase HPA-reactivity to footshock. Here, rats with a history of restriction but no footshock stress (the Restrict group) did not have elevated CORT levels. Therefore, we can only attribute the combination of stress and a history of restriction (S+R) to elevated CORT levels. Binge-eating may be a mechanism by which these rats try to reduce or cope with the increased stress that results from this combination in so far as elevated CORT marks increased stress. Indeed, PF intake has been found to reduce adrenocorticotropic hormone (ACTH) and CORT levels in stressed rats [37], suggesting that PF intake alleviated the adverse effects of stress. In a study done by another laboratory, ACTH levels were also found to be abnormally elevated in their binge-eating S+R group [20]. This suggests that normal negative-feedback regulation of the HPA axis is absent in these rats and that they are hyper-reactive to stress. Binge-eaters report that stress and negative affect are the most powerful triggers of binge-eating [3–7]. Similarly, others have found that stress reinstated cued lever-pressing for palatable pellets after extinction in rats and that treatment with a CRF1 antagonist prevented this ‘relapse’ for PF [38]. Together these studies along with our observation of increased CORT in binge-eating rats warrant a more aggressive research focus and testing of CR1 and CRF2 antagonists in the treatment of binge-eating.
Unfortunately, we cannot confirm that binge-eating attenuated stress in our rats, or that it reduced their elevated CORT levels. Future studies will test for this, but this is also needed in human research. While individuals who binge-eat report that they use binge-eating to escape or cope with stress, this relief may be short lived because the act itself is tied to negative emotions, which serve to fuel the dieting-binge cycle (3, 21, 25). We should also note that varying levels of stress in the early stages of the rats’ development could affect the onset and robustness of the binge-eating in this animal model [39]. Because our rats were raised outside of the laboratory, it is possible that early life stress is a risk factor for binge-eating and that, later, cyclic food restriction (i.e., dieting) and footshock stress (i.e., environmental stressors) are sufficient to express binge-eating.
This animal model highlights the powerful and long lasting effects that a critical experience with caloric restriction can impose on subsequent eating behavior. Binge-eating is a lasting consequence of food restriction in prisoners of war [6] and in the famous Minnesota Semi-starvation study [40]. Our rodent model also yielded similar hormone profiles to binge-eaters in that binge-eating occurs despite normal circulating levels of leptin, insulin, and glucose. Findings of increased CORT levels in our binge-eating rats strengthen the role of increased CORT in human binge eaters as a biological substrate of binge-eating and strengthen the role of stress in binge-eating. Our findings implicate the need to target environmental stress reduction, to include more stress-reduction techniques, and increase testing of novel HPA-targeted compounds in the treatment of eating disorders where binge-eating is a main feature.
Acknowledgments
This work was supported by NIH grant DK066007 (to MMB), a Laureate Young Investigator’s Award from the National Eating Disorders Association (to MMB), and a UAB Clinical Nutrition Research Center grant (NIH PD30-DK056336). These data were presented at the 2006 Society for the Study of Ingestive Behavior (SSIB) in Naples, FL, July 22, 2006.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, IV-TR. Washington, DC: APA; 2000. [Google Scholar]
- 2.Yanovski SZ. Binge eating disorder: current knowledge and future directions. Obes Res. 1993;1:306–324. doi: 10.1002/j.1550-8528.1993.tb00626.x. [DOI] [PubMed] [Google Scholar]
- 3.Hagan MM, Shuman ES, Oswald KD, Corcoran KJ, Profitt JH, Blackburn K, Schwiebert MW, Chandler PC, Birbaum C. Incidence of chaotic eating behaviors in binge-eating disorder: Contributing factors. Behav Med. 2002;28:99–105. doi: 10.1080/08964280209596048. [DOI] [PubMed] [Google Scholar]
- 4.Crowther JH, Sanftner J, Bonifazi DZ, Shepherd KL. The role of daily hassles in binge eating. Int J Eat Disorders. 2001;29:449–454. doi: 10.1002/eat.1041. [DOI] [PubMed] [Google Scholar]
- 5.Heatherton TF, Baumeister RF. Binge eating as escape from self-awareness. Psych Bull. 1991;119:86–108. doi: 10.1037/0033-2909.110.1.86. [DOI] [PubMed] [Google Scholar]
- 6.Polivy J, Zeitlin SB, Herman CP, Beal AL. Food restriction and binge eating: a study of former prisoners of war. Journal of Abnormal Psychology. 1994;103:409–411. doi: 10.1037//0021-843x.103.2.409. [DOI] [PubMed] [Google Scholar]
- 7.Waters A, Hill A, Waller G. Internal and external antecedents of binge eating episodes in a group of women with bulimia nervosa. Int J Eat Disorders. 2001;29:17–22. doi: 10.1002/1098-108x(200101)29:1<17::aid-eat3>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 8.Walsh BT, Kissileff HR, Cassidy SM, Dantzic S. Eating behavior of women with bulimia. Arch Gen Psychiatry. 1989;46:54–58. doi: 10.1001/archpsyc.1989.01810010056008. [DOI] [PubMed] [Google Scholar]
- 9.Hagan MM, Chandler PC, Wauford PK, Rybak RJ, Oswald KD. The role of palatable food and hunger as trigger factors in an animal model of stress induced binge-eating. Int J Eat Disorders. 2003;34:183–197. doi: 10.1002/eat.10168. [DOI] [PubMed] [Google Scholar]
- 10.Hagan MM, Wauford PK, Chandler PC, Jarrett LA, Rybak RJ, Blackburn K. A new animal model of binge-eating: key synergistic role of past caloric restriction and stress. Physiol Behav. 2002;77:45–54. doi: 10.1016/s0031-9384(02)00809-0. [DOI] [PubMed] [Google Scholar]
- 11.Greeno CG, Wing RR. Stress-induced eating. Psych Bull. 1994;115:444–464. doi: 10.1037/0033-2909.115.3.444. [DOI] [PubMed] [Google Scholar]
- 12.Freeman LM, Gil KM. Daily stress, coping, and dietary restraint in binge eating. Int J Eat Disorders. 2004;36:204–212. doi: 10.1002/eat.20012. [DOI] [PubMed] [Google Scholar]
- 13.Oliver G, Wardle J, Gibson EL. Stress and food choice: a laboratory study. Psychosomatic Med. 2000;62:853–865. doi: 10.1097/00006842-200011000-00016. [DOI] [PubMed] [Google Scholar]
- 14.Wardle J, Steptoe A, Oliver G, Lipsey Z. Stress, dietary restraint and food intake. J Psychosomatic Res. 2000;48:195–202. doi: 10.1016/s0022-3999(00)00076-3. [DOI] [PubMed] [Google Scholar]
- 15.Corwin RL, Wojnicki FH, Fisher JO, Dimitriou SG, Rice HB, Young MA. Limited access to a dietary fat option affects ingestive behavior but not body composition in male rats. Physiol Behav. 1998;65:545–553. doi: 10.1016/s0031-9384(98)00201-7. [DOI] [PubMed] [Google Scholar]
- 16.Bello NT, Guarda AS, Hyun J, Moran TH. Reduced caloric availability with intermittent access to sweetened fat leads to binge-type feeding in rats. Soc Study of Ingestive Behav Abstr. 2006:182. [Google Scholar]
- 17.Hagan MM, Moss DE. Persistence of binge-eating patterns after a history of restriction with intermittent bouts of refeeding on palatable food in rats: implications for bulimia nervosa. Int J Eat Disorders. 1997;22:411–420. doi: 10.1002/(sici)1098-108x(199712)22:4<411::aid-eat6>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 18.Hewson AK, Tung LY, Connell DW, Tookman L, Dickson SL. The rat arcuate nucleus integrates peripheral signals provided by leptin, insulin, and a ghrelin mimetic. Diabetes. 2002;51:3412–3419. doi: 10.2337/diabetes.51.12.3412. [DOI] [PubMed] [Google Scholar]
- 19.Ostlund RE, Yang JW, Klein D, Gingerich R. Relation between plasma leptin concentration and body fat, gender, diet, age, and metabolic covariates. J Clin Endocrinol Metab. 1996;81:3909–3913. doi: 10.1210/jcem.81.11.8923837. [DOI] [PubMed] [Google Scholar]
- 20.Kopf S, Di Francesco MC, Casartelli A, Suozzi A, Marini F, Cristofori P, Gerrard P, Heidbreder CH, Melotto S. Ghrelin is involved in stress-induced binge eating in rats exposed to yo-yo dieting. Fed Euro Neurosc Soc Abstr 2006; Vienna, Austria. July 8–12, 2006. [Google Scholar]
- 21.Horvath TL, Diano S, Sotonyi PMH, Tschop M. Minireview: ghrelin and the regulation of energy balance--a hypothalamic perspective. Endocrinology. 2001;142:4163–4139. doi: 10.1210/endo.142.10.8490. [DOI] [PubMed] [Google Scholar]
- 22.Havel PJ, Kasim-Karakas S, Mueller W, Johnson PR, Gingerich RL, Stern JS. Relationship of plasma leptin to plasma insulin and adiposity in normal weight and overweight women: effects of dietary fat content and sustained weight loss. J Clin Endocrinol Metab. 1996;81:4406–4413. doi: 10.1210/jcem.81.12.8954050. [DOI] [PubMed] [Google Scholar]
- 23.Kant GJ, Lenox RH, Bunnell BN, Mougey EH, Pennington LL, Meyerhoff JL. Comparison of stress response in male and female rats: pituitary cyclic AMP and plasma prolactin, growth hormone and corticosterone. Psychoneuroendocrinology. 1983;8:421–428. doi: 10.1016/0306-4530(83)90021-5. [DOI] [PubMed] [Google Scholar]
- 24.van Dijk G, Donahey JC, Thiele TE, Scheurink AJ, Steffens AB, Wilkinson CW, Tenenbaum R, Campfield LA, Burn P, Seeley RJ, Woods SC. Central leptin stimulates corticosterone secretion at the onset of the dark phase. Diabetes. 1997;46:1911–1914. doi: 10.2337/diabetes.46.11.1911. [DOI] [PubMed] [Google Scholar]
- 25.Lowe MR, Gleaves DH, Murphy-Eberenz KP. On the relation of dieting and bingeing in bulimia nervosa. J Abnorm Psychol. 1998;107:263–271. doi: 10.1037//0021-843x.107.2.263. [DOI] [PubMed] [Google Scholar]
- 26.Abbott DW, de Zwaan M, Mussell MP, Raymond NC, Seim HC, Crow SJ, Crosby RD, Mitchell JE. Onset of binge eating and dieting in overweight women: implications for etiology, associated features and treatment. J Psychosom Res. 1998;44:367–374. doi: 10.1016/s0022-3999(97)00261-4. [DOI] [PubMed] [Google Scholar]
- 27.Probst M, Goris M, Vandereycken W, Pieters G, Vanderlinden J, Van Coppenolle H. Body composition in bulimia nervosa patients compared to healthy females. Eur J Nutr. 2004;43:288–296. doi: 10.1007/s00394-004-0473-3. [DOI] [PubMed] [Google Scholar]
- 28.Vaz F, Guisado J, Penas-Lledo E. History of anorexia nervosa in bulimic patients: its influence on body composition. Int J Eat Disord. 2003;34:148–155. doi: 10.1002/eat.10153. [DOI] [PubMed] [Google Scholar]
- 29.Newton J, Freeman C, Hannan W, Cowen S. Osteoporosis and normal weight bulimia nervosa--which patients are at risk? J Psychosom Res. 1993;37:239–247. doi: 10.1016/0022-3999(93)90032-b. [DOI] [PubMed] [Google Scholar]
- 30.Epel E, Lapidus R, McEwen B, Brownell K. Stress may add bite to appetite in women: a laboratory study of stress-induced cortisol and eating behavior. Psychoneuroendocrinology. 2001;26:37–49. doi: 10.1016/s0306-4530(00)00035-4. [DOI] [PubMed] [Google Scholar]
- 31.Tataranni PA, Larson DE, Snitker S, Young JB, Flatt JP, Ravussin E. Effects of glucocorticoids on energy metabolism and food intake in humans. Am J Physiol. 1996;271:E317–E325. doi: 10.1152/ajpendo.1996.271.2.E317. [DOI] [PubMed] [Google Scholar]
- 32.Monteleone P, Luisi M, Colurcio B, Casarosa E, Monteleone P, Ioime R, Genazzani AR, Maj M. Plasma levels of neuroactive steroids are increased in untreated women with anorexia nervosa or bulimia nervosa. Psychosom Med. 2001;63:62–68. doi: 10.1097/00006842-200101000-00008. [DOI] [PubMed] [Google Scholar]
- 33.Koo-Loeb JH, Costello N, Light KC, Girdler SS. Women with eating disorder tendencies display altered cardiovascular, neuroendocrine, and psychosocial profiles. Psychosom Med. 2000;62:539–548. doi: 10.1097/00006842-200007000-00013. [DOI] [PubMed] [Google Scholar]
- 34.Gluck ME, Geliebter A, Hung J, Yahav E. Cortisol, hunger, and desire to binge eat following a cold stress test in obese women with binge eating disorder. Psychosom Med. 2004;66:876–881. doi: 10.1097/01.psy.0000143637.63508.47. [DOI] [PubMed] [Google Scholar]
- 35.Cattanach L, Malley R, Rodin J. Psychologic and physiologic reactivity to stressors in eating disordered individuals. Psychosom Med. 1988;50:591–599. doi: 10.1097/00006842-198811000-00005. [DOI] [PubMed] [Google Scholar]
- 36.Lingswiler VM, Crowther JH, Stephens MA. Emotional reactivity and eating in binge eating and obesity. J Behav Med. 1987;10:287–299. doi: 10.1007/BF00846542. [DOI] [PubMed] [Google Scholar]
- 37.Pecoraro N, Reyes F, Gomez F, Bhargava A, Dallman MF. Chronic stress promotes palatable feeding, which reduces signs of stress: feedforward and feedback effects of chronic stress. Endocrinology. 2004;145:3754–3762. doi: 10.1210/en.2004-0305. [DOI] [PubMed] [Google Scholar]
- 38.Ghitza UE, Gray SM, Epstein DH, Rice KC, Shaham Y. The anxiogenic drug yohimbine reinstates palatable food seeking in a rat relapse model: a role of CRF(1) receptors. Neuropsychopharm. 2005:1–9. doi: 10.1038/sj.npp.1300964. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hancock SD, Menard JL, Olmstead MC. Variations in maternal care influence vulnerability to stress-induced binge eating in female rats. Physiol Behav. 2005;85:430–439. doi: 10.1016/j.physbeh.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 40.Keys A, Brozek J, Henschel A, Mickelsen O, Taylor HL. Behavior and complaints in natural starvation. The Biology of Human Starvation. 1950;II:783–818. [Google Scholar]


